Abstract
In this study, drinking and irrigation water quality in Shirin Darreh dam basin (1750 km2), NE Iran, was assessed. Thirty-three surface water, groundwater, and the reservoir water samples were analyzed for physicochemical parameters, including pH, EC, Ca2+, Mg2+, Na+, K+, CO32−, HCO3−, SO42−, and Cl−, in laboratory of Water Company of North Khorasan. Hydrogeochemical approaches showed that water-rock interaction was the main mechanism controlling the water chemistry in the study area. The major type of the water samples were determined by Mg2+, Na+, Ca2+, SO42−, Cl−, and HCO3− ions mainly originating from the developed marl, shale, and limestone rocky units in the study area. The water flow direction was addressed as one of the effective parameters on the ion content in the surface waters. Based on saturation index, gypsum, and anhydrite were under-saturated in all the water samples, and calcite and aragonite were super-saturated in major part of the water samples. Drinking quality of the water samples was in suitable, intermediate, good and inappropriate categories, and thoroughly unpleasant and non-potable classes were not dominant based on Schoeller classification. According to water quality index, 36 and 64% of the water samples were in unsuitable and suitable drinking water quality ranges, respectively. Among the irrigation water quality indices, EC, MH, SO42−, and Cl− hazards were damaging for agricultural purposes. The lithological frame of the study basin with erosion sensitive rocks is an important natural factor controlling the water resources quality for different purposes, especially drinking and irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality is one of the most important factors in health and sustainable ecosystems. Clean water resources support plants diversity and wildlife, and all human activities on land affect the water quality. The chemical composition of irrigation water influences plant growth directly through toxicity and deficiency, or indirectly by altering the availability of basic nutrients to plants (Qadir et al. 2021; Jha et al. 2022). The major ion concentration in the water resources depends on several factors, including geological setting, especially lithological frame, precipitation and evaporation pattern, temperature, oxidation–reduction reactions in the water, geological processes within the deep and shallow aquifers, and anthropogenic activities leading to pollutants discharging into the water (nitrates, phosphates, heavy metals, etc.) (Wu et al. 2009; Sasakova et al. 2018; Jha et al. 2020; Davraz and Batur 2021; Fida et al. 2022). Several diseases and malfunctions, such as typhoid fever, Giardia, and severe diarrhea, in human body originate from unsafe and polluted water (WHO 2017). Therefore, evaluation of drinking and irrigation water quality is a key process to assure the environmental sustainability and development.
The water quality index (WQI) is an evaluable approach to study the surface and groundwater quality for different purposes, mainly drinking (Kouadri et al. 2021; Patel et al. 2023). Globally, different water quality indices have been proposed, such as the United States National Sanitation Foundation Water Quality Index (NSFWQI), British Colombia Water Quality Index (BCWQI), Oregon Water Quality Index (OWQI), and Canadian Council of Ministers of the Environment Water Quality Index (CCMEWQI) (Mitchell and Stapp 1996; Zandbergen and Hall 1998; Cude 2001; Khan et al. 2004). There are several researches focused on assessing the water quality by WQI, and the results showed that the water quality was considerably affected by population growth, industrial development, and sensitive formations to erosion and weathering (Wu et al. 2017; Othman et al. 2020; Chabuk et al. 2020). The irrigation water quality can be evaluated by different indicators, such as sodium adsorption ratio (SAR), magnesium hazard (MH), residual sodium carbonate (RSC), and residual sodium bicarbonate (RSBC), based on the physicochemical parameters (Murtaza et al. 2021; Nsabimana et al. 2022; Tekile 2023). Each of these indicators implies the hazard of a specific parameter in the irrigation water.
Mean annual rainfall in the world is 813 mm, while it is measured at 217 and 228 mm in the Middle East and Iran, respectively, that is less than one third of the global average. In Iran, the water used in agriculture, drinking, and industrial purposes is annually 90, 6, and 4%, respectively (Marzban et al. 2019). Iran, with 1% of the world’s population, has only 3% of the global fresh water resources (Babran and Honarbakhsh 2019). Despite the suitable water quality in the major part of Iran, agricultural, domestic and industrial wastewater discharging into the surface and groundwater resources has led to decrease in the water quality. The serious problems of anthropogenic pollution have been observed in the rivers, such as Karoun, Kashafroud, and Seymareh in south, northeast, and west (Madadinia et al. 2014; Molavi et al. 2018; Noori et al. 2018). Geological formations with high solubility, such as carbonate and evaporate rocks, are regarded as the major natural concerns affecting the water quality in Iran. Interaction between the water and the soluble rocks changes the chemical composition of surface and groundwater resources, and the water quality can be degraded as a result. Mohammadzadeh et al. (2017) concluded that the cretaceous carbonate and marl were the major sources of carbonate ions discharging and carbonate type in the groundwater resources in Bajestan plain, east Iran. Cheraqi et al. (2019) studied the groundwater quality index for drinking in Khorramabad, western Iran. They found that the high total dissolved solids (TDS) and electrical conductivity (EC) in water resources was due to halite and carbonates dissolution in the area. Valipour (2022) showed the groundwater interaction with shale, marl, dolomite, and evaporate rocks increase the water salinity in southwest Qouchan, Iran, and therefore the water quality was not suitable for agricultural purposes.
Shirin Darreh dam, northeastern Iran, was constructed on Shirin Darreh River in North Khorasan Province, to supply drinking water of Bojnord City, and to store irrigation water for downstream agricultural lands. There are 9577 ha agricultural lands in the area (40% water farming, 60% dry farming). The rocks with high sensitivity to erosion and weathering, like marl, shale, and gypsum, expose in more than 60% of upstream areas of the dam. Moreover, because of arid and semi-arid characteristic, drought phenomenon has adversely affected the water resources, resulting in the water quality decrease (Ahmadian Moqaddam 2012; Khademi 2019). Drinking and irrigation water quality in the Shirin Darreh dam basin were studied in a few researches, in which the development of villages and counties, and natural factors, such as erosion sensitive rocks, were emphasized as the main parameters controlling the water chemistry (Khajehpour et al. 2014; Sadra 2014; Saghi et al. 2015; Teimouri et al. 2018). Planning the management programs on the dam reservoir and the other water resources in the study area can be processed by monitoring and controlling the water quality for various purposes, especially drinking and irrigation. Therefore, because of the lack of sufficient studies, and the vital role of safe drinking and irrigation water in human health, the water quality assessment is of high importance for the study area. This study gives the clear insights in comparing the water quality of surface water, groundwater, and stored water resources by hydrogeochemical tools and water quality indices. Moreover, the findings of this study can fill the gap of studies about the role of lithological units on water quality in the study area. The main objectives of this paper are (1) to determine the main mechanism controlling the dissolved ions discharging into the water resources using hydrogeochemical tools, such as Gibbs and Piper diagrams, and saturation indices (SI) and (2) to evaluate the drinking and irrigation water quality of surface and groundwater resources by WQI approach, Schoeller classification, and irrigation quality indicators.
Materials and methods
Study area
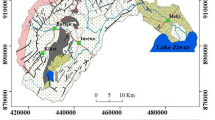
The Shirin Darreh dam basin (57° 6′ 25″–57° 58′ 35″ E, 37° 41′ 31″–37° 59′ 52″ N), with drainage area of 1750 km2, is located in the Koppeh-Daq basin, North Khorasan Province, northeast Iran (Fig. 1a, b). The dam has supplied drinking water for Bojnord City (the capital of the province) and the irrigation water for agricultural lands in the downstream since 2005, when the dam was constructed on the Shirin Darreh River (the main river draining the basin). The total storage capacity of the Shirin Darreh dam (an earth fill dam with a clay core) is 92 million cubic meter water. In the study area, the annual average temperature is about 15.3 °C. The annual average precipitation is 266 mm that the maximum and minimum monthly precipitation was obtained for March (40.8 mm) and September (5.4 mm), respectively. Generally, the wet period was recorded within December to May.
Afshar Harb (1994) reported that the sedimentary rocks of Cretaceous age, such as limestone (Tirgan formation), marl (Sarcheshmeh formation), shale (Sanganeh formation), sandstone (Aitamir formation), and Quaternary deposits (fluvial terraces, eolian, alluvial, and debris sediments) are the main geological units in the study area (Fig. 1b). Marl, sandstone, shale, limestone, and Quaternary deposits outcrop in 37% (647 km2), 30% (525 km2), 24% (420 km2), 8% (140 km2), and 1% (18 km2) of the total area in the basin, respectively (Ahmadian Moqaddam 2012). The Tirgan Formation is observed as massive or thick-layered with shale and marl inter-layers, and mainly forms the heights due to resistance to erosion. The thickness of the formation ranges between 500 and 700 m from east to west in the study area. The Tirgan Formation located at an anticline center and its outcrop can be regarded as the feeding zone of karstic aquifers. The Sarcheshmeh Formation is composed of gray marl and shale with limestone inter-layers, and has 700–1200-m thickness. Black shale and siltstone inter-layers are the main petrographic constituents of the Sanganeh Formation with 1000–1400-m thickness in the basin. High erodibility of the Sanganeh Formation is a result of its soft petrographic nature leading to huge amount of suspended sediment load discharging into the rivers. Thickness of the Aitamir sandstone is about 200–800 m in the study basin.
The Aitamir unit is a cliff forming because of more hardness and resistance to erosion compared to the other geological units in the study area. The Quaternary deposits are mainly fluvial terraces, eolian, alluvial, and debris sediments with rare outcrops of travertine units. The NE-SW trending faults are the consequence of the regional tectonic and have an important role in evolution of hydrologic network and groundwater flow. Based on the topographic map and satellite image of the basin the elevation range is between 697 and 2703 (Fig 2a, b). The elevation decreases from north and northeast to southwest so that the minimum elevation is measured in the dam location (Afshar Harb 1994).
There are three types of the groundwater resources in the study area including karstic aquifers, semi-deep alluvial aquifers, and deep aquifers. The karstic Tirgan Formation with limestone lithology is the main sources of supplying the Shirin Darreh River water. The semi-deep alluvial aquifers are observed in the Quaternary deposits where the faults and fractures facilitate the water infiltration. These aquifers are supplied by the river and local flows in permeable soils and grounds. The deep faults are the major factor to evolution of the deep aquifers with rare distribution in the study area. The bedrock is uniform and completely composed of sandstone and shale covered by marl and dolomite (Fig. 1c). The Sarcheshmeh and Sanganeh Formations are considered as impermeable rocks and surface and rainfall water cannot be allowed to penetrate and create deep and semi-deep aquifers through these formations. In contrast, the fractured and faulted Aitamir and Tirgan Formations are the most effective formations to supply the groundwater (Kazemi 2014). The hydrological properties of the formations in the study area are summarized in Table 1
The surface water distribution system of the study area is made up of five main rivers including Qoroq (east), Asia Zou (northeast), Mian Zou (north), Pasin Darreh (northwest), and Shirin Darreh (southwest) (Fig. 2c). The Shirin Darreh River finally reaches the Caspian Sea in north Iran. The total channel length in the study basin is about 1200 km. The Qoroq River originated from eastern heights has seasonal flows and 60-km length and reaches the Shirin Darreh River in the west. The northern heights between Iran and Turkmenistan are the origin of the Asia Zou and Mian Zou Rivers.
These rivers have constant flows and can be considered as perennial streams. The seasonal Pasin Darreh River is supplied by the western heights. The average flow discharge of the above main rivers is estimated to be about 80–95 m3/s. The depth of water table is increasing from west (dam location) to east and center to north, and is varying between 800 and 1600 m in the study basin (Fig. 2d). Based on topographic map (Fig. 2a), the direction of surface water flow is toward the southwest (dam location). The direction of groundwater flow was also determined in north and northwest to south (Kazemi 2014). It suggests that the dissolved ions in the upstream area where covered by erodible geological units are leached by water resources and can be transported to the south and southwest. Therefore, high ionic content in the south and southwest can be predicted.
Sampling
The surface and groundwater samples (n = 33) were collected from rivers, wells, springs, and the dam reservoir during autumn 2020 (Fig. 2c). The water samples included were 20 river waters, 6 well waters, 6 spring waters, and 1 water sample from the reservoir. The most part of Iran are involved in the severe drought and less precipitation outcomes. Water table falling is leading to dried wells and springs. It was discussed in many previous researches, such as Hejazizadeh et al. (2019), Babaeian et al. (2021), and Teimouri et al. (2022). The groundwater samples were collected after several hours’ fieldwork to find wells and springs with enough water to collect. The average depth of the study wells is about 230 m, as Wwe1, Wwe2, Wwe3, Wwe4, Wwe5, and Wwe6 water samples were collected from a depth of 200, 215, 234, 235, 245, and 255 m, respectively. These water resources are important to supply the stored water in the Shirin Darreh dam reservoir. A 0.45-μm filter was used to filter the water samples, and then the samples were transferred to 500-ml high density polyethylene bottles (HDPE) for the next laboratory analysis. Prior to collection, the bottles were thoroughly cleaned and rinsed with distilled water and then with the collected water sample. pH and EC were measured in situ by portable pH meter (Horiba) and EC meter (con 200), which were calibrated before use. Sampling, preservation and analysis procedure of the water samples were carried out based on the method suggested by APHA (1992) (Mallick 2017; Dixit et al. 2021). Ca2+ and Mg2+ were measured titrimetrically by standard EDTA, and Na+ and K+ were measured using flame photometry. Titration with HCl was used to measure CO32− and HCO3−. SO42−, and Cl− were determined by UV-Visible spectrometer and standard AgNO3 titration, respectively. All the analyses were carried out in laboratory of Regional Water Company of North Khorasan (RWCNK). TDS and total hardness (TH) of the water samples were computed as below (Boyd 2015):
All the water resources in natural ecosystems are electrically neutral. Therefore, sum of anions concentration must be equal to the sum of cations concentration. To ensure the analytical accuracy, a formula called “ion balancing” was proposed (Eq. 4), in which, the concentrations are expressed in meq/l, and %D is the difference percentage. A given water sample is accurately analyzed and is neutral if the %D is in ±10 range (Fuchu et al. 2021; Wang et al. 2022).
Drinking water quality
Drinking quality of the water samples was assessed by Schoeller classification (1967) and WQI suggested by Mishra and Tiwari (1985). Based on five parameters including TDS, TH, Na+, SO42−, and Cl−, Schoeller proposed a drinking water classification, and therefore six water quality categories can be distinguished (Table 2).
WQI provides a single number expressing the overall water quality at a certain location and time, based on several water quality parameters (Chabuk et al. 2020; Patel et al. 2023). The WQI presented by Mishra and Tiwari (1985) is calculated by the content of Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, pH, and TDS by the following equations:
where qi is the quality rating for the ith water quality parameters (i = 1, 2, 3, …, N); Vi is the obtained value of the ith parameter; Si is the standard permissible value for a parameter (Table 3), and Wi is the inverse value of the Si. The information to calculate the WQI is presented in Table 3. The permissible value of the WQI is 100. If WQI > 100 for a water sample it is concluded that it is not suitable for drinking, and values of the WQI lesser than 100 indicate that the water sample is suitable.
Irrigation water quality
Two important indices to reflect the sodium hazard in irrigation water are SAR and sodium percent (%Na). The SAR indicates the proportion of Na+ to Ca2+ and Mg2+. %Na compares Na+ and K+ content with sum of the major cation contents of water. The following equations are used to obtain the SAR and %Na (ions concentration in meq/l) (Richards 1954; Wilcox 1955):
MH is calculated as below (ions concentration in meq/l) (Paliwal 1972):
RSC and RSBC are useful indices for evaluating the quality of irrigation water, because they show the relationship between the quantity of CO32−, HCO3−, Ca2+, and Mg2+. These two indices are calculated as below (ions concentration in meq/l) (Eaton 1950; Ragunath 1987; Gupta and Gupta 1987):
Concentration < 400 mg/l is desired range of SO42− content in irrigation water, and waters with > 400 mg/l SO42− can acidify the soil (Jha et al. 2022). Therefore, the concentration less than 400 mg/l was considered as the desired range of SO42− for irrigation in the study water samples. The chloride hazard in irrigation water can be assessed as below (ions concentration in meq/l) (Grillot et al. 1957):
Hydrogeochemical tools
Gibbs (1970) proposed a diagram, in which the ratio of anions [Cl−/(Cl−+HCO3−)] and cations [(Na++K+)/(Na++K++Ca2+)] are individually plotted against the TDS. In fact, the mutual effects of water and respective aquifers can be easily studied in the Gibbs diagram. The mechanisms controlling water chemistry such as chemical composition of the rock types (rock dominance), chemistry of precipitation (precipitation dominance), and evaporation rate (evaporation dominance) can be detected in the Gibbs diagram (Ren et al. 2020; Mthembu et al. 2021). Piper diagram (or trilinear diagram) is used as an effective graphical representation of water chemistry in hydrogeological studies (Piper 1944). The percentage values of four ion groups including Ca2++ Mg2+, Na++ K+, CO32−+HCO3−, and SO42−+Cl− are considered in the plot. Two separate ternary plots show the cations and anions and both are then projected onto a rhomb (Gad and El Osta 2020; Siddah and Sahu 2022). The main rock types interacting with the water resources can be detected in the Piper diagram (Hounslow 1995).
Natural water resources quality is affected by different dissolved ions, which can be derived from natural or anthropogenic processes. Dissolution of mineral species occurs because of weathering and erosion of soil and rock (Singh et al. 2019; Hori et al. 2021). Generally, the SI is used to evaluate the degree of equilibrium between water and specific mineral and is expressed as below (Apello and Postma 2005):
where IAP is the ion activity product, K is the solubility constant, and SI is the saturation index. If the water is in equilibrium with a mineral, the SI = 0. SI greater than zero indicates super-saturation and the specific mineral would tend to precipitate; if less than zero, this index indicates under-saturation and the specific mineral would tend to be dissolved (Jampani et al. 2020; Ren et al. 2020; Wang et al. 2020).
Arc GIS 10.3 was applied to draw the maps of the study area and produce the spatial distribution of major analyzed parameters. The statistical properties of the parameters in the study water samples were obtained by IBM SPSS 20. To determine the chemical equilibrium between minerals and waters, SI of carbonates (calcite, dolomite, aragonite) and sulfates (gypsum and anhydrite) was calculated using PHREEQC ver.2 software. Piper and Schoeller diagrams were plotted by Aq.QA ver.1.1.5.1. It should be noted that the reservoir water sample (Wre) was regarded as surface water in the analytical procedures.
Results and discussion
Water type
The Shirin Darreh dam basin shows a varied water quality mainly due to its general geology. The groundwater resources are mostly observed in Cretaceous to Quaternary deposits that can also determine the surface water type. As a result, the water resources quality in the study area changes in the basin due to effect of various parameters such as regional geology, different lithological units, aquifer composition and hydrological properties of the formations. To better evaluate the chemical characters of the water resources and the relationship between the ion content and lithological units, the analysis results has been plotted on Piper diagram (Piper 1944) (Fig. 3). According to the majority of different cations and anions in the surface water samples, five hydrochemical water types can be distinguished as Na-SO4, Mg-SO4, Ca-SO4, Ca-HCO3, and Mg-HCO3 (Table 4).
About 38%, 33%, and 19% of the surface water samples fall in the Na-SO4, Mg-SO4, and Ca-SO4 fields, and only 5% of them equally lie in the Ca-HCO3 and Mg-HCO3 dominance. Abundance of the sulfate ion compared to bicarbonate is probably due to water action on gypsum and anhydrite inter-layers of marl and limestone, and sulfate ion in sandstone units. The alkaline and earth alkaline metals in the surface water samples can originate from weathering of Na, Ca, and Mg bearing minerals such as marl, limestone, and clay minerals. Therefore, Na-SO4, Mg-SO4, and Ca-SO4 are the dominant hydrogeochemical types in the surface water samples.
In the groundwater resources, five hydrochemical types can be defined including Na-Cl, Mg-SO4, Mg-HCO3, Ca-HCO3, and Na-HCO3, with sequential decrease in percent. Khosravani Shiri et al. (2017) assessed the water quality for Farouj City, North Khorsan Province, and mostly detected Na-SO4 and Na-Cl types for the study water resources. The latter finding is in accordance with the above result that is due to the same lithological frame of the two study areas (mainly composed of carbonates and evaporates). It is well established that the saturation processes, such as interactions with surrounding rocks, remaining time in an aquifers, and no exchange with atmospheric materials, can change water type to Na-Cl. Therefore, it is predicted when the water infiltrates into deeper aquifers, the water type gradually changes from sulfate or bicarbonate to Na-Cl in water cycling system (Adelana 2014; Tikhomirov 2016). Hence, predominance of Na-Cl type only in the groundwater samples implies that the saturation processes result in evolution of Na-Cl type. Wu et al. (2009), and Kumar et al. (2017), who observed more water samples with Na-Cl type in groundwater resources compared to surface waters in southwest China and Tamil Nadu, India, respectively, reported similar findings. Arifullah et al. (2022) found that the collected water samples in Nankana Sahib District, Punjab province, Pakistan, showed Ca-Cl and mixed Ca-Mg-Cl types because of silicates and carbonate weathering and dissolution. In addition, the abundance of chloride ions was attributed to the addition of fertilizers containing chloride to the soil. Not extended rocks bearing sulfate minerals (mainly gypsum and anhydrite) and presence of carbonate and silicate formations in Catalonia (northeastern Spain) led to distinguish of only Ca-HCO3 and Na-HCO3 by Tapias et al. (2022). Overall, general geology plays an important role in determining the water type by decreasing or increasing special ion contents in the water environment.
Water chemistry
Statistical summary of the analyzed water samples and major ions of water resources in the Shirin Darreh dam basin including maximum, minimum, and average are presented in Table 5. By Kolmogrov-Smirnov, normality of the data was determined and the results indicate that the half of the study samples are normally distributed (significant level >0.05) and remaining are non-normally distributed (significant level <0.05). The skewness and kurtosis results also emphasize on the normality test of the data. %D for all the water samples falls in ±10 range and therefore there was no error in analysis of the major ions. Water pH has an important effect on ion concentration, geochemical equilibrium, oxidation-reduction reactions, and water suitability for different purposes, as a result. Optimum pH ranges for drinking is between 6.5 and 8.5 (WHO 2017). The pH value of the water samples ranges between 7.3 and 8.5, with an average of 7.9, indicating suitable quality for drinking (Figs. 4b and 5b). EC stands for electrical conductivity as it relates to the capacity of water to transport electricity. Maximum allowed value for EC in drinking water is 1500 μS/cm (WHO 2017). EC of the water samples ranges between 612 and 2510 μS/cm with an average value of 1282 μS/cm. EC in 14% and 41% of the surface water and groundwater samples is higher than the allowed limit, respectively (Figs. 4a and 5a). Higher EC values of the groundwater samples show more residence time in the aquifers and salt enrichment in the groundwater. TDS is a measure of the dissolved combined content of all inorganic and organic substances in the water. The permissible value of TDS for drinking is 1000 mg/l (WHO 2017). TDS values of the study water samples (using Eq. 2) range from 364 to 4469 mg/l, with an average of 1195 mg/l. Values of this parameter in 62 and 25% of the surface and groundwater samples are more than the maximum acceptable limit, respectively (Figs. 4d and 5d). As mentioned before, EC and TDS are the quality parameters showing the salinity and ion content in the water. Dissolved ions, EC, and TDS are directly related. Lee et al. (2019) studied the groundwater hydrogeochemistry in Korea with main lithology of metamorphic and volcanic rocks. EC and TDS of the water samples ranges were 48–340 μS/cm and 30–220 mg/l, respectively. Similarly, Apollaro (2019) measured the water EC and TDS about 72–196 μS/cm and 53–95 mg/l, respectively, in Southern Calabria, Italy, where the main lithology is observed as volcanic and metamorphic rocks. In contrast, Nganje et al. (2017) and Tafesse et al. (2022) obtained the water EC and TDS in the higher ranges (5.6–2720 μS/cm and 2.8–1360 mg/l, 600–1120 μS/cm and 310–560 mg/l) for Northern Ethiopia and Nigeria, respectively, covered by sediments especially marl, shale, and sandstone (like the study area). Therefore, abundance of easily eroded rocks (mainly sedimentary units) in the study area can be a major source of higher EC and TDS in the water compared to less eroded rocks such as metamorphic and volcanic formations. TH is the amount of dissolved Ca2+ and Mg2+, and hard water has high dissolved minerals, especially calcium and magnesium bearing minerals (WHO 2017). Generally, TH more than 100–500 mg/l in the water can lead to scale development in pipelines of water distribution systems or water treatment plants. In addition, soap reacts with the calcium (which is relatively high in hard water) to form soap scum in hard water (Oyebode and Omoya 2019; Hori et al. 2021). TH values (using Eq. 3) of the water samples vary between 195 and 1643 mg/l, with an average of 608 mg/l.
TH value exceeds the permissible limit in 67 and 25% of the surface and groundwater samples, respectively (Figs. 4k and 5k). Ca2+ and Mg2+ content varies from 1.9 to 28 and 0.6 to 19.7 meq/l, with an average of 6.7 and 5.3 meq/l, respectively (Figs. 4c, f and 5c, f). The maximum acceptable values of Ca2+ and Mg2+ for drinking are 5–15 and 4–12.5 meq/l, respectively (WHO 2017). Concentration of Ca2+ and Mg2+ is higher than the WHO standard in 9.5 and 16.5% of the surface and groundwater samples, respectively. The above ions can be originated from weathering of Ca-Mg bearing minerals such as calcite (aragonite) and dolomite. Carbonate, marl, and dolomite studied by Raisossadat and Shokri (2010), Javanbakht et al. (2013), and Golafshani et al. (2020) in Tirgan and Sarcheshmeh Formations are the main natural sources for Ca2+ and Mg2+ in the water samples. Calcite and dolomite are two abundant minerals in marl or limestone, and reacts with water to form Ca2+ and Mg2+ ions as below (Langmuir 1997):
Moreover, gypsum and anhydrite were observed in Tirgan limestone and Sarcheshmeh marl in inter-layer heterogenic form (Raisossadat and Moussavi-Harami 2000). Gypsum and anhydrite dissolution results in Ca2+ discharge into the water as below (Hem 1989):
Clay minerals reaction with water is the other source of Ca2+ in the water. Anortite, detected by Khormali and Amini (2015) in the Sanganeh unit, hydrolyze according to Eq. 20, and Ca2+ is released into the water, as a result (Hem 1989):
Mechanism controlling water chemistry such as ion exchange is confidently responsible for low concentration of Ca2+ and Mg2+ in the water resources. During the ion exchange Ca2+ and Mg2+ are replaced by Na+ and K+ on adsorption sites on the clay minerals (Hem 1989). Na+ and K+ concentrations vary from 1.5 to 54.9, and 0 to 1.2 meq/l, with an average of 7.9 and 0.2 meq/l, respectively. The Na+ is lower than the permissible limit for drinking (<8.7 meq/l) in all the surface water samples, while this ion content exceeds the limit in 25% of the groundwater resources (Figs. 4e and 5e). Considering the allowed limit of K+ (<0.3 meq/l) (WHO 2017), 42 and 17% of the surface and groundwater samples are not qualified for drinking, respectively. Albite hydroloization (Eq. 21) can be a source of Na+ releasing into the water, that Khormali and Amini (2015) detected this mineral in Sanganeh Formation.
Halite can be formed in marl or gypsum units as heterogenic layers, and dissolves in the water and disintegrates to Na+ and Cl− ions (Eq. 22). Therefore, halite-bearing units such as Tirgan, Sarcheshmeh, and Aitamir (Sharafi et al. 2012; Jafarzadeh et al. 2020) are regarded as the other sources for Na+ ions in the water.
K+ is a major constituent of clay minerals such as illite, chlorite, and smectite. Chlorite was detected in Sanganeh unit (Tazikeh et al. 2017) and can be a source for K+ releasing into the water. As mentioned before, ion exchange process can increase the Na+ and K+ content in the water. HCO3− content varies between 0 and 9.1 meq/l with an average of 3.9 meq/l. Main natural source of this ion in the water is calcite and dolomite dissolution (Eqs. 16 and 17), or anortite and albite hydration (Eqs. 20 and 21). The interaction of the mentioned minerals with water and silicate weathering can lead to enrichment of Ca2+, Mg2+, and HCO3− ions. In addition, HCO3− can be derived from CO2 dissolution through anoxic biodegradation of organic matter originating from anthropogenic activities (such as industrial, irrigation and domestic wastewater, or waste in landfills) (Henry et al. 2017; Gu et al. 2022). Despite not well-known adverse health effects of HCO3-, it is suggested that its allowed limit in drinking water is 4 meq/l (WHO 2017). Therefore, HCO3− content in 53 and 50% of the surface and groundwater samples exceeds the permissible limit (Figs. 4j and 5j). Sulfate concentration varies from 2.3 to 21.1 meq/l, with an average of 9.2 meq/l. Sulfate levels above 250 mg/l may make the water taste bitter or like medicine. High sulfate levels may also corrode plumbing, especially copper piping. Health concerns regarding high sulfate in drinking water commonly occur in concentrations above 10 meq/l (WHO 2017). Sulfate content in 62 and 17% of the surface and groundwater samples is higher than the allowed limit (10 meq/l), respectively (Figs. 4i and 5i). Weathering of sulfate bearing minerals such as gypsum and anhydrite (Eqs.18 and 19) can be regarded as the major natural sources of sulfate releasing into the water. Chloride content in the water samples ranges from 1.1 to 54.2 meq/l, with an average of 6.3 meq/l. This ion is a natural-occurring ion that is present in both fresh and salt water. The presence of chloride in the water can result from several sources including the soils weathering, salt-bearing geological units, deposition of salt spray, WHO 2017and wastewaters. The chloride content above the permissible limit (7 meq/l) results in a detectable taste (). Chloride content in all the surface waters is less than the permissible limit, while 25% of the groundwater sources contain the higher concentration of chloride (Figs. 4l and 5l). Halite-bearing geological units are responsible for natural content of chloride in the study groundwater samples (Eq. 22).
Based on the spatial distribution of the analyzed parameters in the surface water resources (Fig. 4), concentrations of Ca2+, Mg2+, K+, HCO3−, SO42−, Cl−, TDS and TH in the west and southwest of the basin are higher than the other parts. It is clearly suggests that the surface water direction (toward southwest) has an effective role on ion concentration. Tang and Wu (2014), and Jin et al. (2019), who observed more ions content in surface water accordance with water direction in Xilin River basin and Lake Genggahai Catchment, China, respectively, reached the similar result.
Mechanisms controlling water chemistry
Water-rock interaction is the most important factor controlling the geochemical composition and ion content of water resources. This process includes dissolution/precipitation, ion exchange, oxidation, and reduction providing different ions content in the water (Hem 1989). Gibbs diagram (Gibbs 1970) was applied to better understand the main mechanisms controlling the water chemistry such as precipitation, rock weathering, and evaporation in the water resources of the Shirin Darreh dam basin. In this diagram, Na++K+/Na++K++Ca2+ and Cl−/Cl−+HCO3− ratios are plotted against TDS. Based on Fig. 6, the main part of the water samples falls in the rock dominance suggesting the water chemistry in the study basin is mainly determined by chemical weathering of geological units. A few numbers of the water samples were plotted in the evaporation dominance. As mentioned before, the mean annual temperature and precipitation was obtained 15.3 °C and 266 mm for the study basin. It means that the evaporation (associated with high temperature) and high precipitation cannot significantly affect the water chemistry in the study basin. Therefore, the rock weathering is determined as the most effective controlling mechanism of the water chemistry. Mohamed et al. (2022) studied the water hydrogeochemistry in northeastern Africa with mean annual temperature and precipitation about 21 and 210 mm. Most part of the water samples was plotted in the rock dominance and it was concluded that the moderate temperature and precipitation led to water samples falling in the water-rock interaction field. In contrast, Nazzal et al. (2014) plotted the major part of the water samples, collected from Saq aquifer, Saudi Arabia, in the evaporation field. High mean annual temperature (43–48 °C) and low rainfall (less than 30 mm/year) can be regarded as the main reason for controlling the water chemistry by the evaporation mechanism. Egbueri et al. (2019) in Ojoto Province, southern Nigeria, with mean annual temperature and rainfall about 32 °C and 500 mm, found the role of precipitation in determining water chemistry. Therefore, the effect of climate parameters on water ion content can be simply shown by Gibbs diagram. In the areas with high temperature and low rainfall, the evaporation mechanism controls the water chemistry. Moderate temperature and rainfall (like the study area) can be lead to more effects of water-rock interaction on water chemical composition. The precipitation dominance is mostly observed in areas with humid climate and heavy rainfall (moderate to high temperature and high rainfall). Overall, based on Gibbs diagram (Fig. 6), high temperature and low rainfall results in high TDS and more content of Na++K+ and Cl− compared to Na++K++Ca2+ and Cl−+HCO3− (evaporation dominance). Moderate to high temperature and high rainfall shows the precipitation dominance with low TDS and higher content of Na++K+ and Cl− than Na++K++Ca2+ and Cl−+HCO3− in water. Moderate temperature and rainfall are generally associated with moderate TDS and lower content of Na++K+ and Cl− than Na++K++Ca2+ and Cl−+HCO3− in water sources.
SI
SI calculated values of calcite, dolomite, and aragonite range between −0.79 and 1.31, −2.84 and 2.2, and −0.95 and 1.15, with an average of 0.5, 0.75, and 0.29, respectively. SI for gypsum and anhydrite was calculated between −1.83 and −0.21, and −2.07 and −0.47, with an average of −1.07 and −1.33, respectively. Based on the average SI, most of the water samples are over-saturated with respect to calcite, dolomite, and aragonite (carbonate minerals) (Fig. 7a). In contrast, gypsum, and anhydrite (sulfate minerals) are under-saturated in the water samples. Figure 7b shows that the water samples are almost saturated or over-saturated in carbonate minerals, indicating that carbonate precipitates, especially for dolomite with steeper descending slope (R2 = 0.17). Hence, effect of carbonate minerals on the water sources is obvious. TDS and SI for sulfate minerals are increasing contemporaneously, with two nearly equal increasing slopes of gypsum (R2 = 0.473) and anhydrite (R2 = 0.477). Gypsum or anhydrite dissolution (Eqs. 18 and 19) occurs in a closed system consisting of a confined aquifer with calcite and aragonite remaining at saturation. During this process, delivering sulfate and calcium to the water, calcite and aragonite become oversaturated (Andre et al. 2005). The above observation reveals that the water chemistry in the study basin is considerably affected by carbonate precipitation and sulfate dissolution. Ren et al. (2020) calculated the positive SI values for calcite and dolomite, and negative values for gypsum in groundwater samples collected from Guanzhong Basin, China. This basin is mainly covered by sedimentary rocks with carbonate thick bedrock that can be regarded as a source for calcite and dolomite (Xu et al. 2019; Xu et al. 2022). Similar findings were reported by Adimalla and Venkatayogi (2018), Talangana State, South India, and Davraz and Batur (2021), Yalvac-Gelendost basin, Turkey. In contrast, Lu et al. (2015) obtained the negative SI values for calcite and dolomite in water samples collected from Leizhou Peninsula, China, where mainly covered by volcanic rocks. The under-saturated carbonates showed that soluble carbonates mineral phases were absent in the host aquifer. Therefore, lithological frame of a basin governed the ion content of water and saturated or under-saturated mineral phases in water.
Water drinking suitability
Schoeller classification
Major part of the water samples (84%) are classified as good category based on Cl− content (Table 4). According to SO42− concentration, 54, 19, 15, and 12% of the water samples are in intermediate, inappropriate, suitable, and good categories, respectively, while equal percent of these samples (45%) are classified in good and suitable categories based on Na+ content (Fig. 8). As mentioned before, sulfate bearing minerals are one of the main constituents of the rocky units in the area. Water reaction with these minerals (Eqs. 18 and 19) can lead to sulfate ion release in to the water and make water inappropriate and intermediate for drinking. TDS falls in two major categories as suitable (54%) and intermediate (33%) quality. Suitable (45%), intermediate (39%), and inappropriate (13%) categories are detected for the water samples based on TH. Over all, drinking quality of the water samples is in suitable, intermediate, good and inappropriate categories, and thoroughly unpleasant and non-potable classes are not dominant.
WQI
According to the obtained values, WQI is more than 100 for 12 water samples (36%) which were not suitable enough to drink (Fig. 9). These water samples include 8 surface waters, 2 well waters, and one spring water sample. In contrast, WQI for 21 water samples (64%) (Including 12 surface waters, 4 well waters, and 5 spring water samples) is less than 100 and can be used for drinking. The mean WQI for surface water and groundwater samples is equal to 130 and 89, respectively, and for the reservoir water, it is equal to 106. The reservoir water sample, which can be considered as a surface water resource, (WQI = 106) is almost unsuitable for drinking purposes (Fig. 9). Therefore, it can be concluded that the groundwater resources has higher quality for drinking compared to surface waters in the study area, based on WQI. Through runoff, air fallout, and other sources, surface water can contain significant amounts of natural and anthropogenic contaminants. Because groundwater is connected to surface water systems through soil percolation and seepage, it is also susceptible to contamination. However, the rock and sediment layers below the water table act as natural filters. Therefore, groundwater generally contains fewer contaminants than surface water and requires less treatment processes (Apello and Postma 2005; Singh et al. 2019). This is in good agreement with previous studies, in which groundwater resources were more suitable for drinking compared to surface waters (Sasakova et al. 2018; Jha et al. 2020).
Irrigation water quality
The water quality used for irrigation is essential for the yield and quantity of crops, maintenance of soil productivity, and protection of the environment. Irrigation water quality refers mainly to the chemical composition of the water, or more specifically, to the mineral composition of water (Boyd 2015). The quality criteria for irrigation water are entirely different from the criteria for drinking water. Furthermore, the quality criteria may vary among crops, as different crops have different susceptibilities to certain minerals or properties of the water. Irrigation suitability of the water resources was evaluated by EC, TDS, SAR, %Na, MH, RSC, RSBC, SO42− content, and Clp. Classification of the water samples for irrigation based on calculated parameters and the parameters histograms are shown in Table 6 and Fig. 10, respectively.
Salts in irrigation water are mainly sodium chloride, calcium and magnesium bicarbonates, chlorides, and sulfates. Salty irrigation can affect plant growth in two ways: salinity and toxicity. The first sign of salinity is usually stunted growth, with plant leaves often having a bluish-green color (Ogunfowokan et al. 2013). As salt levels in the soil increase to more toxic levels, scalding, or burning on the tip and edges of the older leaves occurs. Two common indicators of irrigation water salinity are EC and TDS (Zaman et al. 2018). High salinity in the irrigation water potentially associated with soil salinization in arid and semi-arid regions, especially in lowland areas with clay soil. Loess with high clay content is more susceptible to soil salinization, and the farmland development should be prevented in this area (Li et al. 2018). Based on EC, 100 and 75% of the surface and groundwater samples, and the dam water sample are categorized as doubtful irrigation water. 62 and 75% of the surface and groundwater samples indicate moderate and excellent irrigation water quality based on TDS content, respectively. Like the groundwater sources, the dam reservoir is also excellent for irrigation.
High Na+ in water affects the soil permeability and causes infiltration problems. Sodium ion in soil as exchangeable forms replaces calcium and magnesium absorbed on soil clays, and disperses soil particles. As a result, when the soil dries, it becomes hard and compact and reduces water and air infiltration, and its structure is destroyed, as a result (Li et al. 2018; Qadir et al. 2021). According to SAR values, 100 and 75% of the surface and groundwater samples, and the dam water sample fall in excellent class of irrigation water. Seventy-five and 58% of the surface and groundwater samples, and the dam reservoir water sample are classified as good irrigation water quality based on %Na. In natural water resources, Ca2+ and Mg2+ maintain a state of equilibrium. A high level of magnesium in irrigation water leads to soil alkalinity; moreover, a considerable quantity of water is absorbed between Mg2+ and clay minerals that can reduce the soil infiltration ability and adversely affect the crop yields (Tekile 2023). Calculated MH values indicate that 70 and 89% of the surface and groundwater samples, and the dam reservoir water sample fall in unsuitable irrigation quality class. As water in the soil becomes more concentrated, waters with high content of HCO3− have a propensity to precipitate Ca2+ and Mg2+.
In fact, there is tendency for Ca2+ and Mg2+ to precipitate in high carbonate waters (Zaman et al. 2018). Additional Na+, HCO3- and CO32− ions affect the soil properties, such as increase the organic matter. As a result, the relative proportion of Na+ in the water increases in NaHCO3 form, and then, soils irrigated with high carbonate water might become unproductive, because of carbonate deposition (Murtaza et al. 2021). All the study water samples are good for irrigation based on RSC. 65 and 58% of the surface and groundwater samples fall in non-alkaline class irrigation water, while the dam reservoir water sample is qualified as low alkaline water according to RSBC values. Sulfate is relatively common ion in water and has no major impact on the soil other than contributing to the total salt content. Irrigation water high in SO42− reduces phosphorus availability to plants (Zaman et al. 2018). Considering the SO42− content, 80% of the surface water samples are unsuitable, while 83% of the groundwater samples and the dam reservoir sample indicate suitable quality for irrigation purposes. Chloride contributes to salinity of irrigation water, and when concentrations are high enough, can be toxic to plants. This ion cannot be absorbed by soils, and therefore accumulates in the leaves of plants due to movements in the soil (Zaman et al. 2018). Based on chloride hazard, half of the surface waters (50%) fall in the moderate class, and the rest indicate unsuitable quality, while 50% of the groundwater samples and the dam reservoir water sample are very damaging for crop production.
Conclusions
In this research hydrogeochemical evolution and drinking and irrigation suitability of the surface and groundwater resources in the Shirin Darreh dam basin were evaluated. Water type (predominance of Na-SO4, Mg-SO4, Ca-SO4 types) of the study sources indicates that the presence of sulfate and carbonate geological units has an important role on ion content management of the surface waters. In contrast, majority of Na-Cl and Mg-SO4 types shows that the maturity of groundwater in addition to sulfate and carbonates weathering control the chemical composition of the groundwater sources. Calcite, dolomite, gypsum, anhydrite, albite, and anortite bearing units are outlined as the most important factors releasing the major ions through interaction with water resources in the study basin. Spatial distribution of the analyzed parameters in the surface water resources shows that the concentrations of Ca2+, Mg2+, K+, HCO3−, SO42−, Cl−, TDS, and TH in the west and southwest of the basin are higher than the other parts. Therefore, it can be concluded that the direction of the above parameters increases in accordance with the surface water direction (toward southwest) in the basin. A Gibbs diagram demonstrates that chemical weathering of rock-forming mineral controls water chemistry in the study basin. Based on SI values, the water chemistry in the study basin is considerably affected by carbonate precipitation and sulfate dissolution. According to the calculated WQI, water quality of the groundwater samples is more suitable for drinking than the surface waters, and Schoeller drinking water classification dose not determine unpleasant and non-potable classes. High EC and Mg2+ content lead to low quality of the water resources for crop yield. In addition, major part of the surface water resources is unsuitable based on sulfate content, and Cl− concentration makes mostly the surface and ground water samples unsuitable and very damaging for irrigation, respectively.
Overall, it can be concluded that the geological characteristics, especially lithological frame of the study area have an important effect on water quality for irrigation and drinking purposes. Water quality is an essential parameter to sustainable development and environmental stability of the study area and the other dam basins. Therefore, the periodical monitoring (monthly or seasonal) of the water resources, or long-time and non-stop (one or two-year period) assessment of the water quality in terms of major ion concentrations and physicochemical parameters are highly recommended. It can be helpful procedure to identify temporal variation in the quality of water resources. Determination of anthropogenic pollutants, like agricultural or domestic wastewater, is also suggested.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adelana M (2014) Groundwater: hydrogeochemistry, environmental impacts and management practices. Nova Science Publishers Inc, New York
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Talangana State, South India. Appl Water Sci 8(44). https://doi.org/10.1007/s13201-018-0682-1
Afshar Harb A (1994) Geology of Iran: Geology of Koppeh-Dagh. Geological Survey of Iran, Tehran
Ahmadian Moqaddam R (2012) Investigation and susceptibility zonation of lanslide hazard in Shirin Darreh Dam catchment area. Ferdowsi University of Mashhad, Dissertation
American Public Health Association (APHA) (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association (APHA), American Water Works Association (AWWA), and Water Pollution Control Federation (WPCF), New York
Andre L, Franceschi M, Pouchan O, Atteia O (2005) Using geochemical data and modelling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, south-west of France. J Hydrol 305:40–62. https://doi.org/10.1016/j.jhydro.2004.08.027
Apello CA, Postma D (2005) Geochemistry, Groundwater and Pollution. A.A. Balkema Publishers, Amsterdam, the Netherlands
Apollaro C (2019) Geochemical modeling of water-rock interaction in the granulite rocks of lower crust in the Serre Massif (Southern Calabria, Italy). Groundwater Qual Mediterranean Region. https://doi.org/10.1155/2019/5602648
Arifullah CH, Akram W, Rashid A, Ullah Z, Shah M, Alrefaei AF, Kamel M, Aleya L, Abdel-Daim MM (2022) Quality assessment of groundwater based on geochemical modeling and water quality index (WQI). Water 14(3888). https://doi.org/10.3390/w14233888
Babaeian I, Erfani Rahmatinia A, Entezari A, Baaghideh M, Bannayan Aval M, Habibi M (2021) Future projection of drought vulnerability over northeast provinces of Iran during 2021-2100. Atmosphere 12(12). https://doi.org/10.3390/atmos12121704
Babran S, Honarbakhsh N (2019) Water crisis in Iran and the world. Rahbord 16(41):105–133
Boyd CE (2015) Water Quality: an Introduction. Springer, London
Chabuk A, Al-Madholm Q, Al-Maliki A, Al-Ansari N, Hussain HM, Laue J (2020) Water quality assessment along Tigris River (Iraq) using water quality index (WQI) and GIS software. Arab J Geosci 13(654). https://doi.org/10.1007/s12517-020-05575-5
Cheraqi Z, Sarikhani R, Farahpour MM, Qasemi Dehnavi A (2019) Assessment and analysis of water quality index of groundwater in Khorramabad region. Water Resour Eng 11(29):1–14
Cude C (2001) Oregon water quality index; a tool for evaluating water quality management effectiveness. J Am Water Resour Assoc 37:125–137
Davraz A, Batur B (2021) Hydrogeochemistry characteristics of groundwater and health risk assessment in Yalvac-Gelendost basin (Turkey). Appl Water Science 11(67). https://doi.org/10.1007/s13201-021-01401-9
Dixit A, Siddaiah NS, Joshi P (2021) Hydrogeochemical assessment of wetlands of Gurugram, Haryana, India: implications for natural processes and anthropogenic changes. Arab J Geosci 14(199). https://doi.org/10.1007/s12517-020-06423-2
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69(2):123–133
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Model Earth Syst Environ 5:1443–1461. https://doi.org/10.1007/s4008-019-00613-y
Fida M, Lil P, Wang Y, Khorshed Alaml SM, Nsabimana A (2022) Water contamination and human health risks in Pakistan: a review. Expos Health. https://doi.org/10.1007/s12403-022-00512-1
Fuchu Z, Bin W, Fan G, Mingliang D, Liantong X (2021) Hydrogeochemical characteristics of groundwater and evaluation of water quality in arid area of Northwest China: a case study in the plain area of Kuitun River Basin. Arab J Geosci 14(2099). https://doi.org/10.1007/s12517-021-08464-7
Gad M, El Osta M (2020) Geochemical controlling mechanisms and quality of the groundwater resources in El Fayoum depression, Egypt. Arab J Geosci 13(861). https://doi.org/10.1007/s12517-020-05882-x
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Golafshani T, Khanehbad M, Moussavi-Harami SR, Mahboubi A (2020) Carbonate platform evolution of the Tirgan formation during Early Cretaceous (Urgonian) in the eastern Kopet Dagh basin, northeast Iran: depositional environment and sequence stratigraphic significance. Carbonates Evaporates 35(110):1–22. https://doi.org/10.1007/s13146-020-00647-9
Grillot G, Hayward HE, Everett DH (1957) Utilization of saline water. UNESCO, Paris
Gu C, Waldorn S, Bass AM (2022) Anthropogenic land use and urbanization alter the dynamics and increase the export of dissolved carbon in an urbanized river system. Sci Tot Environ 846(157436). https://doi.org/10.1016/j.scitotenv.2022.157436
Gupta SK, Gupta IC (1987) Management of saline soils and water. Oxford and IBM Publication, New Delhi
Hejazizadeh Z, Pazhooh F, Jafari F (2019) The relationship between severe and pervasive droughts and wet years in half east of Iran with synoptic troposphere condition. J Geog Environ Hazards 7(3):135–160. https://doi.org/10.22067/GEO.V0IO.68353
Hem JD (1989) Study and interpretation of the chemical characteristics of natural water. United States Geological Survey (USGS), Washington D.C
Henry IA, Kowarz V, Ostaggard K (2017) Aerobic and anoxic biodegradability of amines applied in CO2-capture. Int J Greenhouse Gas Control 58:266–275. https://doi.org/10.1016/j.ijggc.2017.02.004
Hori M, Shozugawa K, Watanabe Y (2021) A survey of monitoring water hardness in Japan and its distribution pattern. Sci Rep 11:205–216. https://doi.org/10.1038/s41598-021-92949-8
Hounslow AW (1995) Water quality data: analysis and interpretation. CRC Press, Oklahoma
Jafarzadeh M, Choudhury TR, Taheri A, Banerjee S, Jafarian A (2020) Glauconite within Albian-Cenomanian Aitamir faromation, Kopet-Dagh basin, northeastern Iran: origin and implications of cretaceous seawater. Arab J Geosci 13(1236). https://doi.org/10.1007/s12517-020-05920-8
Jampani M, Leidl R, Hulsmann S, Sonkamble S, Amerasinghe P (2020) Hydrogeochemical and mixing processes controlling groundwater chemistry in a wastewater irrigated agricultural system of India. Chemosphere 239(124741). https://doi.org/10.1016/j.chemosphere.2019.124741
Javanbakht M, Ghazi S, Moussavi-Harami SR, Mahboubi A (2013) Depositional history and sequence stratigraphy of Tirgan formation (Barremian-Aptian) in central Kopet Dagh, NE Iran. J Geol Soc India 82(6):701–711. https://doi.org/10.1007/s12594-013-0208-2
Jha MK, Shekhar A, Jenifer MA (2020) Assessing groundwater quality for drinking water supply using hybrid fuzzy-GIS-based water quality index. Water Resour 179(115867). https://doi.org/10.1016/j.waters.2020.115867
Jha R, Singh VP, Singh V, Roy LB, Thendiyath R (2022) Groundwater and water quality. Springer, London
Jin Y, Jin X, Yang D, Mao X (2019) Controlling factors of water ionic composition characteristics in the Lake Genggahai Catchment, NE Qinghai-Tibetian Plateau, China. Water 11(7):96–112. https://doi.org/10.3390/w11071329
Kazemi M (2014) Hydrogeochemical investigation, reason of high temperature of water and present conceptual model of underground condition of “Ayüb and Shafa Hot Springs” (North Khorasan Province). Ferdowsi University of Mashhad, Dissertation
Khademi N (2019) Assessment of sedimentation trend in Shirin Darreh Dam by HEC-RAS 4.1.0 model. Dissertation. Shahrud University of Technology
Khajehpour MA, Karimi L, Shiasi Arani M, Ansari H (2014) Eutrophication check of reservoirs with CE-QUAL-W2 (case study: Shirin Darreh reservoir). Iran J Irrig Drain 8(1):96–107
Khan AA, Paterson R, Khan H (2004) Modification and application of Canadian Council of Ministers of the Environment Water Quality Index (CCMEWQI) for the communication of drinking water quality data in Newfoundland and Labrador. Water Qual Res J Canada 39:285–293
Khormali F, Amini A (2015) Clay mineralogy of the Jurassic-tertiary sedimentary rocks of the Kopet Dagh basin (Northeatern Iran): Implications for paleoclimate and sedimentary environment. Acta Geodyn Geomater 12(4180):1–12. https://doi.org/10.13168/AAG.2015.0036
Khosravani Shiri Z, Mamudy Gharaei MH, Mazaheri A (2017) Hydrogeochemistry and assessment of water resources in Quaternary deposits in southeast Farouj (North Khorasan) for drinking and industry. Iran Quaternary 3(3):265–276
Kouadri S, Elbeltagi A, Islam AR, Kateb S (2021) Performance of machine learning methods in predicting water quality index based on irregular data set: application on Illizi region (Algerian southeast). Appl Water Sci 11(190):125–136. https://doi.org/10.1007/s13201-021-01528-9
Kumar SK, Babu SH, Rao PE, Selwakumar S, Thivya C, Muralidharan S, Jeyabal G (2017) Evaluation of water quality and hydrogeochemistry of surface and groundwater, Tiruvallur District, Tamil Nadu, India. Appl Water Sci 7:2533–2544. https://doi.org/10.1007/s13201-016-0447-7
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, New Jersey
Lee BD, Oh YH, Cho BW, Yun U, Choo CO (2019) Hydrogeochemical properties of groundwater used for Korea bottled waters in relation to geology. Water 11(1043). https://doi.org/10.3390/w11051043
Li P, He S, Yang N, Xiang G (2018) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77(775). https://doi.org/10.1007/s12665-018-7968-3
Lu Y, Tang C, Chen J, Chen J (2015) Groundwater recharge and hydrogeochemical evolution in Leizhou Peninsula, China. J Chem. https://doi.org/10.1155/2015/427579
Madadinia M, Monavari M, Karbasi A, Nabavi MB, Rajabzadeh E (2014) Study on water quality of Karoun River (Ahavaz region) using water quality index. J Environ Sci Tech 16(1):49–60
Mallick J (2017) Hydrogeochemical characteristics and assessment of water quality in the Al-Saad lake, Abha Saudi Arabia. Appl Water Sci 7:2869–2882. https://doi.org/10.1007/s13201-017-0553-1
Marzban H, Sadraei Javaheri A, Zibaei M (2019) Study of the status of resources and water consumption in Iran and improving the situation. J Water Wastewater 30(4):16–32. https://doi.org/10.22093/WWJ.2018.126649.2663
Mishra M, Tiwari TN (1985) A preliminary assignment of water quality index to major rivers. Ind J Environ Protect 5(2):276–289
Mitchell MK, Stapp WB (1996) Field manual for water quality monitoring: an environmental education program for schools. Thomson-Shore Inc, Germany
Mohamed A, Asmoay A, Alshehri F, Abdelrady A, Othman A (2022) Hydro-geochemical applications and multivariate analysis to assess the water-rock interaction in arid environments. Appl Sci 12(6340). https://doi.org/10.3390/app12136340
Mohammadzadeh F, Ekhtesasi MR, Hosseini ZA (2017) The effects of geological formations on groundwater quality with application of Boulian logic, case study: Bajestan watershed plain. Watershed Eng Manag 9(1):11–21
Molavi F, Dehghan H, Alizadeh A, Hosseini-Fatemi M (2018) Study of pollution in the Kashafroud River by the approach of protozoan parasites. Exp Animal Biol 8(1):29–38
Mthembu PP, Elumalai V, Senthilkumar M, Wu J (2021) Investigation of geochemical characterization and groundwater quality with special emphasis on health risk assessment in alluvial aquifers. Int J of Environl Sci Tech 18:3711–3730. https://doi.org/10.1007/s13762-021-3129-0
Murtaza G, Zia-ur-Rehman M, Qadir M (2021) High residual sodium carbonate water in Indian subcontinent: concerns, challenges and remediation. Int J Environ Sci Tech 18(1). https://doi.org/10.1007/s13762-020-03066-4
Nazzal Y, Ahmed I, Al-Arifi N, Ghrefat H, Zaidi F, El-Waheidi M, Batayneh A, Zumlot T (2014) A pragmatic approach to study the groundwater for domestic and agricultural usage, Saq aquifer, northwest of Saudi Arabia. Environ Monit Assess 32(7):15–28. https://doi.org/10.1007/s10661-014-3728-3
Nganje TN, Hursthouse AS, Edet A, Stirling D, Adamu CI (2017) Hydrogeochemistry of surface water and groundwater in the shale bedrock, Cross River basin and Niger Delta region, Nigeria. Appl Water Sci 7:961–985. https://doi.org/10.1007/s13201-015-0308-9
Noori Z, Salajegheh A, Malekian A, Moghaddamnia A (2018) Investigating the effects of best management practices on the reduction of point and non-point source pollution of water using SWAT model (case study: Seymareh River). Iran J Soil Water Rese 48(5):995–1006
Nsabimana A, Li P, Wang Y, Khorshed Alam KA (2022) Variation and multi-time series prediction of total hardness in groundwater of the Guanzhong Plain (China) using grey Markov model. Environ Monit Assess 194:899. https://doi.org/10.1007/s10661-022-10585-9
Ogunfowokan AO, Obisanya JF, Ogunkoya OO (2013) Salinity and sodium hazards of three streams of different agricultural land use systems in Ile-Ife, Nigeria. Appl Water Sci 3:19–28. https://doi.org/10.1007/s13201-012-0053-2
Othman F, Alaaeldin ME, Seyam M, Ahmed AN, Teo FY, Afan HA, Sherif M, Sefelnasr A, El-Shafie A (2020) Efficient river water quality index prediction concidering minimal number of inputs variables. Eng Appl Comput Fluid Mech 14(1). https://doi.org/10.1080/19942060.2020.1760942
Oyebode OJ, Omoya OO (2019) Treatment optimization and performance evaluation of water supply schemes: a case study on Akure and Environs. Int J Environ Sci Tech 16:6873–6884. https://doi.org/10.1007/s13762-019-02276-9
Paliwal KV (1972) Irrigation with saline water. IARI Publication, New Delhi
Patel PS, Pandya DM, Shah MA (2023) A systematic and comparative study of water quality index (WQI) for groundwater quality analysis and assessment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-25936-3
Piper AM (1944) A graphic procedure in geochemical interpretation of water analyses. Trans Am Geophys Union 25(2):914–923
Qadir M, Sposito G, Smith CJ, Oster JD (2021) Reassessing irrigation water quality guidelines for sodicity hazard. Agri Water Manage 225(17054). https://doi.org/10.1016/j.agwat.2021.107054
Ragunath HM (1987) Groundwater. Wiley Eastern, London
Raisossadat N, Moussavi-Harami SR (2000) Lithostratigraphic and facies analyses of the Sarcheshmeh formation (Lower Cretaceous) in the eastern Kopet Dagh Basin, NE Iran. Cretaceous Res 21(4):507–516. https://doi.org/10.1006/cres.2000.0216
Raisossadat N, Shokri M (2010) Biostratigraphic studies of the lower Cretaceous (Upper Barremian-lower Aptian) Sarcheshmeh and Sanganeh formations in the Kopet Dagh basin, NE Iran: an integration of calcareous nannofossil and ammonite stratigraphies. Stratigr Geol Correl 19:188–204. https://doi.org/10.1134/S0869593811020109
Ren X, Li P, He X, Su F, Elumalai V (2020) Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-020-00772-5
Richards LA (1954) Diagnosis and improvement of saline and alkaline soils. In: Handbook No.60. United States Department of Agriculture (USDA), Washington D.C
Sadra B (2014) Quantity and quality investigation of water resources and sediment in Shirin Darreh Dam catchment area (North Khorasan Province). Dissertation. Ferdowsi University of Mashhad
Saghi MH, Khajehpour E, Karimi L (2015) Basin and reservoir water quality improvement methods of dams (case study: Shirin Darreh dam). J Water Soil Sci 25(3/2):211–227
Sasakova N, Gregova G, Takacova D, Kovakova S (2018) Pollution of surface and groundwater by sources related to agricultural activities. Front Sustain Food Syst 2(42):205–215. https://doi.org/10.3389/fsufs.2018.00042
Schoeller H (1967) Qualitative evaluation of groundwater resources. In: H. Schoeller H (ed) Methods and techniques of groundwater investigation and development. UNESCO, Paris, pp 44–52
Sharafi M, Ashuri M, Mahboubi A, Moussavi-Harami SR (2012) Stratigraphic application of Talassinoides ichnofabric in delineating sequence stratigraphic surfaces (Mid-Cretaceous), Kopet-Dagh basin, northeastern Iran. Palaeoworld 21:202–2016. https://doi.org/10.1016/j.palwor.2012.06.001
Siddah S, Sahu P (2022) Understanding the geochemical evolution of groundwater in Central Gujarat, India: and integrated hydrogeochemical and multivariate statistical approach. Arab J Geosci 15(1117). https://doi.org/10.1007/s12517-022-10386-x
Singh SK, Kanga S, Ghosh B (2019) Groundwater quality assessment using geoinformatics. Lambert Academy Publishing (LAP)
Tafesse NT, Konka B, Alemaw BF (2022) Hydrogeochemistry of shale-limestone-mudstone successions aquifers- the case study of the Hantebet sub basin in Northern Ethiopia. J Water Resour Protect 14:433–455. https://doi.org/10.4236/warp.2022.146023
Tang XW, Wu JK (2014) Major ion chemistry of surface water in the Xilin River basin and the possible controls. Huan Jing Ke Xue 35(1):131–142
Tapias JC, Melian R, Sendros A, Font X, Casas A (2022) Geochemical characterization and health concerns of mineral bottled waters in Catalonia (North-Eastern Spain). Water 14(3581). https://doi.org/10.3390/w14213581
Tazikeh H, Khormali F, Amini A, Barani Motlaq M (2017) Changes and evolution of soils composed of different depositional matters in Sheykh synclinal, NE Bojnord. Agric Eng 40(1):101–124
Teimouri M, Asadi Nalivan O, Elahi S (2022) The probabilistic analysis of drought severity-duration in North Khorasan Province using Copula functions. Watershed Manag Res. https://doi.org/10.22092/WMRJ.2022.359052.1479
Teimouri M, Sheikh V, Sadoddin A (2018) Evaluation and comparison of water quality using gray relational analysis and NSFWQI in Shirin Darreh Dam reservoir. J Health Environ 11(2):16–182
Tekile AK (2023) Suitability assessment of surface water quality for irrigation: a case study of Modjo River, Ethiopia. J Environ Public Health. https://doi.org/10.1155/2023/1482229
Tikhomirov VV (2016) Hydrogeochemistry fundamentals and advances: groundwater composition and chemistry. Wiley, Poland
Valipour (2022) Quality evaluation of groundwater pollution based on geological formation. Environ Water Eng 8(2):519–529. https://doi.org/10.22034/JEWE.2022.307605.1637
Wang L, Du T, Hu Q, Lin S, Liu M, Chen H (2020) Assessing major factors impacts on the hydrogeochemistry in a riverside alluvial aquifer, northeast China. Arab J Geosci 13(1072). https://doi.org/10.1007/s12517-020-06058-3
Wang W, Chen Y, Wang W, Yang Y, Hou Y, Zhang S, Zhu Z (2022) Assessing the influence of land use change on groundwater hydrochemistry in an oasis-desert region of central Asia. Water 14(651). https://doi.org/10.3390/w14040651
Wilcox LV (1955) Classification and use of irrigation waters (Circular No. 969). United States Department of Agriculture (USDA), Washington D.C
World Health Organization (WHO) (2017) Guidelines for drinking water quality. WHO, Rome
Wu P, Tang C, Zhu L, Liu C (2009) Hydrogeochemical characteristics of surface water and ground water in the karst basin, southwest China. Hydrol Process 23(14):2012–2022. https://doi.org/10.1002/hyp.7332
Wu Z, Zhang D, Cai Y, Wang X, Zhang L, Chen Y (2017) Water quality assessment based on the water quality index method in Lake Poyang: The largest freshwater lake in China. Sci Rep 7(17999). https://doi.org/10.1038/s41598-017-18285-y
Xu F, Li P, Chen W, He S, Li F, Mu D, Elumalai V (2022) Impacts of land use/land cover patterns on groundwater quality in the Guanzhong Basin of northwest China. Geocarto International. https://doi.org/10.1080/10106049.2022.2115153
Xu P, Feng W, Qian H, Zhang Q (2019) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the central-western Guanzhong basin, China. Int J Environ Res Public Health 16(9). https://doi.org/10.3390/ijerph16091492
Zaman M, Shahid SA, Heng L (2018) Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer, Switzerland
Zandbergen PA, Hall KJ (1998) Analysis of the British Colombia water quality index for watershed managers, a case study of two small watersheds. Water Qual Res J Canada 33:519–549
Acknowledgements
The author would like to thank the Regional Water Company of Northern Khorasan Province for analyzing the water samples. The constructive and supportive comments by anonymous reviewers are highly appreciated.
Funding
This study was conducted as the research project (Doctoral Dissertation), supported by the Ferdowsi University of Mashhad (Grant number 3/47984 (contribution of the author)) approved on 11.7.2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tajbakhshian, M. Hydrogeochemical evolution and water quality assessment of surface and groundwater resources: case study, Shirin Darreh dam basin (NE Iran). Arab J Geosci 16, 544 (2023). https://doi.org/10.1007/s12517-023-11641-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11641-5