Abstract
The study employed an integrated application of electrical resistivity tomography (ERT), soil classifications, and physicochemical parameters of water samples to map the extent and dynamics of leachate migration from the municipal waste dump and its potential impact on groundwater in a crystalline basement complex area, Malete, Nigeria. The ERT model revealed leachate dispersion beneath poorly graded inorganic silt-sand topsoil to about 2 m, hoisting low resistive moisturized soil within the range \(1.8\times {10}^{-3}\) to \(6.4\times {10}^{-1}\) Ωm and underlain by an impervious thin silty-clay layer (about 0.5-m thick) and weathered basement downward. The imprint of induced near-surface leachate percolation is revealed with increased (but acceptable) turbidity, conductivity, DO, and BOD within the shallow wells but prevented by the impervious silt–clay liner from vertical migration to the deeper water source. The concentrations of As, Cd, Cr, Fe, Pb, Zn, and Cu in the water samples and the acceptable TDS and TSS parameters; with low nitrate, sulfate and phosphate concentration are within the recommended range approved by WHO permissible limit for potable water and could have no adverse effects on the public’s health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dumpsite leachate is the most well-known source of pollution in shallow groundwater aquifers. Leaching of both organic and inorganic contaminants from dumpsites is a severe environmental problem for both surface water and aquifers over a long time, posing long-term health and environmental consequences (Macdonald et al. 2012; Bjerg et al. 2014; Oyeyemi et al. 2018, 2019). Since soil and rocks usually are good conductors of electricity, infiltration of leachate or polluted water may include a valuable number of dissolved ions, causing their conductivity to rise from poor to a moderate anomaly up to peak value. Humans are heavily reliant on groundwater, and predominantly, all sub-Saharan African communities obtain their drinking water from privately owned water wells; some of which are only a few meters deep or even deeper and in most cases prone to near-surface contaminants. Groundwater constitutes a higher percentage of the freshwater resources readily available in sub-Saharan Africa, and it forms the largest reservoir in the crystalline basement complex of Nigeria (Bernstone et al. 2000; Karlik and Kaya 2001; Macdonald et al. 2012; Olasehinde and Raji 2007; Abubakar et al. 2014; Adabanija and Alabi 2014; Ayolabi et al. 2015).
Contamination of groundwater can take place through migration of pollutants many meters in either vertical or horizontal distance, depending on the topography (via surface run-off), hydrology situation, and rock type within proximity or locale and poses a major threat to biotic communities (Dimitriou et al. 2008; Mepaiyeda et al. 2019; Moreira and Cesar 2021; Aziz et al. 2010; Fatta et al. 1999; Xing et al. 2013). Noninvasive and cost-effective geophysical techniques such as electrical resistivity tomography, very low-frequency electromagnetic (VLF-EM), and water quality assessment are appropriate for groundwater contamination investigation. The approaches have been effectively utilized to characterize geo-electric layers, landfill evaluation studies, map leachate movement around dumpsites, and groundwater contamination by examining their physical and chemical properties, also bacterial and heavy metals content (Bernstone et al. 2000; Karlik and Kaya 2001; Ogilvy et al. 2002; Porsani et al. 2004; Olasehinde and Raji 2007; Sundararajan et al. 2007; Boudreat et al. 2010; Adewoyin et al. 2019; Akinbiyi et al. 2020).
For identifying the occurrence and movement of leachate and to verify the geotechnical stability, induced polarization methods, electrical conductivity (EC) logging, and seismic surveys have been well utilized and documented (Karlik and Kaya 2001; Porsani et al. 2004; Boudreat et al. 2010; Ramalho et al. 2013; Zume et al. 2006; Osinowo and Olayinka 2012; Naudet et al. 2014), while much attention has been given to the investigation of effect leachate has on the qualities of the soils they are located in, and how these soil features contribute to the movement of the leachate plume, either by assisting or disrupting it (Ogilvy et al. 2002; De Carlo et al. 2013; Moreira et al. 2013; Ayolabi et al. 2015; Park et al. 2016; Maurya et al. 2017; Raji and Adeyoe 2017; Morita et al. 2020). However, hydrochemical analysis for trace element concentration and other microbiological effects is essential for the exact determination of groundwater contamination, and it corroborates the results of mechanical alteration in soil and leachate (Aziz et al. 2010; Ayolabi et al. 2015; Fatta et al. 1999; Xing et al. 2013; Kamble et al. 2020; Farzaneh et al. 2021; Igboama et al. 2021).
Considering the increased population of an ancient rural settlement Malete, which serves as a host for Kwara State University and other federal developmental projects, the increased rate of municipal dumps on the community’s open dumpsite necessitates aggressive preventive and restorative management measures on leachate impacts on surface and groundwater quality of the area. Municipal waste disposed in such open dumpsite is subject to either infiltration from precipitation or groundwater underflow that could result in accumulation of organic and inorganic compounds at bottom of the dumpsite and eventually penetrates through the soil to contaminate the groundwater (Taonameso et al. 2019). The resident of Malete depends on shallow hand-dug wells with low water recharge potential and susceptible to contamination and a few very deep wells (generally above 100 m) for domestic and commercial use. Thus, the aim of this study is to determine the lateral and vertical extent of leachate migration through the sub-base soil and its effect on the quality of the underground water in agreement with the World Health Organization (WHO 2017) standard for drinking water and Nigerian standard of drinking quality water (NSDWQ 2007), using the electrical resistivity tomography, soil sample, and physicochemical analysis.
Location and geology of the area
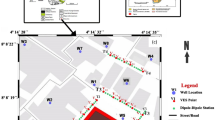
Malete falls within Moro local government area of Kwara State, bounded by geographical coordinates 8° 40′ to 8˚ 45′ N and 4° 25′ to 4° 30′ E, in the southwestern region of Nigeria. The climate is that of Guinea Savannah region with relative humidity of 50–60%, temperature range within 20.8 to 38.7 °C, sufficient untapped agricultural resources and agglomeration of buildings where people live and work. The rocks are of the Precambrian basement complex of southwestern Nigeria, predominantly Migmatite-Gneiss complex (MG) comprising mainly the biotite gneisses, porphyritic and microgranite, migmatite, and grey gneiss which are made up of minerals such as the quartz, biotite, plagioclase feldspar, microcline feldspar, hornblende, and other accessories which signify rocks in the low grade metamorphic facies (Fig. 1). These rocks trend in NE-SW and slightly dipping to the west. The heap of the dumpsite is about 8-m high above the average relief of 308 m above sea level. It has population of about 102,780 people who depends on River Laonu which flows through the southeast, as the main source of water and near surface water tapped from the major NE-SW deformational trend documented in Nigeria (Burke and Dewey 1972); (Obaje 2009). The dumpsite is within a built-up area, accessible by minor roads and several footpaths from the major road, about 25.5 km from Ilorin.
Methodology
Electrical resistivity tomography
The direct current electrical resistivity method involving the Wenner electrode configuration, which allows for better resolution of the subsurface resistivity distribution (Hamzah et al. 2006), was used to determine the mode of occurrence of leachate in the dumpsite area and the near-subsurface earth model. Three profiles were established NW–SE crosscutting the major structural trend and having spread length within 60 to 90 m. Equidistance electrode spacing “a” of 1, 3, 5, 10, and 15 m were deployed and the resistance “R” readings were transformed to apparent resistivity “\({\rho }_{a}\)” data using the geometric factor “K’” (Eq. 1). This method can measure alterations that are associated with rock parameters such as porosity and permeability and are related to discontinuity, fracture, and faults. Direct current is injected through current electrodes, and the potential difference is calculated and measured laterally and vertically by potential electrodes (Loke and Barker 1996). The apparent resistivity data was topographically corrected, and the resultant parameters were processed using Res2DINV resistivity modeling software, which generated two-dimensional “2D” inverse resistivity models of the subsurface of the dumpsite (Fig. 2).
Sampling and laboratory analysis
Soil sample analysis
Permeability is one of the important physical properties of soil, as some of the major problems of soil mechanics are directly connected with it (Rowe et al. 1995; Daniel 1987; Kayadelen 2007). Optimum permeability range to be possessed by geological barriers which are clay rich geological units (soil liners and covers) for reduction of contaminants in dumpsites are considered within 10−6 to 10−8 cm/s (Lambe and Whitman 1991; Allen et al. 1997). In order to determine the porosity, permeability, and the rate of settlement of the sub-base soil of the dumpsite, four (4) disturbed soil samples were collected at selected anomalous zones on the ERT using a hand auger and were labeled appropriately “A, B” for deeper (0.5 to 1.0 m) and “C, D” for shallow (0 to 0.5 m) depths of the two drill pits “PAC and PBD” respectively. The samples were manually sorted and filtered and prepared for grain size, liquid limit, plastic limit, specific soil gravity, linear shrinkage, and compaction analysis at the Geology Departmental Laboratory of University of Ilorin, Nigeria, using the BS ISO 11277:2020 standard. Both mechanical and hydrometer sieve analysis were adopted for the grain size distribution, in order to determine the coarse and fine grain fraction of the soil, useful to characterize the typical soil type. Mass of the soil retained on each dry sieve was used to determine the percentage passing, while the temperature at each hydrometer reading was recorded and then a statistical data sheet was produced showing the results of the analysis, and the clay and silt percentage in the samples were then calculated from the graph obtained by plotting percentage passing against the grain diameters (Fig. 3). The degree of cohesion and adhesion between the soil particles as related to the resistance of the soil to rupture (Atterberg limits) was determined using the clay minerals content, the plasticity, and shrinkage limits (Fig. 4). The soil were further classified into normal and or abnormal (over normal or under normal) specific gravity, calculating the void ratio of the soil and also the determination of porosity of the soil using the Pycnometer method (Table 1).
The coefficient of permeability is obtained from Eq. 3:
where H1 and H2 signifies the initial height and final height of the water in the standpipe above that which is in the container at time t1 and t2 respectively. A signifies the cross-sectional area of the sample, while a is cross-sectional area of the standpipe, and l as the height of the sample.
Water sample analysis
Six (6) samples of water (3 samples from shallow (about 3 m depth) hand-dug wells and 3 samples from boreholes (above 100 m depth)) were acquired around the dumpsite. The well water were labeled AJW1 (x;y: 8.707953°; 4.464948°), AJW2 (x;y: 8.703558°; 4.470159°), and AJW3 (x;y: 8.709678°; 4.465798°), while the boreholes’ are AJB1 (x;y: 8.707801°; 4.464702°), AJB2 (x;y: 8.707508°; 4.466025°), and AJB3 (x;y: 8.70826°; 4.465474°). The in situ parameters viz; temperature, turbidity, electrical conductivity, appearance, odor, and taste (Fig. 5); preservation and transportation of water samples for chemical and heavy metal parameters laboratory analysis were done according to standard prescription by the American Public Health Association (APHA 2005). The hydrogen ion concentration in ground water and the acidity or alkalinity of the water are both key indicators of pH. Water’s acidity is measured by its ability to quantitatively reduce a strong base to a particular pH level, and it is typically caused by carbon dioxide, mineral acids, and hydrolyzed salts like ferric and aluminum sulfates. Acids can have an impact on a variety of activities, including biological processes, chemical reactions, and corrosion (APHA 2005). The main contributors to water alkalinity are the ions hydroxide (OH−), bicarbonate (HCO3−) carbonate (CO32−), or a combination of two of these ions. When softening water, the amount of lime and soda needed must be calculated based on the alkalinity of the water. All of the titratable bases together make up the alkalinity of water, which is its ability to neutralize acids (APHA 2005). It has also been found that temperature affects the metabolic processes of aquatic organisms (Igwemmar et al. 2013), while the chemical processes will be accelerated in a body of water as its temperature rises. Due to the insolubility of gasses like oxygen, effects like a foul odor and taste will result. The turbidity (cloudiness of water) measures how well light can travel through water (APHA 2005). Electrical conductivity measures the water’s ionic content and is directly related to the total amount of dissolved particles.
In water, solids can be suspended or in solution. Glass fiber filter were to distinguish between two main categories of solids (Tchobanoglous et al. 2003) in the samples. First are dissolved solids, which pass through the filter with the water, and second are suspended solids, where particles and colloids are retained on the filter’s top (APHA 2005). One of the other key indicators of water quality is dissolved oxygen (DO), which serves as a crucial measure of water pollution (APHA 2005). Higher dissolved oxygen “DO” concentrations are related to better water quality. However, only very little oxygen is soluble in water, and this has great sensitivity to temperature (Ogbonnaya 2008; Wasiu et al. 2022). The chemical and heavy metals analysis in this study include: total suspended solid (TSS), total dissolved solids (TDS), total hardness (TH), calcium hardness, magnesium hardness, acidity, alkalinity, nitrate (NO−3), chloride (Cl), sulfate \({(SO}^{3-})\), dissolved oxygen (DO), biochemical oxygen demand (BOD), bicarbonate, carbonate, arsenic (As), cadmium (Cd), copper (Cu), chromium (Cr), iron (Fe), lead (Pb), and zinc (Zn). The gravimetric method was used to estimate the TDS from the resultant TSS, while the TH and calcium hardness were determined using teh Erichrome indicator and further used for Mg hardness calculation (Table 2). The acidity and/or alkalinity, NO−3 and Cl of the water samples were determined through titration. Presence of \({SO}_{4}^{2-}\) was determined using the gravimetric Method. Sulfate is precipitated in hydrochloric acid medium as BaSO4 by the addition of BaCl2 solution. The dissolved oxygen was determined using the iodometric titration method on content of the stored water sample kept in a dark room for 7 days, while the BOD was determined by subtracting the dissolved oxygen (DO) of the seventh day from the initial dissolved oxygen for the first day. The analytical technique used to determine the concentration of metal atoms/ions (As, Cd, Cu, Cr, Fe, Pb, Zn) in the water samples was determined after digestion, using the atomic absorption spectroscopy (AAS), and the results are presented in Table 3.
Result and discussion
Leachate mapping
The ERT sections (Fig. 2a–c) delineate the presence of decomposed/inorganic waste, topsoil with very low resistivity within \(1.8\times {10}^{-3}\) to \(6.4\times {10}^{-1}\) Ωm and fresh dump to lateritic cover with relatively high resistivity. The resistivity model corresponds to conductive material to about 2 m, underlined by a thin layer (about 0.5 m thick) of sandy-clay/weathered basement with a resistivity range within 87.033 Ωm to 222.13 Ωm and fresh basement downward with the highest resistivity contrast. The lowest resistivity signature at the western region on traverse 1 (Fig. 2a) is attributable to the topography as it is at a low elevation to other traverses and, thus, linked with the leachate percolation path. The ERT traverse 2 reveal the conspicuous silty-clay liner (depicted with irregular polygon) beneath the inorganic waste topsoil. The high resistivity contrast (above 100 Ωm) on traverse 3 (5 m away from the dumpsite; Fig. 2c) shows a typical basement complex lithology with top lateritic hardpan overlying the sandy-clay or weathered basement rock and the fresh near-surface rocks. The underlying weathered zones and/or fractured zones are considered insignificant to leachate migration in the area because they do not show surface manifestation and could not serve as conduit for contaminant.
The soil classification test corroborated the resistivity model with a result that shows the topsoil as poorly graded (≤ 50% fines and ≤ 3% gravel) inorganic silt-sand (Fig. 3) using specific gravity “SG” range 2.61 to 2.67 in concordance with the standard soil type for SG classification by Lambe and Whiteman (1969); Rowe et al. (1995); Elsbury et al. (1990); NRA (1995). Pit PAC (along traverse 2) revealed a lower plastic index within 22.5–22.6 (Fig. 4) which depicts the thick inorganic silty sand overburden with medium swelling potential and very low permeability within \(2.85\times {10}^{-6}\) to \(2.91\times {10}^{-6}\), while PBD has relatively high swelling potential due to the high PI and low permeability range \(3.28\times {10}^{-5}\) and \(3.01\times {10}^{-5}\) and (Table 1). The characteristics of the sub-surface soil (at greater than/equal to 2-m depth) are attributable to silty-clay with: maximum dry density (MDD) range from 1.75 to 1.87 g/cm3, optimal moisture content (OMC) range from 11.1 to 14.5% (depicted in Fig. 5), low permeability (low hydraulic conductivity), and acceptable liquid limit (> 20%) for liner materials, following the classification as compared to field need by Benson et al. (1994); Rowe et al. (1995); Daniel (1987); O’Flaherty (1998); Layade and Ogunkoya (2018).
Water quality
Physical parameters
The in situ sensory test revealed the groundwater samples as tasteless, colorless, and odorless, while the hydrogen ion concentration and the acidity/alkalinity of the water indicate a pH level range within 6.85 to 7.10 with a temperature range from 27.2 to 27.8 °C (Fig. 6). The pH is within the neutral point, and the temperature is barely below the standard room temperature, which is the permissible limit for portable water (FEPA 1991; WHO 2004; Ukpong and Okon 2013; Wasiu et al. 2022). The turbidity is higher in the samples from the shallow wells (within the range 2.04–2.56 NTU) than in the relatively low range of 1.20 to 1.38 NTU in the borehole samples. Colloidal particles made of clay and silt at shallow depths in the area within the wells’ depth level might be attributed to the increased turbidity of the near-surface water as compared with that of the deeper source. However, the turbidity is generally relatively low as compared with the estimated range of Sunday and Oyinade (2020) and in concordance with the acceptable range stated by WHO and the NSDWQ. The water’s ionic content (obtained from an electrical conductivity test) ranges from 58.50 to 243.00 µS/cm, with well AJW2 (about 2-m deep and near PAC), having the highest conductivity. The high conductivity is attributable to induced near-surface leachate migration of ionic chemicals into the well water, conspicuous in the electrical resistivity model of traverse 2.
Chemical parameters
The biochemical oxygen demand (BOD) is the quantity of oxygen required in a given volume of water to completely degrade or stabilize all biodegradable organic compounds. The “DO” and “BOD” of the water samples range between 88.00 to 129.60 mg/L and 19.20 to 56.00 mg/L respectively (Table 2). Both parameters are higher in the well water samples and quite above the permissible level, indicating some concentration of organic materials. The acidity and alkalinity levels of the water samples range between 0.20 to 0.48 mg/L and 2.80 to 4.40 mg/L, respectively, while the estimated total hardness ranged between 114.40 and 252.03 mg/L (Fig. 7). The concentration of calcium (Ca2+) and magnesium (Mg2+) ions is higher in the well water samples, characteristics of highly mineralized water which might have percolated primarily through the poorly graded inorganic silt-sand and/or silty-clay (with low permeability). Total suspended solids (TSS) viz; calcium, magnesium, sodium, and manganese chlorides, carbonates, sulfates, and nitrates concentrate range within 5.24 to 18.58 mg/L. The total dissolved solids (TDS) concentrate range within 16.41 to 23.78 mg/L which corresponds to fresh water. The samples generally possess low concentrations of total hardness. TDS and TSS are higher in borehole water samples with low nitrate, sulfate, and phosphate concentration, but within the acceptable range for drinking and all irrigation water usage, and could have no negative effects on the public’s health. In very small or trace amounts, water may contain a wide range of toxic inorganic substances. They may pose a risk to public health even in trace amounts (Rowe et al. 1995). Although many toxic substances come from industrial activities or improper management of hazardous waste, some toxic substances come from natural sources (Tchobanoglous et al. 2003). The heavy metal (Ar, Cd, Cr, Fe, Pb, Zn, and Cu) concentrations in the water samples (Table 3) are currently within the WHO standard permissible limit for potable water. However, the slight increase in Cd concentration (above 0.005) in the deeper wells (AJBs) suggests immediate treatment and prevention of prolonged exposure to the drinking water (Fig. 8).
Representation of the chemical parameters examined in the water samples. a Hand-dug well “AJW” and b borehole water “AJB”; Conc, concentration; T.Ac, total acidity; T.Alk, total alkalinity; T.H, total hardness; DO, dissolved oxygen; BOD, biochemical oxygen demand; TDS, total dissolved solids; TSS, total suspended solids; Cl, chloride; SO4, sulphate; N03, nitrate; P04, phosphate
Conclusion
Integrated 2D electrical resistivity tomography, soil classifications, and physicochemical parameters of water samples have been used to determine the near-surface occurrence of leachate plume within a dumpsite in a Precambrian basement rock area, Malete, Nigeria. High resolution of the subsurface resistivity distribution “ERT” revealed leachate dispersion beneath decomposed/inorganic waste topsoil to about 2 m, hoisting low resistive moisturized soil within range \(1.8\times {10}^{-3}\) to \(6.4\times {10}^{-1}\) Ωm and underlain by a thin layer of about 0.5-m thick sandy-clay and weathered basement downward. The corresponding soil classification results indicate the thick overburden as poorly graded inorganic silt-sand with a specific gravity range within 2.61 to 2.67, medium swelling potential, and a very low permeability range within \(2.85\times {10}^{-6}\) to \(2.91\times {10}^{-6}\). The subsurface soil is characterized as silty-clay with a maximum dry density range from 1.75 to 1.87 g/cm3, optimal moisture content range from 11.1 to 14.5%, low permeability, and acceptable liquid limit (> 20%), which indicate a suitable liner material capable of impeding the flow of leachate to prevent groundwater contamination.
The in situ sensory test on six water samples around the dumpsite revealed acceptable physical characteristics of drinking water which are tasteless, colorless, and odorless, neutral pH level range within 6.85 to 7.10, and at the normal room temperature, a temperature range from 27.2 to 27.8 °C. The increased (but within the acceptable limit) turbidity, conductivity, DO, and BOD within the shallow wells are attributed to induced near-surface leachate migration of ionic chemicals or organic materials which have percolated primarily through the poorly graded inorganic silt-sand (topsoil) but prevented by the impervious silt–clay liner from vertical migration to the deeper water source. Also, the concentrations of As, Cd, Cr, Fe, Pb, Zn, and Cu in the water samples are below the recommended concentration approved by the WHO as the permissible limit for potable water. Therefore, increased TDS and TSS in samples of deeper water sources with low nitrate, sulfate, and phosphate concentration are within the acceptable range of drinking water that could have no negative effects on the public’s health.
References
Abubakar H, Raji W and Boyode S (2014) Direct current resistivity and very low frequency electromagnetic studies for groundwater development in a basement complex area of Nigeria. Sci Focus 19(1):1–10
Adabanija M and Alabi T (2014) An integrated approach to mapping the concentration and pathway of leachate plumes beneath a dump site in south-western Nigeria. Int J Sci Eng Res 5 (1):2229–5518
Adewoyin O, Joshua E, Akinyemi M, Omaje M, Akinwumi S (2019) Predicting dynamic geotechnical parameters in near-surface coastal environment. Cogent Eng 6:1588081
Adewuyi AA, Mallam M (2014) The impact of a waste disposal site on soil and groundwater in Dusten-Kura Gwari, Minna, Niger state, Nigeria. J Appl Physics 6(6):1–5
Aizebeokhai A, Ogungbade O & Oyeyemi K (2017) Integrating VES and 2D ERI for near-surface characterization in a crystalline basement terrain. In SEG Technical Program Expanded Abstracts. Society of Exploration Geophysics 5401–5406
Aizebeokhai A, Oyeyemi K (2017) Geoelectrical characterization of basement aquifers: the case of Iberekodo Southwestern Nigeria. Hydrogeol J 26(2):651–664. https://doi.org/10.1007/s10040-017-1679-9
Akinbiyi AO, Sanuade AO, Akanji A, Isah A, Ugwoke LJ & Olaseeni GO (2020) An integrated geophysical approach for post foundation studies at Edunabon town, southwestern Nigeria. Model Earth Syst Environ https://doi.org/10.1007/s40808-020-01001-7
Alamiokuma G & Chaanda M (2020) Geophysical investigation of structural failures using electrical resistivity tomography:a case study of buildings in FUPRE, Nigeria. J Earth Sci Geotech Eng 10(5):15–33
Allen A, Dillion A & O"Brien M (1997) Approaches to landfill site selection in Ireland. In Engneering Geology and the environment Balkema pp. 1569–1574
APHA (2005) Standard methods for the examination of water and waste water. American Public Health Association, 21st edn. APHA, Washington, DC
Ayolabi E, Oluwatosin L, Ifekwuna C (2015) Integrates geophysical and physicochemical assessment of Olushosun sanitary landfill site, southwest Nigeria. Arab J Geosci 8:4101–4115
Aziz H, Yussff M, Adlan M, Adnan N, Alias S (2004) Physico-chemical removal of iron from semi-aerobic leachate by lime stone filter. Waste Manag 24:353–358. https://doi.org/10.1016/j.wasman.2003.10.006
Aziz S, Aziz H, Yusoff M, Bashir M, Umar, M (2010) Leachatecharacterization in semi-aerobic and anaerobic sanitary landfills:a comparative study. J Environ Manage 91:2608–2614
Benson C, Daniel D, Gordon P (1994) Estimating hydaulic conductivity of clay liners. J Geotech Eng ASCE 120(2):366–387
Bernstone C, Dahlin T (1997) DC resistivity mapping of old landfills: two case studies. Eur J Environ Eng Geophys 2:121–136
Bernstone C, Dahlin T, Ohisson T, Hogland W (2000) DC resistivity mapping of internal landfill structure: two pre-excavation surveys. Environ Geol 39:3–4
Bjerg P, Albrechtsen H, Kjeldsen H, Christensen T, Cozzarelli I (2014) The biogeochemistry of contaminant groundwater plumes arising from waste disposal facilities A2-Holland, Heinrich D. In: Turekian KK (ed) Treatise on Geochemistry, 2nd edn. Elsevier, Oxford, pp 573–605
Boudreat J, Dube J, Chouteau M, Winiarski T, Hardy E (2010) Geophysical characteristic of contaminated urban fills. Eng Geol 116:196–206
Brunner D and Keller D (1972) Sanitary landfill design and operation. Environ Protect Agency 59
BSI B 1377 (1990) Methods of testing for soils for civil engineering purposes. British Standard Institution, London
Burke K, Dewey J (1972) Orogeny in Africa. In: Dessauvagie TF, Whiteman AJ (eds) Africa geology. University of Ibadan Press, Ibadan, pp 583–608
Daniel D (1987) Earthen liners for land disposal facilities. Proceeding paper in geotechnical practice for waste disposal '87. American Society of Civil Engineers New York
De Carlo L, Perri M, Caputo M, Deiana R, Vurro M, Cassiani G (2013) Characterization of a dismissed landfill via electrical resistivity tomography and mise-a`-lamasse method. J Appl Geophys 98:1
Dimitriou E, Karaouzas I, Saratakos I, Zacharias I, Bogdanos K, Diapoulis A (2008) Groundwater risk assessment at a heavily industrialized catchment and the associated impacts on a Peri-Urban wetland. J Environ Manage 88(3):526–538
Elsbury B, Daniel D, Sraders G, Anderson D (1990) Lessons learned fom compacted clay liner. J Geotech Eng 116(11):164–166
Etim SE, Okon OE, Akpan PA, Ukpong GI, Oku EE (2013) Prevalence of cockroaches (Periplanata americana) in households in Calabar: public health implications. J Public Health Epidemiol 5(3):149–152. https://doi.org/10.5897/JPHE12.081. March 2013 Available online at, ISSN 2141-2316 ©2013 Academic Journals
Farzaneh G, Khorasani N, Ghodousi J, Panahi M (2021) Assessment of surface and groundwater resources quality close to municipal solid waste landfill using multiple indicators and multivariate statistical methods. Int J Environ Res 2021(15):383–394. https://doi.org/10.1007/s41742-020-00307-9
Fatta D, Papadopoulos A, Loizidou M (1999) A study on the landfill leachate and its impact on the groundwater quality of the greater area. Environ Geochem Health 21:175–190
FEPA (1991) Guidelines and standards for environmental pollution control in nigeria, federal environmental protection agency regulation 1991 on pollution abatement in industries and facilities generated wastes 78(42):38
Goldfarb RJ, Groves DI, Gardoll S (2001) Orogenic gold and geologic time : a global synthesis. Ore Geol Rev 18:1–72
Hamzah U, Yaacup R, Samsudin A, Ayub M (2006) Electrical imaging of the groundwater aquifer at Banting, Selangor, Malaysia. Environ Geol 49(8):1156–1162
Igboama WN, Hammed OS, Fatoba JO, Aremu IE, Aroyehun MT (2021) Geo-electric and hydro-physiochemical investigations of Osogbo central dumpsite, Osogbo, Southwestern Negeria. Arab J Geosci 14:1361
Igwemmar NC, Kolawole SA, Odunoku SO (2013) Heavy Metal Concentration in Fish Species Sold in Gwagwalada Market, Abuja. Int J Sci Res 2(1):7 – 9
Kamble BS, Saxena PR, Kurakalva RM, Shankar K (2020) Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city. India. SN Appl Sci 2:498. https://doi.org/10.1007/s42452-020-2199-0
Karlik G, Kaya A (2001) Investigation of groundwater contamination using electric and electromagnetic methods at an open waste disposal site: a case study from Isparta. Turkey Environ Geol 40(6):34–42
Kayadelen C (2007) The consolidation characteristics of an unsaturated compacted soil. Environ Geol. 54:325334
Kearey P, Broks M and Hill I (2002) An introduction to geophysical exploration. Blackwell Science Ltd., Oxford
Lambe T, Whitman R (1969) soils mechanics. J.wiley and sons, New York
Lambe WT, Whitman RV (1991). Soils Mechanics. John Wiley & Sons, 15:576
Layade G, Ogumkoya C (2018) Geotechnical properties of laterictic soil as subgrade and base material for road construction in Abeokuta, Southwest Nigeria. Int J Adv Geosci 6(1):78–80
Loke M, Barker R (1996) Rapid least-squares inversion of apparent resistivity pseudosections by a quasi-Newton method. Geophys Prospect 44:131–152
Loke MH (2011) Electrical resistivity surveys and data interpretation. in Gupta, H (ed.), Solid Earth Geophysics Encyclopaedia (2nd Edition) “Electrical & Electromagnetic” Springer-Verlag, pp 276–283
Macdonald A, Bonsor H, Dochartaigh E, Taylor R (2012) Quantitative map of groundwater resource in Africa. Environ Res Lett 7:024009
Maurya P, Ronde V, Fiandaca G, Balbarini N, Auken E, Bjerg P et al (2017) Detailed landfill leachate plume mapping using 2D and 3D electrical resistivity tomography–with correlation to ionic strength measured in screens. J Appl Geophys 138:1–8
Mepaiyeda S, Madik K, Gwavava O, Baiyegunhi C, Sigabi L (2019) Contaminant delineation of a landfill site using electrical resistivity and induced polarization methods in Alice, Eastern Cape, South Africa. Hindawi Int J Geophysics 2019:5057832. https://doi.org/10.1155/2019/5057832
Moreira L, Cesar A (2021) Analysis of leachate generation dynamics in a closed municipal solid waste landfill by means of geophysical data (DC resistivity and self-potential methods). Pure Appl Geophys 178:1355–136. https://doi.org/10.1007/s00024-021-02700-7. The Author(s), under exclusive licence to Springer Nature Switzerland AG
Moreira C, Braga A, Godoy L, Sardinha D (2013) Relationship between age of waste and natural electric potential generation in sanitary landfill. Geofısica Int 52(4):375–383
Morita A, de Souza P, Elis V and Wendland E (2020) Longterm geophysical monitoring of an abandoned dumpsite area in a Guarani Aquifer recharge zone. J Contam Hydrol.https://doi.org/10.1016/j.jconhyd.2020.103623
Naudet V, Gourry J, Girard J, Mathieu F, Saada A (2014) 3D electrical resistivity tomography to locate DNAPL contamination around a housing estate. Near Surf Geophys 12(3):351–360
NRA (1995) Landfill liners. internal Guidance Note, North West West Diposal Officer
NSDQW (2007) Nigerian standard for drinking water quality. Standard Organization of Nigeria, Nigerian Industrial Standard NIS 554, pp: 30
Obaje NG (2009) Geology and mineral resources of Nigeria. Lecture Notes in Earth Sciences 120
O’Flaherty C (1998) Highway engineering, 2nd edn. Edward Amold Publisher, London
Ogbonnaya C (2008) Analysis of groundwater pollution from abattoir waste in Minna. Nigeria. Res J Diary Sci 2(4):74–77 ISSN 1993–5277
Ogilvy R, Meldrum P, Chambers J, Williams G (2002) The use of 3D electrical resistivity tomography to characterize waste and leachate distributions within a closedlandfill, Thriplow, UK. J Environ Eng Geophys 7:11–18
Oladunjoye M, Salami A, Aizebeokhai A, Sanuade OA, Kaka S (2017) Preliminary geotechnical characterization of a site in southwestern methods. J Geol Soc India 89(2):209–215. https://doi.org/10.1007/s12594-017-0585-z
Olasehinde P, Raji W (2007) Geophysical studies on fractures of basement rocks at University of Ilorin, southwestern Nigeria: application to groundwater exploration. Water Resour 17:3–10
Onorm S (1990) Geotechnics in landfill consruction-earthworks. Austrian standards Institute, Vienna
Osinowo O, Olayinka A (2012) Very low frequency electromagnetic (VLF-EM) and electrical resistivity (ER) investigation for groundwater potential evaluation in a complex geological terrain around Ijebu-ode transition zone, southwestern Nigeria. J Geophys Eng 9:374–396
Oyeyemi K, Aizebeokhai A, Attat O (2018) Evaluation of groundwater near municipal solid waste landfill site using ERI technique: a case in southwestern Nigeria. Nat Environ Pollut Technol 9(6):765–772
Oyeyemi K, Aizebeokhia A, Ede A, Rotimi O, Sanuade O, Olofinnade O et al (2019) Investigating the near surface leachate movement in an open dumpsite using surficial ERT method. IOP Conf. Ser: Mater Sci Eng 640(2019):012109. https://doi.org/10.1088/1757-899X/640/1/012109
Park S, Myeong-jong Y, Jung-HoSeung-Wook KK et al (2016) Electrical resistivity imaging (ERI) monitoring for groundwater 1366 L. P. I. Helene et al Pure Appl geophysics. contamination in an uncontrolled landfill, South Korea. Geophys J Appl Geophysics 135:1–7
Porsani L, Filho W, Ellis V, Shimlis J, Moura H (2004) The use of GRR & VES in delineating contamination plume in a landfill site. A case study in SE Brazil. J Appl Geophys 155:199–209
Raji W, Adeyoe T (2017) Geophysical mapping of contaminant leachate around a reclaimed open dumpsite. J King Saud Univ Sci 29:348–3359
Ramalho E, Dill A, Rocha R (2013) Assessment of leachate movement in a sealed landfill using geophysical methods. Environ Earth Sci 68(343–354):1742–1748
Rao G, Rao V, Padalu G, Dhakate R, Sarma V (2014) Application of electrical resistivity tomography methods for delineation of groundwater contamination and potential zones. Arab J Geosci 7:1373–1384
Rowe K (1998) Geosynthetics and the Minimization of Contaminant Migration through Barrier Systems Beneath Solid Waste. 1998 Sixth International Conference on Geosynthetics, 27 – 99
Sasaki Y (1992) Resolution of resistivity tomography inferred from numerical simulation. Geophys Prospect. https://doi.org/10.1111/j.1365-2478.1992.tb00536.x
Sundararajan N, Nandakumar G, Ghary M, Raman K, Srinivas Y (2007) VES and VLF an application to groundwater exploration. Leading Edge, Khammam, pp 708–716
Sunday A, Oyinade F (2020) Bacteriological assessment of selected hand pumped boreholes water sources in Malete environs, Kwara State Nigeria. Nat Appl Sci J 3(June 2020):47–58. https://doi.org/10.38061/idunas.731180
Taonameso S, Mudau LS, Traore AN, Potgieter N (2019) Borehole water: a potential risk to rural communities in South Africa. Water Supply 19(1):128–136
Tchobanoglous G, Soller JA, Olivieri AW, Crook J, Cooper RC, Parkin RT, Spear RC, Eisenberg JNS (2003) Risk-based approach to evaluate the public health benefit of additional wastewater treatment. Environ Sci Technol 37(9):1882–1891. https://doi.org/10.1021/es025774p
Waisu A, Salami BA, Ibrahim M, Al-Osta MA (2022) Engineering and green natural pozzolan-nano silica-based alkali-activated concrete; shrinkage characteristics and life cycle assessment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-23424-8
WHO (2017) Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. https://www.who.int/publications/i/item/9789241549950
WHO World Health Organization (2004) Recommendation. Guidelines for Drinking Water Quality, 3 1. WHO, Geneva, p 515
Xing W, Lu W, Zhao X, Zhang X, Deng W, Christensen T (2013) Environmental impact assessment of leachate recirculation in landfill of municipal solid waste by comparing with evaporation and discharge (EASEWASTE). Waste Manag 33:382–389
Ukpong and Okon (2013) Comparative Analysis of Public and PrivateBorehole Water Supply Sources in Uruan Local Government Area of Akwa Ibom State. International Journal of Applied Science and Technology 3(1):76–91
Zume J, Tarhule A, Cherstone S (2006) Subsurface imaging of an abandoned solid waste landfill site in Norman, Oklahoma. Groundw Monit Remediat 26(2):62–69
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olasunkanmi, N.K., Usman, Z.M. & Jimoh, A.A. Investigation of groundwater quality around municipal waste disposal site in Malete southwestern Nigeria. Arab J Geosci 16, 273 (2023). https://doi.org/10.1007/s12517-023-11359-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11359-4