Abstract
Aluminum (Al) toxicity is a limiting factor for crop growth, especially legumes in acid-affected soil of the world. The present study was done with the objective to study the comparative ameliorative property of exogenously added Ca, Mg, P, and salicylic acid on Al stress in pea (Pisum sativum) grown in nutrient media. Treatment of seedling with 24 ppm Al resulted in an inhibition of root/shoot growth with less dry matter production along with an enhanced level of superoxide, H2O2, and lipid peroxidation. Al toxicity in a pea seedling caused significant variation in the activity of an antioxidative enzyme. The application of 1 mM CaCl2, 0.5 mM H3PO4, MgSO4 (0.5 mM and 0.25 mM), and SA (100 μM) reduced the negative effect of Al stress in the pea seedling marked by the restoration of seedling growth and suppression of Al accumulation, superoxide, and H2O2 content. MgSO4 and SA treatment elevated the Al-induced decline in photosynthetic pigment. Exogenously, an addition of Mg, P, and SA elevated the Al-induced decrease in the activity of SOD, catalase, vitamin C, and carotenoids. Application of 0.5 mM H3PO4 resulted in 47% recovery of root growth and 60% of shoot growth along with the highest suppression of Al uptake and ROS accumulation. Among all the treatments, the application of the 0.5 mM P under Al stress appeared a potential tool in restoring the growth and physiological activities in sensitive pea genotype (AP-3) indicating the best ameliorating agent under Al toxic condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is one of the most abundant metals found in the Earth’s crust, contributing nearly 7% of its mass. Generally, it occurs in non-phytotoxic forms like aluminosilicates and precipitates, but its toxicity occurs mainly in acidic soil. Acidic soils constitute 40% of total cultivated land across the world and pose a major threat to agricultural crop production (Kochian 1995). Soil acidification occurs naturally when basic cations are leached out from soils, and the process is accelerated by heavy rainfall. Various plant species are sensitive to micromolar concentration of Al which hampers the physiological processes and growth. The Al-sensitive plant species express oxidative stress and very low antioxidative property under toxic Al concentration which creates abiotic stress and drastically restrict the crop growth and development (Naz et al., 2022). Al-induced acidity causes oxidative injury in leaf and root cells, which leads to permanent damage, or death of the entire plant (Ahmad et al., 2019). In Al-induced abiotic stress acid soils, change in the ion distributions and water availability retraction within plant cells causes the plant to grow slowly, produce less, and be of lower quality (Khalid et al., 2020).
Pea (Pisum sativum L.) is an important legume crop grown all over the world, and it provides important dietary protein to millions of people. Many abiotic stresses affect the production of peas, of which Al toxicity in acidic soil is a major constraint for pea growth and physiology (Kichigina et al. 2017). The primary effect of phytotoxic Al is the inhibition of root growth in peas and injury to root tips making it hard and subtle (Motoda et al. 2010). Due to Al stress, reactive oxygen species (ROS) viz. superoxide radical, hydrogen peroxide (H2O2), and hydroxyl radical is enhanced in plants (Borgo et al. 2020) which causes damage to protein and causes lipid peroxidation. Plants have a mechanism to defend Al toxicity through various methods viz. secretion of organic acids from roots which capture free Al toxic to plants making it unavailable, increasing the soil pH around the roots, internal detoxification by Al chelation inside plant tissues, compartmentalization of Al in the vacuole (Liu et al. 2009), and enzymatic and non-enzymatic antioxidants which scavenge the ROS (Pandey et al. 2014).
It is reported that the growth and productivity of peas may enhance either by screening for Al tolerance (Singh and Choudhary 2010; Kichigina et al. 2017) or by incorporation of remedial measures such as liming and use of boron (Yu et al. 2009) and mucilage (Geng et al. 2012). The macronutrients viz. P, Mg, and Ca have been reported by many researchers as potential ameliorating agents for Al toxicity (Iqbal 2014; Guo et al. 2006; Bose et al. 2011). Calcium is known to ameliorate Al stress in plants by increasing the soil pH and reducing the uptake of Al (Guo et al. 2006). In peas, Ca treatment has decreased the negative effect of Al on K uptake by roots (Matsumoto and Yamaya 1986). Magnesium (Mg) can prevent Al migration through the cytosolic plasma membrane in root tips. Mg plays an important role in the amelioration of Al toxicity by competing for membrane transporters and metal binding sites on enzymes (Bose et al. 2011). Kinraide et al. (2004) have shown that a higher concentration of Mg in the growth medium helps in the amelioration of Al toxicity in plants. Phosphorus is an important macronutrient for root growth in plants and alleviates Al toxicity by forming a complex with Al thus inhibiting the uptake by plants (Iqbal 2014). Salicylic acid (SA), a phyto-protectant and hormone, plays an important role in various kinds of environmental stress. It inhibits the Al uptake in the plant by enhancing the citrate efflux of the roots (Yang et al. 2003). Application of SA in plants has been shown to protect the cell from oxidative stress by activating the enzymatic defense response (Pandey et al. 2014; Nawaz et al. 2021).
In recent years, a lot of work has been put into discovering an efficient method for creating abiotic Al-induced stress tolerance in plants, which will ultimately assist plants in thriving and surviving in situations that are caused by Al-induced acidity. The most promising technique for enhancing plant tolerance under acidic circumstances is the exogenous application of plant phytohormones, particularly the use of salicylic acid and different nutrients. The action of different nutrients and hormones has been studied individually to observe the ameliorative effect on Al toxicity, but comparative studies among them to depict better ameliorative capacity were limited in pea crops.
In order to examine whether Ca, Mg, P, and salicylic acid could be used to alleviate Al toxicity and prevent oxidative damage to pea seedlings the present study was done with the objective to study the comparative effects of exogenously added Ca, Mg, P, and salicylic acid in the growth medium on Al accumulation, ROS production, and the response of antioxidative defense in pea seedling grown under Al stress.
Materials and methods
Plant material and experimental site
The experiment was carried out at the College of Horticulture and Forestry CAU, Pasighat, Arunachal Pradesh, India, during 2019–2020. The plant materials for the present study comprised one susceptible genotype (Azad Pea-3). The susceptibility/tolerance of the genotype was accessed by hematoxylin staining and growth (data not reported here).
Experimental setup and plant growth
The present study was planned under a completely randomized design (CRD) with 9 treatments and three replications (Table 1). For each treatment, 15 seeds were taken, and after sterilization with sodium hypochlorite, 3% (v/v) was soaked in water for 12 h. The seeds were sown in plastic pots of 1.5 l capacity containing inert sand and allowed to germinate and establish for 6 days. On the 6th day after sowing, seedlings were supplied with only the Hoagland nutrient solution (Hoagland and Arnon 1950) served as control or the Hoagland nutrient solution containing 24 ppm Al,1 mM CaCl2+24 ppm Al, 0.25 mM KH2PO4+24 ppm Al, 0.5 mM KH2PO4+24 ppm Al, 0.25 mM MgSO4+ppm Al, 0.5 MgSO4+24 ppm Al, 50 μM SA+24 ppm Al, and 100 μM SA+24 ppm Al which served as treatment (Table 1). The pH of the Hoagland nutrient solution was maintained at 4.5 with 1 N HCl. During the growth period, the plants were kept under artificial light (3000 lux) for 14 h photo-period at a temperature of 24/18 °C (day/night) and 70% relative humidity. Nutrient solution was replenished every 5 days. And after 14 days of treatment, plants were uprooted carefully and stored at −80 °C for further analysis. For dry matter content, the sample was kept in a hot air oven and measured in an analytical balance. The dried sample was also used for Al uptake estimation.
Statistical analysis
The analysis of variance was done according to the standard method given by Gomez K. and Gomez A. (1984). The mean comparison within the treatment was done by Tukey’s HSD test at a 5% probability level using the SPSS software (version 21). In the graph, different letters indicate significant differences at p>0.05, and the error bar = mean ± SD (n = 3).
Protein and lipid peroxidation estimation
The protein in the pea sample was estimated by Lowry’s method (Lowry et al. 1951). The lipid peroxidation was estimated in terms of the thiobarbituric acid reactive substance (TBARS) method as suggested by Heath and Packer (1968). The TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1, and it was expressed as nmol TBARS g−1 fresh weight (FW).
Fresh samples (100 mg) were homogenized in 5 ml of 10% trichloro-acetic acid (TCA) containing 0.25% 2-thiobarbituric acid (TBA). The mixture was heated for 25 min at 95 °C and immediately cooled in an ice bath. Then, the aliquot was centrifuged for 10 min at 10000 rpm, and the supernatant was collected for reading absorbance at 532 nm using 0.25% TBA in 10% TCA as blank. For the values of non-specific absorption recorded at 600 nm, values recorded at 532 nm were subtracted. Lipid peroxide concentration was calculated using the following formula:
Estimation of reactive oxygen species (ROS)
The superoxide anion content in the plant tissue was assayed by the epinephrine method (Mishra and Fridovich 1972) based on its capability to inhibit the autoxidation of adrenaline to adrenochrome. Plant sample (100 mg) was cut into pieces (2–4 mm) and was kept overnight in the reaction mixture containing 100 μmol/l disodium-EDTA, 20 μmol/l NADH, and 20 mmol/l NaH2PO4 NaH2PO4 buffer pH (7.8). Fresh epinephrine was prepared in 0.1 M HCl and was added to the samples for initiating the reaction. The samples were shaken at 150 rpm and 28 °C for 5 min. The absorbance of the supernatant was read at 480 nm for 10 min. The formation of O2 was expressed as ΔA [480 nm] min−1 g−1 tissue fresh weight.
Hydrogen peroxide content was estimated according to Jana and Choudhuri (1981). Hydrogen peroxide was measured both in the shoot and roots using the UV-Vis spectrophotometer (Hitachi U-1900) after a reaction with titanium sulfate. The level of H2O2 production was calculated using an extinction coefficient of 0.28 μM−1 cm−1 and expressed as nmol per g fresh weight of the tissues.
The content of hydroxyl free radical was estimated by the deoxyribose assay given by Halliwell and Gutteridge (2007). The absorbance was measured at 532 nm.
Determination of enzymatic and non-enzymatic antioxidants
The catalase activity in the root and shoot was measured by the procedure outlined by Beers and Sizer (1952). For the assay, 200 μl of the extracted enzyme was taken and mixed with 100 mM potassium phosphate buffer (pH 7.0). The reaction was initiated by the addition of 50 mM H2O2. The change in absorbance was recorded at 240 nm at an interval of 5 min at 25 °C. The enzyme activity was expressed as unit mg−1 protein min−1 and calculated using an extinction coefficient of 0.036 mM−1 cm−1.
The activity of superoxide dismutase (SOD) was estimated based on the photochemical reduction of riboflavin as superoxide and the inhibition of nitroblue tetrazolium (Beauchamp and Fridovich 1971). Samples (50 mg) were grounded in a condition with 100 mM potassium phosphate buffer (5 ml, pH 7.8) containing 0.1 mM EDTA, 2% (w/v) PVP, and 0.5% (v/v) Triton X-100. Centrifugation of homogenate was done for 10 min at 10000 rpm and 4 °C. The supernatant was dialyzed in cellophane membrane tubings for 6 h against the extraction buffer in cold with 3–4 changes of the buffer. SOD activity was determined in supernatants. One unit of enzyme activity is expressed as the amount of enzyme giving a 50% inhibition of the NBT reduction. The absorbance was measured at 560 nm, and the values were expressed in units per mg protein.
APX catalyzes the reduction of H2O2 using the substrate ascorbate, and it was estimated using the method of Nakano and Asada (1981). The reaction mixture (1 ml) contained 50 mM potassium phosphate buffer (pH 7.0), 0.2 mM EDTA, 0.5 mM AsA, and 1 mM H2O2. Diminishing absorbance was recorded at 290 nm at 30-s intervals for 5 min. The enzyme activity was expressed as μmol ascorbate oxidized min−1 mg−1 protein and calculated using an extinction coefficient of 2.8 mM−1 cm−1.
The estimation of guaiacol peroxidase (GPX) was determined by the method given by Egley et al. (1983). The homogenate was centrifuged at 22000×g for 10 min at 4 °C, and the supernatant after dialysis was used for enzyme assay. An increase in absorbance due to the formation of tetraguaiacohinone was measured at 420 nm (extinction coefficient of 26.6 mM−1 cm−1) at 30-s intervals up to 3 min using a spectrophotometer (Hitachi U-1900).
Vitamin C content was estimated by the method of Jagota and Dani (1982), and photosynthetic pigment was done by following Lichtenthaler’s method (Lichtenthaler 1987).
Al uptake estimation
Plant samples were dried at 70 °C, powdered, and sieved through a 0.5-mm sieve. One gram of plant sample was digested in a 10-ml tri-acid mixture (9:4:1 mixture of nitric acid, perchloric acid, and sulfuric acid), and the volume was made up to 100 ml using distilled water.
The aluminum in the plant sample was determined colorimetrically using an aluminon-acetate buffer (Barnhisel and Bertsch, 1982).
Results
Effect of Al on growth and ameliorative capacity of different chemical agents
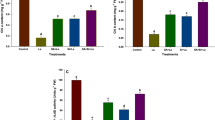
The present study revealed that Al in sand culture had a significant decline in pea seedling growth. Al treatment reduced the root length of the pea seedling by 31% (p<0.05) (Fig. 1). Root length recovery in the presence of 24 ppm Al was nearly 47% (p<0.05)with the application of 0.5 mM KH2PO4 which was statistically similar with 0.25 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, 0.25 mM MgSO4, and 100 μM salicylic acid (Fig. 2). Al treatment reduced shoot length of the pea seedling by 31% (p<0.05) (Fig. 1). Shoot length recovery in the presence of 24 ppm Al was nearly 60% (p<0.05) with the application of 0.5 mM KH2PO4 which was at par with 1 mM CaCl2.
Al treatment reduced the shoot and root dry weight of the pea seedling by 51% (p<0.05) (Fig. 3). In the presence of Al, application of nutrients and SA had significant restoration of dry weight in the pea seedling. The restoration of shoot dry weight was nearly 1.5-fold (p<0.05) with the application of 0.5 mM KH2PO4 and 0.25 mM MgSO4 in the presence of 24 ppm Al.
Photosynthetic pigment and total soluble protein
Pea seedlings grown for 14 days in the presence of Al resulted in nearly 26% (p<0.05) and 29% (p<0.05) decrease in chlorophyll a and chlorophyll b, respectively compared to the control (Fig. 4). In the presence of 24 ppm Al, nearly 105% (p<0.05) chlorophyll a was observed with the application of 50 μM SA (2.24 mg g−1) which was at par with the application of 0.5 mM MgSO4 (2.10 mg g−1), and chlorophyll b was observed highest in 50 μM SA (1.03 mg g−1) Al followed by 0.5 mM MgSO4 (0.86 mg g−1). Presence of 24 ppm Al in the growing media had no significant effect on the protein content of the shoot but a nearly 49% (p<0.05) decrease in soluble protein content in the root, compared to the control (Fig. 5). Application of nutrient and SA along with 24 ppm Al resulted in a significant increase in soluble protein content in the shoot, and the highest was observed with the application of 0.5 mM MgSO4 (29.6 ± 1.3 mg g−1) which was at par with 0.25 mM MgSO4 (28.6 ± 1.4 mg g−1). In the root of pea grown in the presence of 24 ppm Al, application of 1 mM Ca, 0.25 mM and 0.5 MgSO4, 0.5 mM KH2PO4, and 50 μM and 100 μM SA was able to restore soluble protein to normal levels or even higher as compared to control. A 2.4-fold increase in protein was observed with the application of 0.5 mM KH2PO4 in the presence of Al.
ROS generation and lipid peroxidation
Lipid peroxidation content in the shoot and root of pea seedlings in the presence of 24 ppm Al in the growing media significantly increased by nearly 78% (p<0.05) in the shoot and 110% (p<0.05) in the root, respectively compared to no Al (Fig. 6). However, application of 1 mM Ca, 0.5 mM P, 0.25 mM Mg, and 50 μM and 100 μM SA along with Al resulted in an even higher lipid peroxidation than Al treatment alone. However, application of 0.25 mM KH2PO4 in the presence of Al reduced lipid peroxidation by 5.7% (p < 0.05) with respect to 24 ppm Al treatment alone in pea root.
In the presence of 24 ppm Al for 14 days in sand culture, the level of superoxide was enhanced by nearly 29% (p<0.05) and 45% (p<0.05) in pea seedlings, respectively compared to the control (Fig. 7). Application of nutrient and SA reduced the production of Al-induced superoxide anion in root and shoot. In Shoot, application of 50 μM SA was best for reducing Al-induced superoxide by 55% (p<0.05) followed by 0.5 mM MgSO4, 0.25 mM KH2PO4, 0.5 mM KH2PO4, and 1 mM CaCl2. In the root of pea seedling, 0.25 mM KH2PO4 application reduced Al-induced superoxide by 25%.
H2O2 production in the shoot was enhanced by 67% (p<0.05) and a 3.4-fold (p<0.05) in the presence of 24 ppm Al in growing media for 14 days with respect to no Al (Fig. 8). In the shoot of pea seedling, application of 50 μM SA+Al reduced H2O2 production by nearly 42% (p<0.05) with respect to 24 ppm Al treatment alone. The application of nutrients and SA along with Al inhibits the production of H2O2 significantly with respect to 24 ppm Al alone. In The presence of Al, addition of 0.25 mM and 0.5 mM KH2PO4 reduced the production of H2O2 in the root of pea seedlings by nearly 84% (p<0.05) and 72%, respectively compared to 24 ppm Al alone.
Addition of 24 ppm Al in the growing media of pea seedlings for 14 days had nearly 83% (p<0.05) enhanced level of hydroxyl free radical in shoot and a 1.2-fold (p<0.05) increase in root compared to hydroxyl free radical level in control (Fig. 9). In the shoot, only 1 mM CaCl2 was able to reduce Al-induced hydroxyl free radical production by nearly 18%. Root nutrient and SA significantly reduced the production of an Al-induced hydroxyl radical. Application of 100 μM SA reduced Al-induced hydroxyl free radical production by 31% (p<0.05) followed by 0.25 mM KH2PO4 (29%, p<0.05).
Enzymatic antioxidant defense system
Addition of 24 ppm Al resulted in a decrease of nearly 16% (p<0.05) and 21% (p<0.05) in the catalase activity in shoots and roots of pea seedlings compared to the control (Fig. 10). In pea seedlings applied with 0.5 mM P and 50 μM SA with Al, a significant increase of nearly 20% and 32%, respectively, in catalase activity was observed with Al treatment alone. In the root of pea seedlings, the catalase activity was lower with the application of nutrients and SA along with 24 ppm Al with respect to Al-treated seedlings. Pea seedlings grown for 14 days under 24 ppm Al resulted in nearly 8-fold (p<0.05) increased activity of SOD in the shoot and a decrease of 42% (p<0.05) in the root compared to the control (Fig. 11). Addition of KH2PO4, MgSO4, and SA along with Al reduced the Al-induced SOD activity in the shoot significantly. The highest reduction in the Al-induced SOD activity in the presence of Al was observed with the addition of 0.5 mM MgSO4 in the shoot. In the root of peas, SOD activity was found highest with the application of 0.25 mM KH2PO4+24 ppm Al (59.11 U/mg protein). Addition of 24 ppm Al in media increased by nearly 22% (p<0.05) and 2.8-fold (p<0.05) in the APX activity of shoots and roots of pea seedlings, respectively compared to the control (Fig. 12). Application of 0.25 mM and 0.5 mM KH2PO4, 0.25 mM and 0.5 mM MgSO4, and 50 μM and 100 μM SA resulted in a significant decline in Al-induced APX activity in shoots of peas (Fig. 12), whereas in the root of peas, application of 1 mM CaCl2, 0.25 mM KH2PO4, 0.25 mM KH2PO4, and 0.5 mM and MgSO4 50 μM SA reduced Al-induced APX significantly. Application of 0.5 mM KH2PO4+24 ppm Al resulted in the lowest APX activity in the root. Pea seedlings grown for 14 days under 24 ppm Al had a non-significant effect on GPX activity in the shoot and nearly 20% (p<0.05) decrease in GPX activity of root compared to no Al (Fig. 13). Application of different treatments could not restore the GPX activity to a normal level.
Non-enzymatic antioxidants
Vitamin C content of pea seedlings grown for 14 days in the presence of 24 ppm Al significantly reduced by nearly 74% (p<0.05) in the shoot and nearly 36% (p<0.05) in the root compared to vitamin C in the control (Fig. 14). In the presence of 24 ppm Al, a 3.5-fold (p<0.05) increase in vitamin C content in the shoot was observed with the application of 0.5 mM KH2PO4 (19.04 mg/100 g FW) which was significantly at par with 0.25 mM KH2PO4. In the presence of Al application of 0.25 mM MgSO4, an increase of 58% in vitamin C was observed in the root which was at par with 100 μM SA (10.42 mg100 g−1 FW) (Fig. 14).
Garden pea var. AP-3 grown for 14 days in the presence of 24 ppm had a nearly 18% (p<0.05) decline in the total carotenoid content of leaves (Fig. 15). In the presence of 24 ppm Al, the total carotenoid content of leaves increased nearly 42% (p<0.05) with the application of 50 μM SA which was at par with 0.5 mM MgSO4 (0.290 mg g−1 FW).
Growing pea seedlings for 14 days in the presence of 24 ppm Al in the media resulted in nearly 12-fold (p<0.05) and 24-fold (p<0.05) increase in Al content of shoot and root on dry matter basis, respectively compared to the control (Fig. 16). In the presence of 24 ppm Al in media, application of 0.25 mM MgSO4 resulted in the lowest content of Al in the shoot (18.4 μg/g DM) which was at par with 0.25 mM KH2PO4 (30.2 μg/g DM) followed by 0.5 mM KH2PO4 (80.0 μg/g DM). In the root, lowest content of Al was observed with the application of 0.5 mM KH2PO4+24 ppm Al (69.0 μg/g DM).
Discussion
The present study revealed that Al in the growth medium resulted in a significant decline in pea seedling growth. Inhibition of plant growth especially root growth is a distinctive characteristic of Al toxicity which is caused due to hindrance in the cell division mechanism resulting in small and brittle roots which can easily break (Panda et al. 2009). We found that the application of Ca, P, Mg, and SA recovered the growth of peas from Al stress. Exogenous application of P was found best for the recovery of root and shoot length than other chemical agents. The application of 0.5 mM KH2PO4 and 0.25 mM MgSO4 was better for the recovery of the dry weight of Al-susceptible genotype AP-3. Earlier reports suggested that the incorporation of P in soil fully mitigated the toxic effect of Al in sensitive wheat genotype, the detoxification process of Al by P primarily happens in soil rhizosphere instead of plant tissue (Iqbal, 2014), and adding Mg2+ to the growth medium alleviated Al stress in plants (Bose et al. 2011).
Al stress caused a decrease in photosynthetic pigments in pea seedlings. The photosynthetic pigment decrease is caused due to damage of chloroplast and destruction of the primary matter of chlorophyll synthesis. In different stresses, existing chlorophyll in the chloroplast is broken down resulting in the loss of thylakoid structure (Rout et al. 2001; Ghani 2022). With Al in the growing media, the application of Ca, P, Mg, and SA restored the chlorophyll content in pea seedlings. Out of which, 50 μM SA and 0.5 mM Mg addition was better in maintaining chlorophyll pigment in the leaves. Magnesium ion (Mg2+) is a major metal in the chlorophyll, and a substantial quantity is essential for chloroplasts. Therefore, the application of MgSO4 alleviated Al stress in several species of plants by maintaining chlorophyll stability (Kinraide et al. 2004). SA plays a significant role in the uptake of nutrients and enhances the growth and yield of plants.
Al treatment caused a substantial drop in the soluble protein content of root in Al-susceptible genotype AP-3. The outcome is in agreement with the findings of Sabat et al. (2016) in mung bean seedlings where Al treatment decreased the protein in the shoot. Hirpa et al. (2015) also reported that Al addition significantly reduced the protein contents of common bean genotypes; a greater reduction was detected for the sensitive genotypes. However, the application of P and Mg in the presence of Al enhanced the soluble protein content in pea seedlings. Protein is a part of various enzymes which play a significant role in the defense mechanism against various types of stress. The increase in soluble protein content along with better growth indicated the ameliorative capacity of P and Mg.
ROS generation and lipid peroxidation
The lipid peroxidation in the shoot/root was high due to the addition of Al in the growth media. However, application of 1 mM Ca, 0.5 mM P, 0.25 mM Mg, and 50 μM and 100 μM SA along with Al resulted in an even higher lipid peroxidation than the Al treatment alone. It is because, solubilizing of the cell wall lipid increases the cell wall permeability for the entry of cations inside the protoplasm. In the pea seedlings grown under Al stress for 14 days, there was an enhanced level of ROS in the shoot/root, and the generation of oxidative stress is a vital part of the manifestation of Al stress in peas (Matsumoto and Motoda, 2013). Superoxide anion production and H2O2 got shot up due to Al stress in both shoot and root which led to lipid peroxidation in pea seedlings. Application of Ca, P, Mg, and SA was able to minimize the production of H2O2 and superoxide anion with better root elongation depicting an ameliorative effect against Al toxicity. It inhibited the buildup of ROS in the root and shoot tissues thus subduing the oxidative damage to pea seedlings and maintained a satisfactory enzymatic and non-enzymatic defense in the pea seeding. Under enzymatic antioxidant, the first role is played by SOD which transforms the superoxide into H2O2 followed by catalase and peroxidases which neutralize H2O2 in the plant cell (Darko et al. 2004). Al stress leads to oxidative stress due to a higher production of ROS in tomato (Borgo et al. 2020) and rice (Pandey et al. 2013). Among the substance examined, KH2PO4 and SA appeared to be superior to CaCl2 and MgSO4 in neutralizing the accumulation of ROS due to Al stress.
Enzymatic defense system
Application of Al increased the production of H2O2 and superoxide anion, an elevation in the activity of the enzymatic antioxidant. Among the enzymatic defense system, the level of SOD and APX shot up due to Al stress. SOD is a vital enzyme, and its enhancement is directly related to the conversion of superoxide into H2O2 to keep it in check (Pandey et al. 2014; Ghani et al. 2019), signifying that superoxide production may possibly be a reason for an enhanced level of SOD activity. In the root and/or shoot, P and Mg performed better for buffering the SOD activity due to Al-induced damage to the seedling. Less production of superoxide anion was associated with lower SOD production in 0.5 mM Mg+Al- and 0.5 mM P+Al-treated plants as compared to Al treatment alone, suggesting that P and Mg were able to reduce the Al-induced superoxide production in seedlings which led to low SOD activity. In the present study, on Al exposure, among the enzymes involved in the removal of H2O2 the activity of CAT and GPX declined, whereas only APX activity got increased. These findings give light that APX activity has a vital role in neutralizing H2O2 production in stressed pea seedlings. In the previous study on rice, the activity of CAT got reduced due to Al treatment representing that H2O2 accumulation inactivated the enzyme (Pandey et al. 2013). The application of Ca, Mg, P, and SA with Al in the growth medium lowered the Al-led activity of APX, and 0.5 mM P and 50 μM SA enhanced the Al-stimulated decline in CAT in the shoot of pea seedlings. This indicated that the chemical agents were able to ameliorate the damage caused by Al stress by buffering the activity of antioxidant enzymes. Peroxidases are considered stress enzymes, and the activity of peroxidases increases under abiotic stress including Al toxicity (Sharma and Dubey 2007). In the present studies, GPX activity decreased due to Al stress, but nutrient and SA could not enhance the level of GPX. This suggests that CaCl2, KH2PO4, MgSO4, and SA do not play any role in restoring GPX activity in Al-stressed pea seedlings. It has been reported in rice that SA lowered the Al-induced activity of APX and GPX and imparted a positive effect on the growth and development of plants (Pandey et al. 2013). In several plant species, Mg has been shown to alleviate Al toxicity (Chen and Ma 2013). SA plays an important role in plants under a variety of environmental stresses by retaining the redox state of the antioxidant pool and inducing antioxidative defenses (Song et al. 2011).
Vitamin C (ascorbic acid) and carotenoid are frequently studied non-enzymatic antioxidant compounds and play an important role in the growth and development of plants (Pignocchi and Foyer 2003) and, for the relevance in this research, act as neutralizing agents for ROS generated due to plant under stress (Qian et al. 2014). The concentration of non-enzymatic antioxidants got reduced with Al stress. The application of P and Mg was able to restore the level of vitamin C and carotenoid to normal levels possibly by lowering the accumulation of ROS and better growth.
The decline in the carotenoid content could be due to the enhanced level of ROS and lipid peroxidation which destroys carotenoids in the susceptible variety (Ravi et al. 2011).
Application of Al in the growth media significantly enhanced the content of Al in plant tissue and causes imbalances in the nutrient content in root and shoot. The accumulation of Al in the root was more than the shoot of a pea seedling. Ahn et al. (2002) reported that the root inhibition in squash was related to Al concentration and the period of exposure and damage that occurred in the region with high Al accumulation. With the content of Al by pea roots, the growth of seedlings decreased marked by a reduction in length and dry weight. The Al content in roots was reduced when Ca, P, and Mg were added to the medium. The lowest accumulation of Al in pea seedlings was observed with the application of P in the growth medium. P forms Al-phosphate complexes in solution and makes the Al unavailable in the media thus reducing the content by the plants (Iqbal, 2014). In soybean, it has been shown that both Ca and Mg have a protective effect against Al-induced inhibition of root elongation (Silva et al., 2001). It is suggested that the addition of Ca to the growth medium reduces Al activity at the plasma membrane surface of root cells (Silva et al., 2001). The addition of Mg2+ ions increases the ionic strength of the media (Noble and Sumner, 1988) resulting in a reduction in Al3+ saturation on the apoplastic exchange sites.
Conclusion
The Al stress caused a marked reduction in the growth and accumulation of Al along with the buildup of ROS in pea seedlings. The exogenous application of CaSO4, H2PO4, MgSO4, and salicylic acid as ameliorating agents for Al stress in pea genotypes indicated restoration of ordinary growth and inhibited the buildup of ROS in the root and shoot tissues thus subduing the oxidative damage and maintaining a satisfactory enzymatic and non-enzymatic defense in the pea seedlings. Application of 0.5 mM H3PO4 resulted in 47% recovery of root growth and 60% of shoot growth along with the highest suppression of Al uptake and ROS accumulation. Among the four substances used, amelioration effects of Al toxicity appeared superior at 0.5 mM P treatment. This indicated that 0.05 mM P is more efficient than other nutrients (Mg and Ca) and phytohormone, SA, in the amelioration of Al toxicity.
References
Ahmad R, Hussain S, Anjum MA, Khalid MF, Saqib M, Zakir I, Hassan A, Fahad S, Ahmad S (2019) Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In: Plant Abiotic Stress Tolerance. Springer, Cham, Switzerland, pp 191–205. https://doi.org/10.1007/978-3-030-06118-0_8
Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H (2002) Aluminium-induced growth inhibition is associated with impaired efflux and influx of H+ across the plasma membrane in root apices of squash (Cucurbita pepo). J Experiment Botany 53(376):1959–1966
Barnhisel R, Bertsch PM (1982) Aluminium. In: Page AL Methods of soil analysis part 2. Agronomy,. 9 (2). American Society of Agronomy and Soil Science Society of America. Madison, Wisconsin, pp 275-300
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. JBC 195:133–139
Borgo L, Rabêlo FH, Carvalho G, Ramires T, Righetto AJ, Piotto FÂ, Azevedo R (2020) Antioxidant performance and aluminum accumulation in two genotypes of Solanum lycopersicum in response to low pH and aluminum availability and under their combined stress. Sci Hortic 259:108813
Bose J, Babourina O, Rengel Z (2011) Role of magnesium in alleviation of aluminium toxicity in plants. J Exp Bot 62(7):2251–2264
Chen ZC, Ma JF (2013) Magnesium transporters and their role in Al tolerance in plants. Plant Soil 368(1):51–56
Darko E, Ambrus H, Stefanovits-Bányai E, Fodor J, Barnabás BF (2004) Aluminium toxicity, Al tolerance and oxidative stress in an Al -sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci 166:583–591
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta 157:224–232
Geng M, Xu M, Xiao H, Wang H, He L, Zhao Z, Min YM (2012) Protective role of mucilage against Al toxicity to root apex of pea (Pisum sativum). Acta Physiol Plant 34:1261–1266
Ghani MI, Ali A, Atif MJ, Ali M, Amin B, Anees M, Cheng Z (2019) Soil amendment with raw garlic stalk: a novel strategy to stimulate growth and the antioxidative defense system in monocropped eggplant in the north of China. Agronomy 9(2):89
Ghani M, Ali A, Atif M, Ali M, Amin B, Cheng Z (2022) Arbuscular mycorrhizal fungi and dry raw garlic stalk amendment alleviate continuous monocropping growth and photosynthetic declines in eggplant by bolstering its antioxidant system and accumulation of osmolytes and secondary metabolites. Front Plant Sci 13:1
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. John Wiley and Sons, Inc., U.K., pp 84–96
Guo TR, Ying CHEN, Zhang YH, Jin YF (2006) Alleviation of Al toxicity in barley by addition of calcium. Agric Sci China 5(11):828–833
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford, U.K.
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hirpa L, Nigussie-Dechassa R, Setegn G, Bultosa G (2015) Chemical quality of common beans as influenced by genotype and aluminium rates under two soil liming regimes. African J Food, Agric Nutr Dev 15(2):9872–9891
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. In: Circular 347. California Agricultural Experiment Station, Berkeley, CA
Iqbal MT (2014) Acid tolerance mechanisms in soil grown plants. Malays J Soil Sci 6:1–21
Jagota SK, Dani HM (1982) A new colorimetric technique for estimation of vitamin C using Folin-phenol reagent. Anal Biochem 127(1):178–182
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquatic Botany 12:345–354
Khalid MF, Hussain S, Anjum MA, Ahmad S, Ali MA, Ejaz S, Morillon R (2020) Better salinity tolerance in tetraploid vs diploid Volkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J Plant Physiol. 244:153071
Kichigina NE, Puhalsky JV, Shaposhnikov AI et al (2017) Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol Mol Biol Plants 23(4):851–863
Kinraide TB, Pedler JF, Parker DR (2004) Relative effectiveness of calcium and magnesium in the alleviation of rhizotoxicity in wheat induced by copper, zinc, aluminum, sodium, and low pH. Plant Soil 259:201–208
Kochian LV (1995) Cellular mechanisms of aluminium toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu D, Zou J, Meng Q, Zou J, Jiang W (2009) Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicol 18(1):134–143
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Matsumoto H, Motoda H (2013) Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 363(1-2):399–410
Matsumoto H, Yamaya T (1986) Inhibition of potassium uptake and regulation of membrane-associated Mg2+-ATPase activity of pea roots by aluminium. Soil Sci Plant Nutr 32:179–188
Mishra HP, Fridovich I (1972) The role of superoxide anion in auto-oxidation of the epinephrine and sample assay for SOD. J Biol Chem 247:3170–3175
Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H (2010) Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil 333:49–58
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawaz M, Ashraf MY, Khan A, Nawaz F (2021) Salicylic acid–and ascorbic acid–induced salt tolerance in mung bean (Vigna radiata (L.) Wilczek) Accompanied by Oxidative Defense Mechanisms. J Soil Sci Plant Nutr 21:2057–2071
Naz S, Bilal A, Saddiq B, Ejaz S, Ali S, Ain Haider ST, Sardar H, Nasir B, Ahmad I, Tiwari K et al (2022) Foliar application of salicylic acid improved growth, yield, quality and photosynthesis of pea (Pisum sativum L.) by improving antioxidant defense mechanism under saline conditions. Sustainability 14:14180. https://doi.org/10.3390/su142114180
Noble AD, Sumner ME (1988) Calcium and Al interactions and soybean growth in nutrient solutions. Comm Soil Sci Plant Anal 19:1119–1131
Panda SK, Baluška F, Matsumoto H (2009) Aluminum stress signaling in plants. Plant Signal Behav 4(7):592–597
Pandey P, Srivastava RK, Dubey RS (2013) Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicol 22(4):656–670
Pandey P, Srivastava RK, Dubey RS (2014) Water deficit and aluminum tolerance are associated with a high antioxidative enzyme capacity in Indica rice seedlings. Protoplasma 251:147–160
Pignocchi C, Foyer CH (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Curr Opin Plant Biol 6(4):379–389
Qian HF, Peng XF, Han X, Ren J, Zhan KY, Zhu M (2014) The stress factor, exogenous ascorbic acid, affects plant growth and the antioxidant system in Arabidopsis thaliana. Russian J Plant Physiol 61:467–475
Ravi RK, Krishna K, Naik GR (2011) Effect of polyethylene glycol induced water stress on physiological and biochemical responses in pigeonpea (Cajanus cajan L. Mill sp.). Recent res sci technol 3:148–152
Rout G, Samantaray S, Das P (2001) Aluminium toxicity in plants: a review. Agronomie 21:3–21
Sabat G, Mahapatra M, Patro L, Padhy R, Mohanty BK (2016) Studies of aluminum (Al2O3) stress with biochemical parameters of Vigna radiata L seedling. Int J Adv ResBiol Sci 3(2):54–60
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminium. Plant Cell Rep 26:2027–2038
Silva IR, Smyth TJ, Israel DW, Raper CD, Rufty TW (2001) Magnesium ameliorates aluminum rhizotoxicity in soybean by increasing citric acid production and exudation by roots. Plant Cell Physiol 42:546–554
Singh D, Choudhary AK (2010) Inheritance pattern of aluminum tolerance in pea. Plant Breeding 129:688–692
Song H, Xu X, Wang H, Tao Y (2011) Protein carbonylation in barley seedling roots caused by aluminum and proton toxicity is suppressed by salicylic acid. Russian J Plant Physiol 58(4):653–659
Yang ZM, Wang J, Wang SH, Xu LL (2003) Salicylic acid-induced aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 217:168–174
Yu M, Shen R, Xiao H, Xu M, Wang H, Wang H, Zeng O, Bian J (2009) Boron alleviates aluminum toxicity in pea (Pisum sativum). Plant Soil 314:87–98
Acknowledgements
The authors acknowledge the Vice Chancellor of Central Agricultural University, Imphal, India, for providing necessary research facilities.
Author information
Authors and Affiliations
Contributions
M. T. Ansari carried out the experiment. Md. Ramjan conceptualized the experiment and methods. Vivek Yadav has done the drafting and editing of the manuscript. M. H. Ansari did the data analysis. M. F. Ali has done the editing of the manuscript. All authors contributed for the preparation of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There was no ethical concern in the publication.
Consent for publication
All the authors agreed to the publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Haroun Chenchouni
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, M.T., Ramjan, M., Yadav, V. et al. Ameliorative capacity of salicylic acid and nutrients (Ca, P, and Mg) against aluminum toxicity in sensitive pea (Pisum sativum L.). Arab J Geosci 16, 348 (2023). https://doi.org/10.1007/s12517-023-11445-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11445-7