Abstract
Soil salinization affects several soil properties as soil organic matter (SOM), aggregation, and microbe diversity, which may threaten the soil quality and its capacity for greenhouse gas sequestration. Relationship between soil salinity and soil respiration was extensively studied, but little is known about soil aggregation and soil organic carbon (SOC) distribution within aggregates in arid saline soils despite their importance in SOM dynamics. Non-saline soil’s EC (control, ECe = 0.65 dS/m) was adjusted using saline solutions resulting in soil-S2 (ECe = 11.98 dS/m) and soil-S3 (ECe = 20.62 dS/m). After adding wheat straw (20 g/kg soil), soils were incubated at room temperature for 225 days. Soil respiration was measured after 15, 30, 90, 195, and 225 incubation days. At the end of experiments, the aggregate size distribution and aggregate-associated SOC were determined. Cumulative respiration increased with increasing EC and time. The results showed that in all soils, the 50–250-μm fraction contained 60.8–70.6% of the total soil mass. Aggregate-associated SOC concentrations were greatest in the <50-μm fraction followed by the >250-μm fraction. Increasing EC slightly reduces the >250-μm fraction-associated SOC and increases the <50-μm fraction-associated SOC. Thus, aggregation and aggregate-associated SOC were strongly related to polysaccharide production which is controlled by microbial activity. Therefore, we can suggest that the SOC distribution within aggregation was affected by soil respiration, polysaccharide production, and aggregation processes. Results can be integrated in the sustainable SOM management in saline soils to enhance their fertility and their soil ecosystem services.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinization is one of the serious land degradation problems facing the world (Yan et al. 2015). The problem of salinity is spreading widely throughout the world, especially in arid and semi-arid regions (Cao et al. 2021; Taghizadeh-mehrjardi et al. 2016). According to Butcher et al. (2016), it is estimated that 50% of the world’s arable land will be affected by salinization by 2050. In Tunisia, about 1.5 Mha roughly 10% of the country’s area is salt-affected (Maatoug et al. 2019). This area is projected to increase further in the future because of climate change. These soils are mainly extended in the southern regions where the climate’s aridity is intensified.
Salt accumulation in the soil is a major threat to ecosystem sustainability (Chowdhury et al. 2011) and agricultural production in the world’s arid regions (Gao et al. 2021). It is well known that excess salt leads to reduce water and nutrient uptake (Machado and Serralheiro 2017; Munns and Tester 2008) which lower plant growth and lower soil microbial biomass and biochemical process essential for maintaining the soil organic matter (SOM) (Chowdhury et al. 2011; Mavi and Marschner 2012; Tripathi et al. 2006; Xiao et al. 2020). Hence, soil organic matter content, which is a function of organic matter input and turnover, is strongly affected by salinity.
The influence of salinity on soil microbial biomass and activity and carbon mineralization has been widely studied (Egamberdieva et al. 2010; Mavi et al. 2012; Muhammad et al. 2008) with contradictory results. Some studies showed harmful effects of salinity on soil microbial biomass and activity and carbon mineralization (Egamberdieva et al. 2010; Setia et al. 2010; Tripathi et al. 2007), though, other studies reported contrary results (Muhammad et al. 2008; Wong et al. 2009).
Besides investigating the effect of salinity on soil respiration, it is pivotal to study the aggregate distribution and aggregate-associated organic carbon in saline soils. Aggregates, which are special organic-inorganic complexes, are the basic unit of soil structure (Six et al. 2000), while excessive salts in soil solution, especially sodium (Na), induce several adverse phenomena (clay dispersion and aggregate swelling or slaking) which destabilize soil structure (Kohler et al. 2009; Sou/Dakouré et al. 2013).
In arid environments, soil aggregation is crucial property (Kohler et al. 2010) controlling soil’s aeration, erosion, and water permeability (Cheng et al. 2017). According to Muhammadi and Motaghian (2011) and Wang et al. (2021); aggregates and their associated organic carbon determine the capacity of soil to store and retain carbon as well as the stability of soil organic carbon (SOC) pools. Thus, aggregation and SOC distribution have an important influence on soil structure and fertility, which are known to be low in salt-affected soils. In arid regions, such as Tunisia, where agriculture is the main activity of its population, climate changes and the degradation of water resources intensify soil salinization. However, studies investigating aggregation and aggregate-associated organic carbon in saline soils still scarce (Cheng et al. 2017).
Therefore, this work aims to assess the soil organic matter dynamics in salt-affected soils by (i) determining the effect of salinity on CO2 emissions, as a measure of soil basal respiration, and (ii) comparing soil aggregate size distribution and aggregate-associated SOC concentration along different salinity levels.
Material and methods
Soils
The studied soils were classified as Aridisol and were developed under a millet cover crop from Boughrara, Southeastern Tunisia (33° 35′ 52″ N, 10° 48′ 12″ E). This area has an arid Mediterranean climate, where the average temperature reaches 45 °C in summer and 20 °C in winter with mean annual rainfall of 237 mm. The irrigation water resources of the studied area originate, mainly, from the Djeffara aquifer. The chemical composition of that water reveals a dominance of Na+, Ca2+, Cl−, and SO42− ions (Chihi et al. 2015; Zouari et al. 2011). The studied soils with a sandy loam texture were sampled from the upper 30 cm layer. Soil samples were taken with a shovel from a soil pit at the early announced depth. Then, samples were air-dried, sieved at 2 mm and analyzed for pH, soil’s saturated paste electrical conductivity (ECe), total calcium carbonate (CaCO3) (Nelson 1982), and particle size distribution (Robinson 1992) (Table 1).
Soil salinity adjustment
The EC of the air-dried soils was adjusted, based on Mavi et al. (2012) using two saline solutions prepared by dissolving different amounts of NaCl in water. The EC of the solutions were 5.75 and 11.18 dS/m corresponding respectively to 3 and 6 g/L of NaCl. The experimental soils (approximately 400 g) were leached 3–4 times with these solutions. At each leaching event, about 60–80 ml of the prepared saline solution was added after which soils were dried at 25 °C for 72–96 h. The drying maximizes the soil-solution contact (Mavi et al. 2012). After drying, soils were mixed thoroughly to break the clods and then analyzed for saturated paste ECe. This process was repeated until the ECe was adjusted to achieve a range from non-saline to extremely saline soil.
Thus, three soils were obtained and referred to as control (initial soil), soil-S2 and soil-S3 with the ECe were, respectively, 0.65 dS/m, 11.98 dS/m, and 20.62 dS/m. After adjustment of EC, the soils were kept dry at room temperature until the onset of the experiment.
Experimental design
Pre-incubation
Soil microbial activity is strongly affected by soil water availability, which is a function of water content, texture, and SOC content (Chowdhury et al. 2011). The decrease in soil water content restricts the substrate and nutrient diffusion to microbes. For this reason, experimental soils were wetted based on the study of Setia et al. (2010) which reported that maximum respiration for the loamy sand textured soil resulted in 75% water holding capacity (WHC), which corresponds in this study to the water content of 0.10 g/g soil. The wetted soils were pre-incubated for 10 days at 25 °C. The pre-incubation period of 10 days was chosen based on Yan and Marschner (2013), who indicated that microbial respiration stabilized 7–10 days after the rewetting of air-dry soil. Throughout the pre-incubation and subsequent incubation period, water was added on a mass basis to maintain the target water content.
Incubation
Mature wheat straw (ground and sieved to 0.25–2 mm) was added to the pre-incubated soils at 20 g/kg soil and thoroughly mixed. Wheat straw was added as a nutrient source for soil microbes and to enhance the carbon supply. Based on Wong et al. (2009) method, approximately 100 g of amended soils (pre-incubated soil with residues) was placed into air-tight 1 L glass incubation jars. In addition to the soil, the incubation jars had a petri dish with 20 g of soda lime granules to trap the CO2 evolved, and a small vial of water (10 ml) to maintain the humidity. The soda lime traps were oven-dried at 105 °C for 16 h before incubation. Blank was also prepared in the same conditions described above without the soil to account for the amount of CO2 absorbed by the soda lime in the headspace of the incubation jars. The glass jars were incubated in the dark at room temperature for 225 days. There were three replicates per EC level as well as for blank.
Soil respiration
Soil respiration was measured based on the modified soda lime trap method originally developed by Edwards (1982). CO2 evolution was determined after 15, 30, 90, 195, and 225 days of incubation. The traps were oven dried at 105 °C for 24 h after removal from the incubation jars, and reweighed. The amount of evolved CO2 was determined according to the following equation (Wong et al. 2009).
where SLa is weight of soda lime after incubation, SLb is weight of soda lime before incubation, SLc is weight of soda lime blank after incubation, and SLd is weight of soda lime blank before incubation. According to Keith and Wong (2006), a correction factor of 1.69 was used to correct for chemical water loss during the drying process following its reaction with CO2.
Aggregate size fractionation
Soils were fractionated into aggregates at the end of the incubation period using the wet sieving method as described by Six et al. (2000). Three aggregate fractions were obtained: (i) >250 μm (macroaggregates; M), (ii) 50–250 μm (microaggregates; m), and (iii) <50 μm (silt plus clay size particles, s + c). The aggregate-size classes were oven dried (50 °C), weighed, and stored in plastic bags. The process was repeated to obtain three replications. The aggregate fractions were analyzed to determine SOC.
Soil organic carbon
Total soil organic carbon (SOC) was determined in bulk soil samples and aggregate fractions at the end of the incubation experiment by the K2Cr2O7-H2SO4 oxidation method (Dabin 1967).
Polysaccharides
At the end of the incubation experiment, both bulk soils and aggregate fractions were analyzed for polysaccharides using the diluted acid extraction method of Dubois et al. (1956).
Statistical analysis
Data were analyzed with the XLSTAT 2018 software. Principal component analyses (PCA) and correlation test (Pearson coefficient) and ANOVA test were used to determine the effect of soil’s electrical conductivity on soil aggregation, and distribution of soil organic carbon (SOC) and polysaccharides within aggregate size fractions.
Results
Soil respiration
As shown in Fig. 1, the cumulative respiration per gram of soil increased significantly with increasing soil’s EC and incubation time. Cumulative CO2-C is slightly higher at EC 11.98 dS/m than at EC 20.62 dS/m. It increased, at EC 11.98 dS/m, by 1, 20, 47, 34, and 57%, respectively, after 15, 30, 90, 195, and 225 days of incubation. Cumulative CO2-C at EC 20.62 dS/m increased by 0.5% after 15 days, 11% after 30 days, 36% after 90 days, 27% after 195 days, and 55% after 225 days of incubation.
Soil aggregate distribution and aggregate-associated SOC
Soil aggregates are divided into macroaggregates (>250 μm), microaggregates (50–250 μm), and silt + clay fraction (<50 μm). The aggregate size distribution showed a similar pattern (Fig. 2). The microaggregate size class constitutes the major fraction within the studied soils independently of the soil EC. Microaggregates accounted for 63–71% of the total soil mass, followed by macroaggregates which accounted for 17–22 % and silt + clay fraction which accounted for 10–14% of the total soil mass.
At the end of the incubation experiment, the total soil organic carbon (SOC) concentration in bulk soil samples varied slightly (8.4–8.8 g/kg). Aggregate-associated SOC concentrations were generally greatest in silt + clay fraction (8.6–13.9 g/kg) followed by macroaggregate fractions (11.2–13.3 g/kg); however, microaggregates showed very less SOC contents (Fig. 3). Increasing soil’s EC slightly decreased SOC concentration within macroaggregates, while it increased the organic carbon content within silt + clay fraction (Fig. 3).
Polysaccharide contents
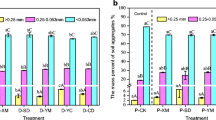
Polysaccharides within bulk soil samples varied slightly. Regardless of soil’s EC, polysaccharides were mainly concentrated in macroaggregates (>250 μm) followed by silt + clay fraction (<50 μm) and microaggregates (50–250 μm) (Fig. 4). Macroaggregates accumulated between 57.74 to 236.03 mg/L of polysaccharides, and the <50-μm fraction contained 38.76–54.56 mg/L, while microaggregates enclosed 9.22–18.19 mg/L of polysaccharides.
Polysaccharide contents within soil aggregates. The results comprise means of three replicates ± SD. Numbers followed by a different letter are not significantly different (p < 0.05). Upper case letters represent the effect of EC on means of polysaccharides within aggregates. Lower case letters represent the effects of aggregate size on polysaccharide contents
Relationship between soil’s EC and aggregation, SOC, and polysaccharide contents within aggregates
The principal component analyses (PCA) showed that EC is strongly and positively correlated with macroaggregates and <50 μm fraction proportion. The correlation coefficients that correspond, respectively, are (r = 0.85) and (r = 0.970) (Fig. 5). However, soil’s EC is significantly and negatively correlated with microaggregate proportion (r = −0.896) (Table 2). Soil’s EC is also significantly correlated with macroaggregates-associated SOC (r = −0.788) and silt + clay-associated SOC (r = 0.936). Nevertheless, EC has no pronounced effect on microaggregate-associated SOC and aggregate-associated polysaccharides. These results confirm those shown in the previous section.
Principal component analysis between soil electrical conductivity (EC); macroaggregates proportion (>250 μm); microaggregates proportion (250–50 μm), silt + clay fraction proportion (<50 μm); macroaggregate-associated soil organic carbon (>250 μm-SOC); microaggregate-associated soil organic carbon (250–50 μm-SOC); silt + clay fraction-associated soil organic carbon (<50 μm-SOC); macroaggregate-associated polysaccharides (>250 μm-poly); microaggregate-associated polysaccharides (250–50 μm-poly); silt + clay fraction-associated polysaccharides (<50 μm-poly)
Discussion
Influence of salinity on soil respiration
This study shows a significant increase in cumulative CO2-C, indicating a rise in soil respiration, with increasing EC. Our results are in disagree with previous studies (Setia et al. 2010; Wong et al. 2009; Yuan et al. 2007b) which described a negative relationship between EC and soil respiration. The negative relationship can be attributed to the toxic and inhibitor effects of salts on soil microorganisms. According to Llamas et al. (2008) and Mandeel (2006), excessive accumulation of salts in soil solution reduces water uptake due to low osmotic potential which lowers the size and activity of soil microbial biomass (Andronov et al. 2012; Rousk et al. 2011) and then reduce soil’s cumulative CO2-C.
Nevertheless, the positive effect of salinity on cumulative respiration can be explained by the presence of salt-tolerant microorganisms. According to Asghar et al. (2012), a small subset of microbes can adjust to soil salinity. Salinity-resistant microorganisms can rapidly accumulate salts or organic osmolytes to adjust their intracellular osmotic potential (Kawasaki et al. 2001). Those osmolytes can be synthesized within a few hours after exposure to salts (Hagemann 2011), which could allow the salt-tolerant microbes to remain active. According to Asghar et al. (2012), in all soils in salt-affected landscapes, there is a small subset of microbes that decompose added substrate even if the EC strongly increases.
We can suggest that the way cumulative CO2-C rises in this research may confirm the existence of salt-tolerant microbes as reported previously. In fact, the soil respiration varies in three modes: up to 90 days of incubation, the raise was gradually traducing the activation of “dormant” microorganisms after the soil’s amendment with an organic substrate. From 90 to 195 days, soil cumulative respiration increases abruptly by the increase of mineralization activity of microbes, while after 195 days of incubation, the slowdown of soil respiration is probably due to the decrease of incubated nutrients.
Therefore, in this study, the increase of cumulative respiration, as a measure of microbial activity, with increasing EC reflects the presence of a subset of salt-tolerant microbes. In the presence of freshly added residues, these salt-tolerant microbes are stimulated (Wong et al. 2009). Thus, in saline soils, microbial activity seems to be reduced mainly by a lack of available substrate.
Influence of salinity on soil aggregation and organic carbon distribution
Soil aggregates are a main component of soil structure, which is used to describe the size, shape, and arrangement of solids and pores, hence affecting pore continuity, water holding capacity, and infiltration (Leifheit et al. 2014). According to Li et al. (2014), the distribution of soil aggregates, in arid and semi-arid regions, is essential in resisting soil erosion. The distribution of aggregates partly reflects soil quality in these regions (Cheng et al. 2017). In this study, microaggregates (50–250 μm) predominate the aggregate size distribution. Similar result is found by Cheng et al. (2017) in saline soils under different halophyte types. Thus, soil salinity influence on aggregate size distribution was not very pronounced. However, the increase of soil salinity slightly decreases the microaggregate proportion, while it increases the proportion of macroaggregates and <50 μm size fraction. Aggregate size distribution is influenced by several factors including the primary particle size distribution (Schweizera et al. 2019), calcium carbonate (Bronick and Lal 2005), and SOM content (Ukalska-Jaruga et al. 2018). The studied soil is a loam sand textured soil where sand content reaches 79% and those of silt and clay are respectively 8 and 12%. In such conditions, we recorded weak soil aggregation, which can be enhanced by the presence of SOC (Bouajila et al. 2022; Bouajila et al. 2023) and calcium carbonate contents (Tatarko 2001). Therefore, soil aggregation and aggregate size distribution in saline soils seem to be largely controlled by texture and amount of CaCO3 (Bouajila et al. 2023).
Soil organic carbon is preferentially accumulated in silt + clay fraction (<50 μm) and macroaggregates (>250 μm) than in microaggregates (50–250 μm). This result is in coherence with those of Brodowski et al. (2006) who found that aggregate-associated SOC was greatest in the <53-μm fraction, which accounts for 44 to 88% of the total mass. Thus, SOC contents increased with the decrease of aggregate diameter (Liu and Yu 2011). In contrast, some studies have shown that aggregate-associated SOC concentrations are higher in macroaggregates than in microaggregates (Bouajila et al. 2021; Xie et al. 2008; Zhao et al. 2006).
In the present study, the enrichment of <50 μm fraction and macroaggregates with organic carbon could be mainly attributed to the synthesis of polysaccharides which is related to the incorporation of organic residues into the soil samples. Polysaccharides were preferentially concentrated in silt + clay fraction and macroaggregates. According to Cosentino et al. (2006), organic residues that enter the soil and mix with mineral particles act as a “nucleus” for aggregate formation, feeding microbes, and increasing their activity. In turn, this activity results in the production of polysaccharides, mucilages, and fungal hyphae that play an important role in soil aggregation (Oades 1984). Polysaccharides, which are mainly derived from plant and animal tissues and exudations of plant roots, fungal hyphae, and bacteria, are negatively charged and relatively immobile as they interact with clay particles (Tisdall 1996). Polysaccharides also act as glue to connect soil aggregates and bind clay and silt particles into macroaggregates (Kumar et al. 2013). The relative decrease in macroaggregate-associated SOC contents with increasing soil salinity could be attributed to the effect of salts on fungi. Those microorganisms play an essential role in the formation and stabilization of macroaggregates (Tisdall 1994). However, fungi are more sensitive to salt stress than bacteria (Wichern et al. 2006) which reduces macroaggregate formation (Denef et al. 2001) and its associated SOC. Therefore, the increase of soil organic carbon (incorporation of organic matter, the proliferation of bacteria, production of microbial polysaccharides…) in aggregates mainly accumulated in silt + clay fraction (Liao et al. 2006) which may explain the increase of <50 μm fraction associated SOC with increasing salinity.
To summarize, in arid saline soils, SOC is preferentially accumulated in silt + clay fraction and macroaggregates reflecting the chemical and physical protection of SOM. Organic matter is not easily accessible to soil microbes due to chemical adsorption onto the surfaces of clay minerals (Sissoko and Kpomblekou-A 2010) and physical occlusion within macroaggregates (Six et al. 2002). According to Feng et al. (2013) and Matus et al. (2008), the stabilization of organic carbon in soil increases with increasing clay or silt + clay content. However, macroaggregates are known to exert a minimal amount of physical protection. Increasing retention of crop residues (roots, shoots…) provides considerable quantities of polysaccharides and other soluble organic compounds (Six et al. 2004) responsible for slowing down the turnover rate of macroaggregates (Du et al. 2009). The increase of macroaggregate formation in soils enhances SOC physical protection. We can suggest that the SOC sequestration in saline soils (far from saturation with SOC) is possible through the enhancement of organic matter content (compost, mulch, reducing tillage..) because the mineralization rate is relatively high. The continuously amendment of soil by organic residues and sustainable management of SOC stocks reduce the impacts of dormant population of salt-tolerant microorganisms responsible of high mineralization rate.
Conclusion
In this study, we found that soil respiration increased with increasing salinity. This suggested the existence of a dormant population of salt-tolerant microorganisms that become active and can multiply rapidly when an organic substrate is available. This finding confirms that microbial activity in saline soils is mainly restricted by a lack of substrate. Despite this has no apparent effect on aggregate size distribution, increasing soil salinity promotes the accumulation of SOC in the silt + clay fraction and macroaggregates which is mainly related to polysaccharide production and microbial activity. In arid saline soils, chemical and physical protection of SOM by adsorption onto soil minerals and occlusion within aggregates enhance the soil’s capacity to sequester organic carbon. Thus, saline soils may play an essential role in reducing the atmospheric concentration of greenhouse gases and then mitigating climate change. While it is important at this point to determine the time necessary to the formation of the aggregates to protect SOM.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Andronov EE, Petrova SN, Pinaev AG, Pershina EV, Rakhimgalieva SZ, Akhmedenov KM et al (2012) Analysis of the structure of microbial community in soils with different degrees of salinization using T-RFLP and real-time PCR techniques. Eur Soill Sci 45:147–156. https://doi.org/10.1046/j.0960-7412.2001.01247.x\
Asghar HN, Setia R, Marschner P (2012) Community composition and activity of microbes from saline soils and non-saline soils respond similarly to changes in salinity. Soil Bio and Bioch 49:106–113
Bouajila A, Omar Z, Essayeh W, Brahim N (2023) Soil properties and organic carbon stock of soils under arid coastal ecosystem oasis in Southern East of Tunisia. Arid Ecosys 13(2):167–179
Bouajila A, Omar Z, Saoud R, Rahmani R (2022) Soil contamination by phthalate esters in cultivated and non-cultivated soils in North African arid regions: a Tunisian case study. Env and Nat Res Journal 20(6):553–562
Bouajila A, Omar Z, Magherbi G (2021) Soil aggregation, aggregate-associated organic carbon, and total nitrogen under different land use in Regosols of coastal arid lands in Gabes Tunisia. Arabian J of Geosci 14:1933. https://doi.org/10.1007/s12517-021-08289-4
Brodowski S, John B, Flessa H, Amelung W (2006) Aggregate-occluded black carbon in soil. Eur J of Soil Sci 57:539–546
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geode 124:3–22
Butcher K, Wick AF, DeSutter T, Chatterjee A, Harmon J (2016) Soil salinity: a threat to global food security. Agron J 108:2189–2200
Cao C, Tao S, Cui Z, Zhang Y (2021) Response of soil properties and microbial communities to increasing salinization in the Meadow Grassland of Northeast China. Microb Ecol 82:722–735
Cheng Z, Wang J, William JG, Yuang H, Zhang F (2017) Soil aggregation and aggregate-associated organic carbon under four typical halophyte communities in Northwest China. Pedo. https://doi.org/10.1016/S1002-0160(17)60330-8
Chihi H, de Marsily G, Belayouni H, Yahyaoui H (2015) Relationship between tectonic structures and hydrogeochemical compartmentalization in aquifers: example of the “Jeffara de Medenine” system, south–east Tunisia. J Hydro: Regional Studies 4:410–430
Chowdhury N, Marschner P, Burns RG (2011) Soil microbial activity and community composition: impact of changes in matric and osmotic potential. Soil Bio and Biochem 43:1229–1236
Cosentino D, Chenu C, Bissonnais YL (2006) Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil. Soil Bio and Bioch 38:2053–2062
Dabin B (1967) Application de dosage automatique à l’analyse des sols, 3éme partie. Cahier ORSTOM, série pédologie, volume V, n° 3:257–263
Denef K, Six J, Bossuyt H, Frey SD, Elliott ET, Merckx R, Paustian K (2001) Soil Biol and Biochem 33:1599–1611
Du ZL, Liu SF, Li KJ, Ren TS (2009) Soil organic carbon and physical quality as influenced by long-term application of residue and mineral fertilizer in the North China Plain. Aust J of Soil Res 47:585–591
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Edwards NT (1982) The use of soda-lime for measuring respiration rates in terrestrial systems. Pedobio 23:321–330
Egamberdieva D, Renella G, Wirth S, Islam R (2010) Secondary salinity effects on soil microbial biomass. Bio and Fertil of Soils 46:445–449
Feng W, Plante A, Six J (2013) Improving estimates of maximal organic carbon stabilization by fne soil particles. Biogeochem112: 81–93.
Gao Y, Liu X, Hou W, Han Y, Wang R, Zhang H (2021) Characteristics of saline soil in extremely arid regions: a case study using GF-3 and ALOS-2 Quad-Pol SAR data in Qinghai. China. Rem Sens 13:417. https://doi.org/10.3390/rs13030417
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35:87–123
Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S et al (2001) Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13:889–905. https://doi.org/10.1105/tpc.13.4.889
Keith H, Wong SC (2006) Measurement of soil CO2 efflux using soda lime absorption: both quantitative and reliable. Soil Bio and Biochem 38:1121–1131
Kohler J, Hernández JA, Caravaca F, Roldán A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Env and Expert Botany 65:245–252
Kohler J, Caravaca F, Roldán A (2010) An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Bio and Biochem 42:429–434
Kumar R, Rawat KS, Singh J, Singh A, Rai A (2013) Soil aggregation dynamics and carbon sequestration. J of Appl and Nat Sci 5(1):250–267
Leifheit EF, Veresoglou SD, Lehmann A, Morris EK, Rilling MC (2014) Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—a meta–analysis. Plant and Soil 374(1–2):523–537
Li X, Feng G, Sharratt BS, Zheng Z, Pi H, Gao F (2014) Soil wind erodibility based on dry aggregate-size distribution in the Tarim Basin. Soil Sci Soc of Amer J 78:2009–2016
Liao JD, Boutton TW, Jastrow JD (2006) Organic matter turnover in soil physical fractions following woody plant invasion of grassland: evidence from natural 13C and 15N. Soil Bio and Biochem 38:3197–3210
Liu ZL, Yu WT (2011) Review of researches on soil aggregate and soil organic carbon. Chin J of Eco-Agri (in Chinese) 19:447–455
Llamas DP, Gonzales MD, Gonzales CI, Lopez GR, Marquina JC (2008) Effects of water potential on spore germination and viability of Fusarium species. J Indy Microbiol Biotechnol 35(11):1411–1418
Maatoug S, Brahim N, Hatira A (2019) Amendment of saline soils by adding sand in the old oasis of Nefzaoua in Tunisia. Res J of Appl Sci, Eng and Techno 16(4):153–159. https://doi.org/10.19026/rjaset.16.6019
Machado R, Serralheiro R (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticl 3:30. https://doi.org/10.3390/horticulturae3020030
Mandeel QA (2006) Biodiversity of the genus Fusarium in saline soil habitats. J of Bas Microbio 46(6):480–494
Matus F, Lusk C, Maire CR (2008) Effects of soil texture, C input rates and litter quality on free organic matter and N mineralization in Chilean rain forest and agricultural soils. Comm Soil Sci Plant Anal 39:187–201
Mavi MS, Marschner P (2012) Drying and wetting in saline and saline-sodic soils-effects on microbial activity, biomass and dissolved organic carbon. Plant and Soil 355:51–62
Mavi MS, Marschner P, Chittleborough DJ, Cox JW, Sanderman J (2012) Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol and Biochem 45:8–13
Muhammad S, Muller T, Joergensen RG (2008) Relationships between soil biological and other soil properties in saline and alkaline arable soils from the Pakistani Punjab. J of Arid Env 72:448–457
Muhammadi J, Motaghian MH (2011) Spatial prediction of soil aggregate stability and aggregate-associated organic carbon content at the catchment scale using geostatistical techniques. Pedo 21:389–399
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Anal Review Plant Bio 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nelson RE (1982) Carbonate and gypsum. In: Page AL (ed) Methods of soil analysis, Part 2, Agronomy Monographs, vol 9, 2nd edn. ASA and SSSA, Madison, pp 181–197
Oades JM (1984) Soil organic matter and structural stability: mechanisms and implications for management. Plant and Soil 76:319–337
Robinson GW (1992) A new method for mechanical analysis of soil and other dispersion. J of Agric Food Chemist 12:306–321
Rousk J, Elyaagubi FK, Jones DL, Godbold DL (2011) Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Bio and Biochem 43:1881–1887
Schweizera SA, Buckaa FB, Graf-Rosenfellnerb M, Kögel-Knabner I (2019) Soil microaggregate size composition and organic matter distribution as affected by clay content. Geoder 355:113901
Setia R, Marschner P, Baldock J, Chittleborough D (2010) Is CO2 evolution in saline soils affected by an osmotic effect and calcium carbonate? Bio and Fert of Soils 46:781–792
Sissoko A, Kpomblekou-A K (2010) Carbon decomposition in broiler litter-amended soils. Soil Bio and Biochem 42:543–550
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil & Till Res 79:7–31
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of organic matter: Interpretations for C-saturation of soils. Plant and Soil 241:155–176
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Bio and Biochem 32:2099–2103
Sou/Dakouré MY, Mermoud A, Yacouba H, Boivin P (2013) Impacts of irrigation with industrial treated wastewater on soil properties. Geoder 200-201:31–39
Taghizadeh-mehrjardi R, Ayoubi S, Namazi Z, Zolfaghari AA, Sadrabadi FR (2016) Prediction of soil surface salinity in arid region of central Iran using auxiliary variables and genetic programming. UASR 30:49–64. https://doi.org/10.1080/15324982.2015.1046092
Tatarko J (2001) Soil aggregation and wind erosion: processes and measurements. Annals Arid Zone 40(3):251–263
Tisdall JM (1994) Possible role of soil microorganisms in aggregation in soils. Plant and Soil 159:115–121
Tisdall JM (1996) Formation of soil aggregates and accumulation of soil organic matter. In: Structure and Organic Matter Storage in Agricultural Soils, pp 57–96
Tripathi S, Kumari S, Chakraborty A, Gupta A, Chakrabarti K, Bandyapadhyay BK (2006) Microbial biomass and its activities in salt-affected coastal soils. Bio and Fert of Soils 42:273–277
Tripathi S, Chakraborty A, Chakrabarti K, Bandyopadhyay B (2007) Enzyme activities and microbial biomass in coastal soils of India. Soil Bio and Biochem 39:2840–2848
Ukalska-Jaruga A, Debaene G, Smreczak B (2018) Particle and structure characterization of fulvic acids from agricultural soils. J Soils Sediments 18:2833–2843. https://doi.org/10.1007/s11368-018-2008-1
Wang C, Pan Y, Zhang Z, Xiao R, Zhang M (2021) Effect of straw decomposition on organic carbon fractions and aggregate stability in salt marshes. Sci of the Total Env 777:145852
Wichern J, Wichern F, Joergensen RG (2006) Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoder 137:100–108
Wong VNL, Dalal RC, Greene RSB (2009) Carbon dynamics of sodic and saline soils following gypsum and organic material additions: a laboratory incubation. Appl Soil Eco 41:29–40
Xiao L, Yuan G, Feng L, Bi D, Wei J (2020) Soil properties and the growth of wheat (Triticum aestivum L.) and maize (Zea mays L.) in response to reed (phragmites communis) biochar use in a salt-affected soil in the Yellow River Delta. Agr Ecosyst Environ 303:107124
Xie JS, Yang YS, Cheng GS, Zhu JM, Zeng HD, Yang ZJ (2008) Effects of vegetation restoration on water stability and organic carbon distribution in aggregates of degraded red soil in subtropics of China. Acta Eco Sinica (in Chinese) 28:702–709
Yan N, Marschner P (2013) Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Bio and Biochem. https://doi.org/10.1016/j.soilbio.2013.06.008
Yan N, Marschner P, Cao W, Zuo C, Qin W (2015) Influence of salinity and water content on soil microorganisms. Int Soil Water Conserv Res 3:316–332
Yuan BC, Xu XG, Li ZZ, Gao TP, Gao M, Fan XW, Deng HM (2007) Microbial biomass and activity in alkalized magnesic soils under arid conditions. Soil Bio and Biochem 39:3004–3013
Zhao SW, Su J, Wu JS, Yang YH, Liu NH (2006) Changes of soil aggregate organic carbon during process of vegetation restoration in Ziwuling. J of Soil and Water Conser (in Chinese) 20:114–111
Zouari K, Trabelsi R, Chkir N (2011) Using geochemical indicators to investigate groundwater mixing and residence time in the aquifer system of Djeffara of Medenine (southeastern Tunisia). Hydrogeo J 19:209–219
Acknowledgements
We appreciate the help of Kawther Ardhaoui during the incubation experiment. Samir Aydi and Rami Rahmani are acknowledged for help with polysaccharide analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Disclaimer
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Responsible Editor: Stefan Grab
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omar, Z., Bouajila, A., Chniguir, N. et al. Organic matter mineralization, aggregation, and aggregate-associated organic carbon in saline soil of arid region of Tunisia: a laboratory incubation. Arab J Geosci 16, 554 (2023). https://doi.org/10.1007/s12517-023-11676-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11676-8