Abstract
Studies on the effect of biochar, manure and manure ash on soil nutrient kinetics are rare. Therefore, an incubation study was conducted to investigate nitrogen (N), phosphorus (P), and sulphur (S) kinetics in soils amended with biochar (B), B + cattle manure (BCM), B + goat and sheep manure (BGSM), B + poultry manure (BPM), farm yard manure (FYM), CM ash (CMA), GSMA, PMA, and wood ash (WA). Manure and manure ash were applied at 120 kg N ha−1; B was applied at 5 tha−1.Total mineral nitrogen (TMN), available P and S were determined at 2 weeks interval. Results revealed that soils treated with WA and PMA had the highest liming (pH 9.88) and salinity (820 μS m−1), respectively. The overall cumulative release of TMN, P and S in soil treated with PMA was 1, 7 and 16% higher than BPM amended soils, respectively. The least percentage of TMN, P and S mineralized was in soil treated with BCM (13.5%), BPM (12.3%) and WA (9.3%), respectively. The first order and power functions were able to capture the kinetic release of N, P and S in the soil and amendments. TMN had significant correlation with nitrate–N (r = 0.925, p ≤ 0.001) ammonium-N (r = 0.737, p ≤ 0.01), sulphate (r = 0.823, p ≤ 0.01) and phosphate (r = 0.702, p ≤ 0.01). The study concluded that the application of manure ash or manure with biochar encourages the timely release of TMN, P and S as well as improving the salinity and pH of the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Animal manure has been reported as a rich source of plant nutrients, however, its application in the soil to supply nutrients such as nitrogen (N) (Roosta et al. 2016; Ashworth et al. 2020) can also lead to excessive application of phosphorus (P) (Rogeri et al. 2016; Bi et al. 2019; Bankole and Azeez 2024) and sulphur (S) (Hoang et al. 2020; Olawale et al. 2023; Bankole and Azeez 2024). Improper management of manure poses a serious challenge to the environment, agronomy and human health (Ashraf et al. 2021), such as odour emissions, greenhouse gas emissions such as methane and ammonia into the atmosphere, soil acidification, eutrophication (Zhang et al. 2020), leaching, and polluting underground water (Ding et al. 2016). An alternative method to mitigate the negative effect of animal manure on the environment is by converting it to manure ash (Song et al. 2020). The use of manure ash has been reported to be cost effective, attractive, and alternative source of chemical fertilizers that is free of pathogens and toxic substances, eliminates biological hazards (Tánczuk et al. 2019) and environmentally friendly (Codling 2006). The application of manure ash has been reported to increase mineralization and availability of nutrients due to the priming effects of the increased mineral input from soil organic materials (Doetterl et al. 2016) or decrease them due to the immobilization effect (Guo et al. 2018) due to the high amount of cations.
Another approach to minimizing nutrient losses and improving nutrient use efficiency is through the application of biochar (Ariyaratne 2000). Lehmann et al. (2011) defined biochar as the product of thermal degradation of organic materials in the absence of air (pyrolysis). As a soil amendment (Butnan et al. 2015), it acts as an adsorbent (Umeugochukwu 2016) and improves nutrient retention capacity (Laird et al. 2010). Biochar itself does not contain sufficient nutrients for plant growth (Yang et al. 2016), however, recent studies showed that biochar can adsorb nutrients (Ding et al. 2016) for it has a strong affinity for inorganic ions such as phosphate, sulphate, and nitrate (Kammann et al. 2015; Schmidt et al. 2015). It has been considered a potentially applicable material to mitigate nutrient loss due to its adsorption capacity (Gai et al. 2014).
Applications of biochar may be a crucial component for maintaining production while concurrently lowering pollution and fertilizer dependency, according to prior studies (Lehmann and Joseph 2015; Stavi and Lal 2013). More recently, numerous studies have shown that the addition of biochar to soils can increase crop yields and alleviate plant stresses associated with drought (Akhtar et al 2014), salinity (Dugdug et al. 2018), and heavy metals (Karunanayake et al. 2018; Rizwan et al. 2018). However, despite the increasingly diverse benefits of biochar application, impediments to the adoption of biochars in sustainable agriculture. Of concern is the great variability in biochars; not only in their nutrient availability and pH but also in their chemical (Gwenzi et al. 2014), and physical characteristics (Downie et al. 2009), depending on the nature of the feedstock and pyrolysis conditions (Barrow 2012).
Nitrate, phosphate, and sulphate are anions and may compete with each other for adsorption or colloidal sites, and their interactions may affect their availability, leading to nutrient loss if not properly managed (Brady and Weil 2010). Measuring the amount of nutrient released during a specified period under uniform conditions is an assessment of the mineralization potential of a soil or any organic material (Abbasi et al. 2003).
However, it remains to be established what mechanisms of interaction exist between biochar and manure for enhanced availability, long-term capture, and storage of nutrients in soils. Also, the availability of nutrients stored in soils as a function of biochar and manure application over time needs to be properly elucidated. Previous studies indicate that there has been very little or no retention of nutrients on biochars (Kameyama et al. 2016; Hollister et al. 2013; Gai et al. 2014).
Several approaches and methods have been used to evaluate mineralization kinetics as indicators of nutrient availability over time. Stanford and Smith (1972) proposed calculating the amount of N mineralized directly. Smith et al. (1980) improved the model and reported that a nonlinear least-squares equation gave a more accurate estimation of k and N0. Azeez and Van Averbeke (2010) used the first order, second order, and power function models developed by Dang et al. (1994) for abiotic systems to estimate nitrogen mineralization kinetics in animal manures. It was reported that the model did not capture the N kinetics. Hence, there is a need to investigate the models used by Azeez and Van Averbeke (2010) on N, P, and S kinetics in soil treated with biochar, manure and manure ash. Lentz et al. (2014) highlighted the need to address the potential effects of biochar and manure ash on nutrient release in soils amended with various fertilizer sources by incubating under controlled conditions. However, scanty studies have been conducted to elucidate these. Therefore, this research was carried out to investigate the influence of biochar, manure, and animal manure ash on N, P, and S mineralization kinetics.
Materials and Methods
Soil samples collection, preparation and analyses
Soils were collected from fallow land within the depth of 0 to 20 cm at the Federal University of Agriculture Abeokuta (7° 14′ 21'' N, 3° 26′ 89'' E) with the aid of a shovel. The collected soil samples were air dried, pulverized, and composite samples passed through a 2 mm mesh sieve. The physicochemical properties were determined (Table 1). The prepared soil samples were analyzed for pH in a 1:2 soil-to-water ratio using a glass electrode pH meter, and exchangeable acidity was determined by the titrimetric method according to McLean (1982). Soil electrical conductivity (EC) was measured in the suspension by the method of Jackson (1973). Particle size distribution was determined by the hydrometer method described by Bouyoucos (1951). Total organic carbon (TOC) was determined using the acid dichromate wet oxidation procedure of Nelson and Sommer (1996). Total nitrogen (TN) was determined by a modified micro-Kjeldahl digestion technique described by Jackson (1964). Nitrate and ammonium—nitrogen were determined spectrophotometrically (Cataldo et al. 1975). Available phosphorus (P) was extracted by Bray-1 procedure (Bray and Kurtz 1945) and determined colourimetricallly. Available S was determined in 0.01 M CaCl2 extract (Williams and Steinbergs 1959) and was determined turbidimetrically by the procedure of Chesnin and Yien (1951). Exchangeable sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg) were extracted with 1 N NH4OAc buffered at pH 7. The extracted Ca and Mg were determined using an Atomic Absorption Spectrophotometer (AAS) while extracted Na and K were determined using flame photometer. The effective cation exchange capacity (ECEC) was expressed as the sum of exchangeable cations and exchangeable acidity.
Manures Collection, Combustion and Analyses
Poultry, cattle, goat and sheep manures were collected from the College of Animal Science and Livestock Production Farm (FUNAAB). The management practices of these animals were semi-intensive except for poultry, which was intensive. Animal manures were stored as heaps in the animal units within the livestock building. The collected manures were air-dried and stored at room temperature. Poultry, cattle, goat and sheep manures were converted to manure ashes by combustion at a temperature of 600 °C in a closed furnace. Manure ashes were analyzed for nutrient composition by standard procedures (Kaira and Maynard 1991; Cater 1993).
Biochar Collection, Preparation and Analysis
Wood shavings were used for the production of biochar. The feedstock was collected from a commercial saw mill factory and air-dried before charring. The essence of air drying is to reduce the moisture content of the feedstock and hasten the charring process. Feedstock was weighed before and after charring so as to determine the biochar yield. Biochar yield is the proportion of the weight of the pyrolyzed product to the weight of the feedstock. The charring process will be carried out in a Top-lit-up-draft kiln (pyrolyzer). Feedstock was fed into the combustion chamber, and the temperature was set at 350 °C. Biochar produced was air dried, ground with a mortar and pestle to reduce their particle size, sieved to < 2 mm, and homogenized prior to routine analysis, according to Kaira and Maynard (1991) and Cater (1993).
Laboratory Experiment
The incubation experiment was arranged in a completely randomized design with three replications. The treatments consisted of biochar, biochar + cattle manure (BCM), biochar + goat and sheep manure (BGSM), biochar + poultry manure (BPM), farmyard manure (FYM), cattle manure ash (CMA), goat and sheep manure ash (GSMA), poultry manure ash (PMA), wood ash (WA), and control (soil only). Soil samples were weighed at 300 g and transferred into 500 mL plastic containers. Manure ashes and manure were applied at 120 kg N ha−1 according to Azeez and Averbeke (2010), while biochar was applied at 5 t ha−1, and incorporated into the soil. The samples were covered with a perforated thin plastic layer for ventilation, moistened to 50% of the soil’s moisture capacity. The temperature was regulated between 25 and 27 °C throughout the experiments. Samples were incubated in an incubator and analyzed fortnightly for 16 weeks for NO3–N, NH4–N, PO43−, SO42−, while total mineral N (TMN) was estimated as the sum of the NO3–N and NH4–N.
Calculations
The percentage of total N, P, and S released from an applied source at time t was calculated as
Where Mt is TMN, P and S released at time t, M0 is the total amount of N, P and S in manure sources, respectively.
First order: (Qiu et al. 2009)
Second order: (Qiu et al. 2009)
Power function
- K1:
-
first-order rate constant (day−1).
- K2:
-
second-order rate constant (mg M kg−1).
- A:
-
initial N release rate constant (mg M kg−1 day−1).
- B:
-
release rate coefficient (mg M kg−1).
Qt (mg M kg−1) is the amount of mineral M released after t days; Qe (mg M kg−1) is the amount of mineral M released at equilibrium. M in the equations refers to mineral N, P, and S. Mineralization was assessed routinely by destructive sampling.
Statistical Analysis
Data were statistically analyzed by Analysis of Variance (ANOVA) using the GENSTAT statistical package (12th edition). Treatment means were separated for significant differences using Duncan’s Multiple Range Test at 5% level of probability. The Microsoft Excel software package was used to make graphs. The correlation analysis coefficients between soil pH and EC, ammonium-N, nitrate–N, phosphate, and sulphate were analyzed using the Pearson nonparametric test.
Results
Soil and Amendments Characteristics
The textural class of the soil used for the study was sandy loam (Table 1). The pH of the soil was slightly acid (6.15) while the organic carbon content was low (6.49 g kg−1). The soil was low in total nitrogen (0.43 g kg−1), available P (7.43 mg kg−1), and S (5.32 mg kg−1), while the exchangeable cations of the soil were moderate, and their abundance was in this order: Ca > Mg > K > Na. The pH values of the amendments (Table 2) ranged from 13.18 in WA to 9.64 in BPM. The pH values of the biochar with or without manures were in the order of BGSM > BCM > B > BPM, while manure ashes were in the order of GSMA > PMA > CMA. The EC of the amendments ranged from 14.58 dS m−1 in WA to 3.77 dS m−1 in biochar.
Wood ash (WA) had significantly higher pH and EC values than other amendments; similarly, manure ashes had higher pH and EC than biochar mixed with manure. Biochar had significantly higher TOC compared to other amendments, however, there was no significant difference between the TOC of biochar mixed with manure and also between manure ashes; though biochar, BCM, BGSM, and BPM recorded significantly higher TOC than WA, CMA, GSMA, and PMA by 39.1, 13.6, 16.2, and 20.7%, respectively.
Biochar mixed with poultry manure had the highest total N, P, and S contents, followed by BCM, while biochar amendment had the least. Wood ash had the highest Ca and Mg contents, while the highest K and Na were recorded in PMA. Biochar recorded significantly higher C:N, C:P, and C:S while PMA had the least C:P and C:S, and BPM had the least C:N.
Effect of Amendments Mineralization on Soil pH and EC
The change in the soil pH with amendments over the incubation period is shown in Table 3. There was a significant increase in soil pH with the application of the amendments to the soil throughout the incubation period, except with BGSM and FYM at 10 and 14 weeks, respectively. Soil treated with WA recorded the highest pH values and was significantly higher than other amendments except CMA from 6 to 10 weeks of incubation. Soil treated with CMA, GSMA, and PMA consistently recorded higher pH values than BCM, BGSM, and BPM, respectively. In addition, the pH of soils amended with manure ash was significantly higher compared to soils treated with biochar mixed with or without manure and FYM from 10 to 16 weeks of the study. Soil pH consistently increased from 1 to 10 weeks in WA and CMA, and from 1 to 12 weeks in GSMA, while a continual decrease in soil pH was recorded in soils treated BCM and PMA throughout the entire incubation periods. The pH of soil treated with FYM slowly decreased from 4 to 16 weeks, while the pH of biochar amended soil was fairly consistent all through the study periods.
The EC in the control ranged from 77 to 120 μS m−1 and amendments had a significant increase in soil EC over the control (Table 3). Soil treated with WA had the highest EC for the first 2 weeks, while PMA amended soil recorded significantly higher EC compared to other amendments from 4 to 16 weeks, except soil treated with GSMA. All manure ash treatments (CMA, GSMA, and PMA) had significantly higher EC values than soils treated with biochar mixed with manures. However, BCM, BGSM, and BPM recorded significantly higher EC than biochar amended soil. A gradual increase in EC occurred as the weeks of incubation increased from 0 to 12 in soils treated with BPM, FYM, CMA, GSMA, and PMA, and decreased afterward until 16 weeks. Soils treated with BCD, BGSM, and WA were characterized by a gradual decrease in EC from 10 to 16 weeks of the incubation periods.
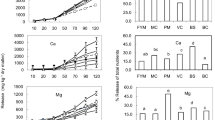
Effect of Biochar, Manure and Manure Ashes on the Cumulative Release of a. Total Mineral N, Phosphate and Sulphate
The results of cumulative release of total mineral N (TMN) release in soil increased significantly (p ≤ 0.05) with the addition of manure ashes and biochar mixed with manures compared to control (unamended soil), biochar, and WA amended soils from 6 to 16 weeks of incubation (Fig. 1a). The cumulative release of TMN in soil treated with WA and biochar was not significantly different from the control.
Soil treated with FYM had the highest cumulative release of TMN, ranging from 42.4 to 474.9 mg kg−1 followed by PMA (33.4 to 433.9 mg kg−1) and GSMA (33.1 to 337.9 mg kg−1) while the least was recorded in the control (16.2 to 143.99 mg kg−1) followed by soil treated with wood ash (19.5 to 169.19 mg kg−1) and biochar (21.4 to 204.79 mg kg−1). The addition of manure ashes had a significantly higher TMN cumulative release than biochar and biochar mixed with manure, respectively. Soil treated with BPM, BGSM, and BCM had 52.4, 33.9, and 20.4% higher cumulative releases of TMN than biochar, respectively, while soil treated with PMA, GSMA, and CMA had 61.1, 49.9, and 47.8% higher cumulative releases of TMN than WA, respectively.
As shown in Fig. 1b, the application of manure ashes and biochar mixed with manure significantly increased the cumulative release of P compared to the control, biochar and WA treated soils. In general, soil treated with PMA had significantly the highest cumulative P release, ranging from 25.3 to 259.2 mg kg−1 compared to other treatments, followed by BPM (19.2 to 245.2 mg kg−1) while the control recorded the least (15.6 to 107.3 mg kg−1). Soil treated with BPM, BGSM, and BCM had 51, 44.7, and 39.1% higher cumulative releases of P than biochar, respectively, while soil treated with PMA, GSMA, and CMA had 53.8, 38.3, and 47.4% higher cumulative releases of P than WA, respectively. The application of PMA and CMA recorded a significantly higher cumulative release of P than BPM and BCM from 2 to 16 weeks of incubation.
Soil treated with PMA had significantly (p ≤ 0.05) higher cumulative S (Fig. 1c) release at all the incubation periods than other treatments, ranging from 29 to 302.9 mg kg−1, while the least was observed in the control, biochar, and WA treated soils, which recorded significantly lower cumulative S release compared to other treatments. The application of BPM recorded a significantly higher cumulative release of S than other treatments except FYM. The overall cumulative release of S in soils treated with PMA and CMA was 14.1 and 27.6% higher than BPM and BCM amended soils, respectively, while BGSMA treated soil recorded a 3.6% higher cumulative release of S than GSMA.
Percentage of total mineral N, available P and S mineralized
Figure 2a shows soil treated with biochar recorded significantly higher TMN mineralization by 31.4%, followed by FYM (26.4%) and PMA (23.1%), while the least percentage of TMN mineralized was recorded in soil treated with BCM (13.5%), followed by BGSM (16.8%) and BPM (17.1%). Soils treated with BPM and BCM had significantly lower TMN mineralized compared to PMA and CMA, respectively. A significant high percentage (27.5%) of P mineralized from organic P (Fig. 2b) was recorded in soils amended with FYM, followed by GSMA (26.6%), compared to other treatments, while soil treated with BPM had a significantly lower percentage (12.3%) of P mineralized than other amendments. The percent of P mineralized in soils treated with BGSMA, GSMA, PMA, and CMA was significantly higher than soils amended with biochar and WA, respectively. The percentage of S mineralized (Fig. 2c) was higher in soils treated with BGSM (41.9%) and biochar (41.1%), followed by BPM (38.1%) and FYM (36%), while soil treated with WA had a significantly lower S mineralized compared to other amendments. However, soils treated with biochar mixed with manures had a significantly higher percentage of S mineralization than manure ashes.
Nitrogen, Phosphorus, and Sulphur Mineralization Kinetics
Table 4 shows the parameter estimates of the equation used to describe mineral N, P, and S kinetics in the control (soil only), soils treated with biochar mixed with manures and manure ashes. The coefficient of determination (R2) was used as the criterion for fit. Table 4 shows that the models were able to capture the mineralization pattern of mineral N in the amendments. The first and second-order constants (K1 and K2) were low and negative for all the amendments with the highest found in soil treated with BPM and the control, respectively. The first order’s R2 for all the amendments was higher than the second order though, soil treated with FYM recorded the highest R2 in both orders. For the power function, soil treated with BGSM had the highest R2 and coefficient (b); although the initial N release constant (a) was positive for all the amendments, the highest amount was observed in soil FYM, followed by GSMA, and the least in the control. The initial N release constant was observed to be higher in FYM and manure ashes than soil treated with biochar with or without manures.
The first and second-order constants for P and S mineralization were low and negative, respectively. In addition, the R2 in the P and S kinetic models was higher in the first order compared to the second order in all the amendments; however, soil treated with BGSM had the highest R2 in K1 and K2 for P mineralization, while soil treated with WA had the highest R2 in K1 and K2 for S mineralization. For the power function model, the initial release rate constant (a) was highest in PMA for P and S, while the lowest was observed in the control. Additionally, the initial release rate in PMA and CMA was higher than that in BPM and BCM, respectively.
Relationship between soil nutrients, electrical conductivity and soil pH
The data on the correlation coefficient between soil pH, electrical conductivity, nitrate, ammonium, total mineral N, phosphate, and sulphate are presented in Table 5. From these results, it was indicated that electrical conductivity had a positive and significant relationship with soil pH (r = 0.687*), nitrate (r = 0.431*), TMN (r = 0.451*), and sulphate (r = 0.445*). There was a positive and significant relationship among all forms N with phosphate and sulphate, however, TMN had highly significant correlation with nitrate N (r = 0.925***) compared to ammonium-N (r = 0.737**). A positive and significant relationship between sulphate and phosphate (r = 0.738**) was observed.
Discussion
The soil properties indicated that the pH was slightly acidic which could be due to low exchangeable bases, especially in tropical regions. The considerable amount of accumulated litter and plant debris may have contributed to the relatively moderate amount of organic carbon content in the soil. The EC of the soil in its natural state was moderate; however, the total N, P, (Aghorunse et al. 2023) and S contents of the soil were low (Bankole et al. 2022) and slightly below the critical levels.
The high pH and EC values in soil amended with wood ash could be due to the presence of a considerable amount of neutralizing compounds and hydro-oxides of Ca, Mg, and K (Mandre et al. 2006) which are the main contributors to alkalinity in wood ash. Organic carbon is the main constituent of biochar, and in this order of abundance, BCM > BGSM > BPM > FYMA > CMA > GSMA > PMA, and the least was in wood ash (WA). Manure properties showed that manure from ruminant animals seems richer in organic carbon than non-ruminant animals (poultry manure). The combined application of biochar with poultry manure (BPM) was richer in micronutrients, total N, S, and P than other amendments; this revealed that animal type, management system, diet or feed quality, additives such as growth stimulants and antibiotics, and the methods of manure storage could have accounted for higher nutrients in poultry manure compared to cattle, goat and sheep manures. Similar result was also reported by Bankole and Azeez (2024). The high carbon content of biochar materials was ascribed to the high C:N, C:P, and C:S ratios in biochar. In comparison to biochar, BCM, BGSM, FYMA, CMA, GSMA, and wood ash, the high nutrient content and low C:N:P:S ratio in PMA and BPM may accelerate the breakdown and mineralization of manure and enhance the availability and release of N, P, and S. It's possible that the biochar's high C:N:P:S ratio was caused by the usage of wood shavings and high-temperature pyrolyzing at 350 °C.
Applying wood ash or manure ash to the soil consistently resulted in a considerable increase in pH values, which were greater than those of biochar with manure additives. Due to the high concentrations of Ca, Mg, and K, which are the main contributors to the soluble alkalinity in ash, the liming effect and neutralizing capability of ash were most likely the mechanism causing this rise (Etiegni and Campbell 1991).
The application of biochar is believed to improve soil pH; in this study, the pH of biochar was almost static and consistent, however, the combined application of biochar with manure raised the pH values. This may be attributed to ion exchange reactions that occur when the terminal OH− of Al3+ and Fe2+ hydroxyl oxides are replaced by organic anions (Dikinya and Mufwanzala 2010) of the manure. Therefore, the ability of the combined application of biochar with manure to raise pH is due to the presence of exchangeable bases in the manure. Whalen et al. (2000) also reported that the increase in pH due to manure application was due to the buffering effect of cations in the manure. The highest pH was within 10 to 12 weeks of incubation in CMA, GSMA, and wood ash. In contrast, the combined application of biochar and manure had reater Ca, Mg, and K levels than the manure ashes and exhibited a more consistent, low, slow, and static liming effect throughout the incubation study. This could be attributed to the slow release of dissolved salts of Ca, Mg, K, and Na from the manures into the soil. This demonstrated that manure ash has a similar liming effect to wood ash. However, a sharp and gradual decrease in soil pH was characterized in manure ashes.
Nottidge et al. (2009) and Nweke (2018) have reported that exchangeable bases, effective cation exchange capacity (ECEC), and electrical conductivity (EC) increased in ash amended soil. All amendments significantly increased soil EC over the control; the increase in soil salinity was due to manure properties and the amount of basic cations. Soil salinization was initially greater in wood ash amended soil due to the readily available dissolved salts of Ca, Mg, K, and Na from the wood ash. Consequently, compared to other amendments, PMA, GSMA, and CMA had greater soil salt levels. The increased concentration of basic cations in the manure prior to burning, which led to a slow and continuous release of dissolved salts in the soil, may be the cause of the high EC in manure ashes.
Azeez and Averbeke (2010) reported that the increasing EC was a reflection of the amount of dissolved salts in the manures. This further buttressed the fact that liming and salinization effects are functions of manure quality and characteristics. Wood ash and manure ashes impacted a greater salinity effect than biochar, and the combined application of biochar and manure implies that the potential for soil salinization is higher in manure ashes, especially poultry manure ash. Moreover, it had been suggested that the release of base cations from wood ash and manure, sulphur mineralization, and nitrification of excess N were all factors in the rise in soil salinity. According to study, adding wood ash or biochar to organic manure may enhance microbial activity and nutrient release; this may be connected to the increase in salinity levels. The salinity of the soil is favorably impacted by the concentrations of nitrate, sulfate, and total mineral N. This might be explained by the possibility that the addition of salt from the amendments and the mineralization of manure, caused by the use of wood ash or biochar, increased the salinity of the soil.
The combined incorporation of biochar with manure or manure ashes resulted in higher total mineral N, sulphate, and phosphate compared to biochar and wood ash treated soil; this could be attributed to the high proportion of total N, P, and S in the manures before they were converted to manure ash. The incineration of manure to manure ash resulted in the greater availability of TMN, P, and S in their inorganic forms than the combined application of manure with biochar. Also, the differences in the fineness, surface area, and sizes between manure ash and manure with biochar played an important role in nutrient release and availability. Abbasi et al. (2007) reported that particle size plays a vital role in N mineralization as it affects the surface area of the N source and contact with microorganisms. Manure ashes also had a higher phosphate and sulfate cumulative release than the combined application of biochar and manure; PMA > BPM and CMA > BCM throughout the study. In addition, manure ashes had a higher TMN cumulative release than the combined application of biochar and manure, GSMA > BGSM, and PMA > BPM. The ability of the manure to mineralize and the nutrients released being adsorbed on the large surface area of biochar could be the reasons for the low release of TMN, phosphate, and sulfate in soil treated with a combined application of biochar and manure.
This had proven to be efficient at reducing dissolved soluble nutrients such as ammonium, nitrate, phosphate, and sulphate by adsorption; a similar finding was reported by Yao et al. (2012). A recent study showed that biochar, as an adsorbent (Umeugochukwu 2016), it has the ability to retain nutrients. Kammann et al. (2015) and Schmidt et al. (2015) also reported, that biochar has a strong affinity for inorganic ions such as phosphate, sulphate, and the total mineral N. Codling (2006) reported that the amount of P and S in the manure ash is similar to that in manure, while manure ash is more concentrated. This explains the rapid release of phosphate and sulphate in poultry and cattle manure ashes than in combined application of biochar with manure. Bauer et al. (2019) and Ervin (2019) observed that manure ash had similar efficiency to chemical fertilizers such as triple superphosphate and potassium sulphate.
However, the use of these chemical fertilizers further acidifies the soil. In the case of soils with relatively low pH, according to this study, the use of manure ashes may increase and maintain the soil pH to a desired level, likewise improving the release rate of soil nutrients. The significant and highly positive relationships between mineralized total mineral N (ammonium-N and nitrate–N) and sulphate and phosphate demonstrated the ability of manure or manure to increase N, P, and S availability. The rate of organic N, P, and S mineralization as well as the cumulative release that may be sustained in the soil over time at their application are important factors to consider when evaluating manure ash and biochar with manure nutrient release efficiency. Despite the role of soil microorganism activities in the manure, the proportion of organic N and P that mineralized was higher in manure ashes compared to biochar mixed with manure. The low TMN and phosphate mineralized in BCM, BGSM, and BPM could be attributed to the high C:N:P ratio and properties of biochar.
However, Xu et al. (2018) reported that the addition of biochar decreases microbial activity and invariably could also reduce the amount of TMN and P availability; a similar finding was reported by Chintala et al. (2014). Contrary to the findings observed in S mineralization, the study showed that the combined application of biochar and manure resulted in a high percentage of sulphate mineralization. The influence of biochar application with manure on the increase or decrease proportion of nutrients mineralized in sandy loam soil may not be well understood, however, several authors (Naeem et al. 2017; Motaghian et al. 2019; Song et al. 2019; Herrmann et al. 2019; Purakayastha et al. 2019) have reported an increase in soil nutrient availability with the application of biochar.
The application of kinetics in estimating potentially available and release rate coefficients of N in soils was first proposed by Stanford and Smith (1972). The estimates of mineralization and availability made by incubating biochar with manure or manure ash-amended soil under controlled conditions in this study showed the N, P, and S mineralization–immobilization dynamics of amended soil. The first and second order constants (K1 and K2) of the estimated total mineral N, phosphate, and sulphate kinetics were negative and low in all the amendments, however, the wide variation in the mineralization rate constant between biochar mixed with manure and manure ashes could be attributed to the properties of the manure and manure ashes. The initial TMN, phosphate, and sulphate release rate constants were positive for all the amendments and higher in manure ashes compared to the combined application of biochar with manure. This could be due to the lower C:N:P:S ratios of manure ashes, which release nutrients faster due to their inorganic form. Similar results have been reported by Olowoboko et al. (2019). The first order and power functions were able to capture the release of N, P, and S in the soil and amendments, since the R2 used as the criterion of fit was high compared to the second order. The present findings obeyed the model proposed by Smith et al. (1980), but contrary to the findings of Azeez and Averbeke (2010) and Olowoboko et al. (2019) that the first order, second order, and power functions were unable to capture the release pattern of N due to the non-conformity of the data to the models, which may be ascribed to the inconsistent nutrient release patterns, mineralization, immobilization and fixation. Nitrogen, P, and S were readily mineralized in soil treated with either manure ashes or a combined application of biochar and manure; this can be attributed to the inorganic form of N, P, and S in manure ashes or organic forms in manure, which were later mineralized by soil microbes. The application of manure has been reported to reduce P fixation, (Azeez and Averbeke 2010) by replacing the sites that fix P with organic anions and increasing P, N (Wu et al. 2017) and S availability.
Conclusion
The findings from the study revealed that the application of manure ash or the combination of biochar and manure improved the chemical properties of the soil. Soils treated with manure ash had a greater liming and salinization effect in the incubation periods compared to the combined application of biochar with manure. The amendments quality and properties are the pronounced determinate of N, P, and S release. The fineness, larger surface area, and concentrated nutrient composition in the mineral form of manure ash improved and increased the cumulative release of N, P, and S and the percentage mineralization of N and P over the combined application of biochar with manure. From the incubation study, the second and power function exponential models fitted well with the observed N, P, and S mineralization data of the amended soil. Further research is needed to investigate the long-term impact of manure ashes and biochar powered by manure on the kinetic release of soil nutrients in cultivated soils. The study concludes that the application of biochar with poultry manure or poultry manure ash is a good liming material in acidic soils, improves soil salinity, and gradually increases the release of N, P, and S.
Data Availability
Data available at reasonable request.
References
Abbasi MK, Shah Z, Adams WA (2003) Effects of nitrification inhibitor nitrapyrin on the fate of nitrogen applied to a soil incubated under laboratory conditions. J Plant Nutr Soil Sci 166:513–518
Agbede TM, Adekiya AO, Eifediyi EK (2017) Impact of poultry manure and NPK fertilizer on soil physical properties and growth and yield of carrot. Journal of Horticultural Research, 25 (1), 81–88.Rizwan M, Ali S, Abbas T, Adrees M, Ziaur-Rehman M, Ibrahim M, Abbas F. Qayyum M, Nawaz R. (2017). Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J Environ Manage 206:676–683. https://doi.org/10.1016/j.jenvman.2017.10.035
Aghorunse AC, Bankole GO, Odelana TB, Adewuyi S, Adejuyigbe CO, Azeez JO (2023) Comparative Evaluation of Fe-Impregnated Filter Paper and Some Conventional Phosphorus Extractants for Assessing Phosphorus Availability in Some Amended Soils of Southwest Nigeria. Commun Soil Sci Plant Anal. https://doi.org/10.1080/00103624.2023.2211095
Akhtar SS, Li G, Andersen MN, Liu F (2014) Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag 138:37–44. https://doi.org/10.1016/j.agwat.2014.02.016
Anda M, Shamshuddin J, Fauziah CI, Omar SS (2008) Mineralogy and Factors Controlling Charge Development of Three Oxisols Developed from Different Parent Materials. Geoderma 143:153–167
Ariyaratne RM (2000) Integrated Plant Nutrition Systems (IPNS) Training Manual (Sri Lanka); The Fertilizer Advisory, Development Information Network for Asia and the Pacific (FADINAP): Bangkok. Thailand 2000:140
Ashraf M, Ramzan N, Khan RU, Durrani AK (2021) Analysis of mixed cattle manure: Kinetics and thermodynamic comparison of pyrolysis and combustion processes. Case Studies in Thermal Engineering 26:101078
Azeez JO, Van Averbeke W, Okorogbon, AOM (2009) Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresource Technology. https://doi.org/10.1016/j.biortech.2009.10.095
Azeez JO, Van Averbeke W (2010) Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Biores Technol 101:5645–5651. https://doi.org/10.1016/j.biortech.2010.01.119
Bankole GO, Azeez JO (2024) Nitrogen, phosphorus and sulphur mineralization patterns of different animal manures applied on a sandy loam soil-an incubation study. Arab J Geosci. https://doi.org/10.1007/s12517-024-11905-8
Bankole GO, Sakariyawo OS, Odelana TB, Aghorunse AC, Adejuyigbe CO, Azeez JO (2022) Sulfur Fractions, Distribution and Sorption Characteristics in Some Soils of Ogun State, Southwestern Nigeria. Commun Soil Sci Plant Anal, https://doi.org/10.1080/00103624.2022.2069798
Barrow CJ (2012) Biochar : potential for countering land degradation and for improving agriculture. Appl Geogr 34:28. https://doi.org/10.1016/j.apgeog.2011.09.008
Bauer PJ, Szogi AA, Shumaker PD (2019) Fertilizer Efficacy of Poultry Litter Ash Blended with Lime or Gypsum as Fillers. Environments 6:50
Bi Q, Li KJ, Bang-Xiao Z, Liu X, Zhu YG (2019) Partial replacement of inorganic phosphorus (p) by organic manure reshapes phosphate mobilizing bacterial community and promotes p bioavailability in a paddy soil. Sci Total Environ 703:134977
Brady NC, Weil RR (2002) The Nature and Properties of Soils, 13th edn. Pearson Prentice Hall Upper Saddle River, New Jersey
Brady NC, Weil RR (2008) The Nature and Properties of Soils, 14th edn. Pearson Education, Upper Saddle River, NJ, USA
Brady NC, Weil RR (2010) Elements of the Nature and Properties of Soils; Pearson Educational International: Upper Saddle River. NJ, USA
Buss SR, Herbert AW, Morgan P, Thornton SF, Smith JWN (2004) A Review of Ammonium Attenuation in Soil and Groundwater. Q J Eng GeolHydrogeol 37:347–359
Butnan S, Deenik JL, Toomsan B (2015) Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 237–238:105–116. https://doi.org/10.1016/j.geoderma.2014.08.010
Chintala R, Schumacher TE, McDonald LM, Clay DE, Malo DD, Papiernik SK, Clay SA, Julson JL (2014) Phosphorus porption and availability from biochars and soil/biochar mixtures. CLEAN- Soil Air Water 42(5):626–634
Codling EE (2006) Laboratory characterization of extractable phosphorus in poultry litter and poultry litter ash. Soil Sci 2006(171):858–864
Dang YP, Dalal RC, Edwards DG, Tiller KG (1994) Kinetics of zinc desorption from Vertisols. Am J Soil Sci Soc 58:1392–1399
Dikinya O, Mufwanzala N (2010) Chicken manure-enhanced soil fertility and productivity: effects of application rates. J Soil Sci Environ Manag 1(3):46–54
Ding Y, Liu Y, Liu S (2016) Biochar to improve soil fertility. A Review Agronomy Sustain Dev 36:36. https://doi.org/10.1007/s13593-016-0372-z
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar Biochar for Environmental Management. Sci Technol 2009:416
Dugdug AA, Chang SX, Ok YS, Rajapaksha AU, Anyia A (2018) Phosphorus sorption capacity of biochars varies with biochar type and salinity level Environ Sci Pollut Res, pp. 1–14, https://doi.org/10.1007/s11356-018-1368-9
Ervin CR (2019) Poultry Litter Ash as an Alternative Fertilizer Source for Corn. Ph.D. Thesis. Virginia Polytechnic Institute and State University, Blacksburg, VA, USA
Etiegni L, Campbell AG (1991) Physical and chemical characteristics of wood ash. Biores Technol 37:173–178
Gai XP, Wang HY, Liu J, Zhai LM, Liu S, Ren TZ, Liu HB (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 9:e113888
Gwenzi W, Chaukura N, Mukome FND, Machado S, Nyamasoka B (2014) Biochar production and applications in sub-Saharan Africa: opportunities, constraints, risks and uncertainties. J Environ Manage 150:250–261. https://doi.org/10.1016/j.jenvman.2014.11.027
Hale SE, Alling V, Martinsen V, Mulder J, Breedveld GD, Cornelissen G (2013) The sorption and desorption of phosphate-P, ammonium N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 91:1612–1619
Herrmann L, Lesueur D, Robin A, Robain H, Bräu L (2019) Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci Total Environ 689:970–979
Hoang TTH, Do DT, Nguyen HN, Nguyen VB, Mann S, Bell RW (2020) Sulfur management strategies to improve partial sulfur balance with irrigated peanut production on deep sands. Archiv Agronom Soil Sci 1–14:1465–1478. https://doi.org/10.1080/03650340.2020.1798412
Hollister CC, Bisogni JJ, Lehmann J (2013) Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (L.) and oak wood (spp.). J Environ Qual 42:137–144
Kameyama K, Miyamoto T, Iwata Y, Shiono T (2016) Influences of feedtock and pyrolysis temperatureon the nitrate adsorption of biochar. Soil Science Plant Nutrition 62:180–184. https://doi.org/10.1080/00380768.2015.1136553
Kammann CI, Schmidt HP, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro HW, Conte P, Stephen J (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep 5:11080
Karunanayake AG, Todd OA, Crowley M, Ricchetti L, Pittman CU, Anderson R, Mohan D, Mlsna T (2018) Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem Eng J 331:480–491. https://doi.org/10.1016/j.cej.2017.08.124
Laird DA, Fleming P, Davis DD (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449. https://doi.org/10.1016/j.geoderma.2010.05.013
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836
Lentz RD, Ippolito JA, Spokas KA (2014) Biochar and manure effects on net nitrogen mineralization and greenhouse gas emissions from calcareous soil under corn. Soil Sci Soc Am J 78:1641–1655. https://doi.org/10.2136/sssaj2014.05.0198
Liu D, Huang Y, Yan H, Jiang Y, Zhao T, An S (2018) Dynamics of Soil Nitrogen Fractions and Their Relationship with Soil Microbial Communities in Two Forest Species of Northern China. PLoS ONE 13:e0196567
Lu P, O’Connor GA (2001) Biosolids effects on phosphorus retention and release in some sandy Florida soils. J Environ Qual 30:1059–1063. https://doi.org/10.2134/jeq2001.3031059x
Mandre M, Parn H, Ots K (2006) Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For Ecol Manage 223:349–357
McNeill AM, Eriksen J, Bergstro€m L, Smith KA, Marstorp H, Kirchmann H, Nilsson I (2005) Nitrogen and sulfur management: Challenges for organic sources in temperate agricultural systems. Soil Use Manag 21:82–93
Motaghian H, Hosseinpur A, Safian M (2019) The Effects of Sugarcane- Derived Biochar on Phosphorus Release Characteristics in a Calcareous Soil. J Soil Sci Plant Nutrition:1–9. https://doi.org/10.1007/s42729-019-00101-3
Naeem MA, Khalid M, Aon M, Abbas G, Tahir M, Amjad M, Murtaza B, Yang A, Akhtar SS (2017) Effect of wheat and rice straw biochar produced at different temperatures on maize growth and nutrient dynamics of a calcareous soil. Archive Agron Soil Sci 63(14):2048–2061
Naz MY, Sulaiman SA (2016) Slow Release Coating Remedy for Nitrogen Loss from Conventional Urea: A Review. J Control Release 225:109–120
Nottidge DO, Balogun RB, Njoku NR (2009) Effect of rice-husk ash on exchange acidity, growth and yield of groundnut (Arachis hypogaea L.) in an acid ultisol. Global J Agricultural Sci 8:1–6
Nweke IA (2015) Effect of Land Use on Organic Matter Concentration of Aggregate Fractions of Fallow and Cultivated Soils. Indian J Appl Resources 5:507–511
Nweke IA (2018) Contrasting tillage systems and wood ash effect on soil chemical properties. British J Environ Sci 7:8–25
Olawale BG, Blessing OT, Courage AA, Oladipupo AJ (2023) Available sulfur release and sulfur uptake as influenced by animal manures and wood ash under maize-grown soils. Arab J Geosci 16(10):563
Olowoboko TB, Azeez JO, Olujimi OO, Babalola OA (2019) Nitrogen mineralization kinetics in some tropical soils amended with ashed and un-ashed animal manures. Jordan J Earth Environ Sci 10(4):210–220
Purakayastha TJ, Bera T, Bhaduri D, Sarkar B, Mandal S, Wade P, Kumari S, Biswas S, Menon M, Pathak H, Tsang DC (2019) A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 227:345–365
Qiu H, Lv L, Pan BC, Zhang QJ, Zhang WM, Zhang QX (2009) Critical review in adsorption kinetic models. J Zhejiang Univ, Sci, A 10(5):716–724. https://doi.org/10.1631/jzus.A0820524
Schmidt HP, Pandit BH, Martinsen V, Cornelissen G, Conte P, Kammann CI (2015) Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture 5:723–741
Sika MP, Hardie AG (2014) Effect of pine wood biochar on ammonium nitrate leaching and availability in a South African sandy soil. Eur J Soil Sci 65:113–119. https://doi.org/10.1111/ejss.12082
Smith JL, Schnabel RB, McNeal BL, Campbell GS (1980) Potential errors in the first-order model for estimating soil nitrogen mineralization potentials. Soil Sci Soc Am J 44:996–1000
Song D, Xi X, Zheng Q, Liang G, Zhou W, Wang X (2019) Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol Environ Safety 180:348–356
Song T, Zhu C, Xue S, Li B, Ye J, Geng B, Li H (2020) Comparative effects of different antibiotics on antibiotic resistance during swine manure composting. Biores Technol 315:123820
Stanford G, Smith SJ (1972) Nitrogen minerialization potentials of soils. Soil Sci Soc Am Proceed 36:465–472
Stavi I, Lal R (2013) Agroforestry and biochar to offset climate change: a review. Agron Sustain Dev 33:81–96. https://doi.org/10.1007/s13593-012-0081-1
Tánczuk M, Junga R, Kolasa-Wiecek A, Niemiec P (2019) Assessment of the energy potential of chicken manure in Poland. Energies 12:1244
Umeugochukwu OP (2016) Mitigation of soil and ground water pollution caused by on-land disposal of olive mill wastewater. Stellenbosch, South Africa, pp 161
Wang G, Deng F, Zou X, Ruan H, Chen HYH (2018) Responses of soilmicrobial biomass, diversity andmetabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manag 34(4):597–605
Whalen JK, Chang C, Clayton GW, Carefoot JP (2000) Cattle manure amendment can increase the pH of acid soils. Soil Sci Soc Am J 64:962–966
Wu YP, Shaaban M, Deng CJ, Peng QA, Hu RG (2017) Changes in the soil N potential mineralization and nitrification in a rice paddy after 20 yr application of chemical fertilizers and organic matter. Can J Soil Sci 97(2):290–299. https://doi.org/10.1139/cjss-2016-0065
Yang D, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016) Biochar to improve soil fertility A review. Agronomy Sustainable Development 36:36
Zhang T, He X, Deng Y, Tsang DCW, Jiang R, Becker GC, Kruse A (2020) Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. The Science of the Total Environment 698:134240. https://doi.org/10.1016/j.scitotenv.2019.134240
Acknowledgements
The authors wish to acknowledge the staff of the Soils of Forest Island in Africa (SOFIIA) Laboratory for the research facilities and the Directorate of University Farms (DUFARMS), Federal University of Agriculture Abeokuta, for their permission to collect animal manures. The authors also appreciate Professor Chris Adejuyigbe for his supervision during the preparation and processing of biochar.
Funding
No monetary assistance, grant or resources was received to support the research and its supporting information files, all expenses were carried out by the corresponding author.
Author information
Authors and Affiliations
Contributions
Dr. Bankole Ganiyu Olawale initiated the research, collected and analyzed data, and wrote the original draft. Dr. Aghorunse Adeoba assisted in proofreading and editing the manuscript. Prof. Jamiu Azeez supervised the research and reviewed the manuscript before submission. All authors have read and approved the final and revised version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors certify do not have any conflicting interest or affiliation with any organization or entity with any financial or non-financial interest in the subject matter or material discussed in this manuscript.
Additional information
Responsible Editor: Stefan Grab
Highlights
1. The application of wood ash or manure ash to the soil instead of biochar with manure consistently increased the pH and salinity of the soil.
2. The availability of phosphorus, sulphur, and total mineral nitrogen increased as a result of the conversion of manure to manure ash.
3. The release of TMN, available P, and S in the soil treated with biochar and manure-ash additions was captured by the first-order and power functions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bankole, G.O., Aghorunse, A.C. & Azeez, J.O. Nitrogen, phosphorus and sulphur mineralization kinetic as affected by biochar, manure and ash amendments—An incubation study. Arab J Geosci 17, 148 (2024). https://doi.org/10.1007/s12517-024-11942-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-024-11942-3