Abstract
Irrational use of pesticides and poor storage management may result in contamination of the surrounding environment including soil, water sources, and crops. Such contamination may pose hazards to human health and the environment. The objective of the current study is to investigate soil (around main pesticide stores) and seasonal stream sediments (Khor Abuhabel sediments) contamination with insecticide residues from nearby pesticide stores and local farms in South Kordofan State, Sudan. The study targeted residues of organochlorines, organophosphates, and pyrethroid insecticides. Soil samples were taken to a depth of 10 cm. Three soil samples were randomly taken representing each of the locations assessed. Samples were analyzed for pesticide residues using gas chromatography (GC) equipped with an electron capture detector (ECD) and mass spectrometry (GC–MS). The results revealed the presence of higher levels of organophosphate residues than organochlorines and pyrethroids. Heptachlor, malathion, and dimethoate were present in all samples tested, while no detectable levels of p.p-DDT and β endosulfan were found. Endosulfan α and deltamethrin were detected in some samples. The highest concentration detected in the soil corresponds to average dimethoate (up to 13.1 ppm), while the lowest level detected belongs to endosulfan sulfate (0.517 ppm). The total concentrations of all detected pesticides in the soils from the vicinity of pesticide stores are higher (23.4 ppm) than the corresponding level in Khor Abuhabel sediment (11.6 ppm). Possible sources of contamination may include drift from recent applications, dust, rains, and runoff water during autumn, the rainy season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insecticides play an important role in controlling pests of crops and vectors of plants, humans, and animal diseases, particularly in tropical countries. However, their mismanagement may lead to severe problems, such as food, water, and environmental contamination (Burger et al. 2008; Aktar et al. 2009; Damalas, 2011; Mahmoud et al. 2016), thus posing hazards to human health, non-target organisms, and the environment (FAO and Itps 2017). Therefore, monitoring of pesticide contamination is very important to prevent environmental pollution and protect human health and the environment from expected hazards (Damalas, 2011; Mahmoud et al. 2016; Meena et al. 2020). Soil and sediment are the final depots for many environmental contaminants including pesticides (Vryzas 2018). Although most pesticides degrade fairly quickly on crops and in the soil, however, some may persist in one form or another for a longer time. Oudejans (1994) and Saha et al. (2021) mentioned that when pesticides are applied in the field, they can move into the soil, air, or water constituents of the environment. Pesticide residues in the plant also can be released into the soil through litter breakdown (Kreutzweiser et al. 2009). The mobility and persistence of pesticides are controlled by their adsorption into soil particles and that was affected by organic matter, clay, soil minerals, soil pH, and temperature (Vryzas et al. 2007). The interaction between soil organic matter and pesticides can form bound residues in soil and generally decreases the leaching, runoff, uptake, action, and bioavailability of pesticides (Lerch et al. 2009; FAO and ITPS 2017). Most pesticide residues found in the sediment are hydrophobic with low water solubility and therefore accumulate at higher frequency and concentration in the sediment compared to the water (Masia et al. 2013; Okoya et al. 2013).
Sudan was considered among the main pesticide users in the Middle East and Africa. The annual imports reached about 5000 tons were used for crop protection and control of vector-borne diseases. However, this amount decreased to an average of 2000–3000 tons (Abdelbagi et al. 2000; 2003) due to the change in agricultural policy and the significant reduction in the area allotted for cotton production, the main crop which receives the highest rate of pesticide application every year. Cotton is the main cash crop in Sudan and is grown under either irrigated or rain-fed systems. Nuba Mountains Cotton Corporation (NMCC) which is operating within South Kordofan State is one of the most important rain-fed cotton-growing areas in Sudan. Besides cotton, this state is famous for the production of cereals, oilseeds, vegetables, and fruits. During the period 1986–2003, chemical insecticides were heavily used for pest control in NMCC (NMCC 2008) due to the increase in the population density of many agricultural and public health pests.
Khor Abuhabel, a seasonal stream that flows from the Nuba Mountains down to Kordofan sandy dunes, passes through the NMCC area. The main pesticide store of NMCC was located within the vicinity of Khor Abuhabel valley. Therefore, pesticide contaminants may be washed with runoff water from nearby fields of NMCC and/or the main pesticide store and end up in the sediments of this seasonal stream and/or nearby soil. Drift during active spray season could provide another source of contamination. Measurable levels of pesticide residues were detected in the soil, human samples, and water sources in the rain-fed areas (Abdelbagi et al. 2000; 2003; 2015; Nesser et al. 2016; 2020). However, most of the previous studies were directed towards irrigated areas and very meager studies covered the residue distribution in the rain-fed areas and/or water streams.
Therefore, the current study was initiated to investigate the residue levels of some of the commonly used pesticides in soil and sediment samples from Khor Abuhabel and the surrounding areas of the main pesticide store of the NMCC. The absence of studies pertinent to pesticide residues in this area which is famous for the production of many crops including cotton, cereals, fruits, vegetables, forest, and animal products further justifies our goals. Such data can help officials and decision-makers to design suitable mitigation measures to any possible hazards to human health and the environment.
Materials and methods
Study area
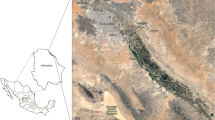
Study areas are located in South Kordofan State. The total area of the State is about 123,700 km2; 7% of it is a mountain and 20% is sand-dunes. The area is characterized by semi-arid conditions and summer rains. The mean maximum temperature ranges between 36 and 40 °C while the minimum temperature is between 17 and 20 °C. Rainfall ranges from 600 to 850 mm per year and starts early in April with 5–10 mm. Half of the area is covered by dark cracking clay soil moderately suited for sorghum, cotton, and sesame as well as grazing plants. Considerable area in the Northern parts of the state where sandy soil is scattered is cultivated with millet (Mohamed 2013). Khor Abuhabel and its associated network of seasons cover most of the area. This stream originates from the Nub Mountains and flows through the cotton production schemes in the area and ends up in Alrahad seasonal lake (locally called Alrahad Fola) in the northern part of Kordofan State. The soil of study is originally formed from the stream sediments (Mohamed 2013).
Chemicals and reagents
Analytical standard (purity 99%) of heptachlor CAS No. 76–44-8, DDT CAS No. 50–29-3, malathion CAS No. 121–75-5, endosulfan CAS No. 115–29-7, dimeton CAS No. 60–51-5, deltamethrin CAS No. [52918–63-5]; [52820–00-5], and dimethoate CAS No. 60–51-5 were provided by the National Chemical Laboratory, Ministry of Health, Khartoum. Sodium chloride and sodium sulfate were supplied by Cculab Scientific Co. Ltd, Khartoum, Sudan.
Sample collection, extraction, and cleanup
The method described by Mohamed et al. (2021) was followed for sample collection while the AOAC-International (1996) official method number 5 was followed for the extraction and cleanup. Seven locations (three along Khor Abuhabel for sediments sampling and four in the vicinity of the main pesticide stores for soil sampling) were selected for sampling (Fig. 1). Three samples were collected per location. Plant debris were removed from the soil surface before taking any soil samples. A soil auger of 17-cm length and 5-cm diameter was used to collect the samples. Ten sub-surface soil (0.0–10 cm) samples were randomly taken from different places in each location and mixed thoroughly to make a composite sample (1 kg each). The size of samples, as well as the sample collection method, was carried out according to the grid pattern method (AOAC 2019) with a total of ten sample proportions. Each sample represents one portion of the total sample. Two soil plugs were taken from each site. The two soil plugs were combined to form the sample portion of that sample (AOAC 2019). The collected samples were kept separately on polyethylene bags, labeled with date and samples code, and sent to the Pesticide Laboratory of the Faculty of Agriculture, University of Khartoum.
Fifty grams of dried soil and sediment of each sample was placed into a glass bottle (capacity 250 ml) and wetted with 15 ml of distilled water, and then 50 ml of re-distilled acetone and 50 ml of n-hexane were added. The bottle was tightly closed and placed firmly in an end-over-end shaker for 3 h. The samples were then left to stand for 5 min and then filtered through a 180-mm filter paper. The filtrate was collected in a round-bottom flask. The round-bottom flask with its content was placed in a rotary evaporator (BüCHIRotavapor R-200) at 40 °C to reduce the filtrate volume to about 80 ml. The content was then transferred to a separatory funnel (capacity 1000 ml) then 600 ml distilled water and 10 ml of sodium chloride solution were added and shaken for 1 min (the cock was opened several times to release pressure). The organic phase was transferred to the 500-ml separatory funnel, while the aqueous phase was re-extracted twice with 30 ml n-hexane. The combined n-hexane extracts were washed twice with 100 ml of distilled water and 5 ml of saturated sodium chloride solution. The organic phase was dried in a rotary evaporator to reduce the volume to 10 ml then filtered through a filter paper containing 10 g of anhydrous sodium sulfate to absorb the moisture. The dried filtrate was then collected in10-ml glass vials tightly closed with a screw-cap, sealed with Teflon tape, and stored in the refrigerator at 4 °C for gas chromatography (GC) analysis.
Recovery test
Spiked samples were used to check for recovery of the method. Sixteen grams of soil was weighted, divided into four groups each containing four grams. Each soil was placed in a glass bottle as previously mentioned, and then treated with analytical standard of candidate insecticides (Mohamed et al. 2021). Fortified samples were subjected to the same extraction and cleanup procedures desorbed previously. The recoveries of the method are greater than 91% as given in Table S1.
Gas chromatography (GC) analysis
All extracts of the soil and sediment were analyzed according to the method described by Mohamed et al. (2021) using a Shimadzu GC device model 2010 Japan, equipped with a Ni63 electron capture detector (ECD) and capillary column DB-5, 30-m length, 0.25-mm i.d. (internal diameter), Part No. 122–5032, and Serial No. us6537762H. The stationary phase was 5% phenylmethyl polysilexane (J & W Scientific). The operating temperatures of the oven, detector, and injection port were 240 °C, 300 °C, and 280 °C, respectively. Nitrogen (99.999% pure) was used as the carrier gas at a flow rate of 3.33 ml/min.
Analytical standard (99% pure) of the candidate pesticides (α and β endosulfan, endosulfan sulfate, malathion, p.p-DDT, heptachlor, deltamethrin, and dimethoate) was injected using a ten-microliter (10-µl) syringe with a needle length of 5 cm (HAMILION BONADUZ SCHWEIZ). Each standard was injected three times or until approximately two consecutive similar peaks at the same retention time were obtained, followed by injection of sample extract (1µ/µl). Each sample was injected three times. The concentration of candidate pesticides was estimated from the peak area and expressed as mg/kg (ppm). The limits of detection (LODs) of p.p-DDT, heptachlor, malathion, α endosulfan, β endosulfan, endosulfan sulfate, dimethoate, and deltamethrin were 0.15, 0.45, 0.33, 0.015, 0.018, 0.307, 0.149, and 0.063 ppm (Table S2).
Gas chromatography with mass spectroscopy (GC–MS)
Three representative samples were reanalyzed according to the method described by Mohamed et al. (2021) using a Shimadzu GC–MS Qp2010 system (Japan) with an AOC-5000 autosampler. The gas chromatography was fitted Rtx5-Ms capillary column 30 m × 0.25 mm id, 0.25-µm film thicknesses from a Restek (UK) analytical column. Helium (purity ≥ 99.999%) was used as a carrier gas at a flow rate of 1.83 ml/min. The splitless injection temperature was 200 °C. The oven temperature was programmed from an initial temperature of 35 °C (held for 3 min) then increased at 5 °C min−1 to 200 °C at which it was held for 14 min. The mass spectrometer was operated with an electron impact (EI) source in the scan mode. The electron energy was 70 eV, and the interface temperature was maintained at 280 °C. The solvent delay was set to 2 min.
Statistical analysis
Collected data were tabulated, statistically analyzed using MINITAB software statistical package. Descriptive statistics was used in terms of means linked with standard deviation, in addition to median and range.
Results
Levels of insecticide residue were investigated in the soil samples in 7 locations from South Kordofan State: Khor Abuhabel sediments and soils around the main pesticide store in the area (Fig. 1).
Pesticides detected in Khor Abuhabel sediment
Analysis of sediment samples of Khor Abuhabel revealed the presence of detectable levels of heptachlor and α endosulfan which were found at an average concentration of 0.505 ppm and 1.467 ppm, and ranges of 0.448–0.611 ppm and 0.070–3.000 ppm, respectively. The result showed the absence of detectable levels of p.p-DDT, β endosulfan, endosulfan sulfate, and deltamethrin (Table 1). The organophosphate, malathion, and dimethoate were found at an average concentration of 0.773 ppm and 8.752 ppm, and ranges of 0.383–1.483 ppm and 8.590–8.970 ppm, respectively. The highest levels detected corresponded to dimethoate, while the lowest concentration detected belongs to deltamethrin (Table 1).
Pesticides detected in soil of main pesticide store
The result in Table 2 showed the presence of detectable levels of heptachlor, endosulfan sulfate, deltamethrin, malathion, and dimethoate. Their respective averages were 1.082 ppm; 0.517 ppm; 0.877 ppm; 7.890 ppm; and 13.110 ppm, while their respective ranges were 0.561–1.988 ppm; ND–0.854 ppm; ND–2.441 ppm; 0.390–1.592 ppm; and 6.790–17.250 ppm. On the other hand, the levels of p.p-DDT, α endosulfan, and β endosulfan were below the detection limit. The highest level detected corresponds to dimethoate, while the lowest level detected belongs to deltamethrin.
Summary of residue levels detected in the two locations
The results showed the presence of detectable levels of heptachlor, malathion, and dimethoate in the analyzed samples of soil and sediment in the 7 locations, while the levels of p.p-DDT and β endosulfan were below the detection limits of the GC-ECD, although they were detected by the GC–MS, but not quantified. The highest levels detected correspond to dimethoate (13.100 ppm), followed by malathion (7.890 ppm) and heptachlor (1.082 ppm) in soils of main pesticide store. The lowest concentration detected belongs to heptachlor (0.505 ppm) in Khor Abuhabel sediment. On the other hand, α endosulfan was found in Khor Abuhabel sediment only, while deltamethrin was found in soils of main pesticide store only. The highest levels detected correspond to dimethoate while the lowest level detected belongs to endosulfan sulfate. Results indicated that the total levels of OPs were higher than those in other groups (Fig. 2).
Discussion
The current study investigated the residue levels of the commonly used organochlorines, organophosphates, and pyrethroid insecticides in and soil sediment samples from seven locations in the traditional rain-fed areas of South Kordofan State. The limited number of comprehensive and systematic studies on the topic in this area claim for such investigation to protect human health, local environment, and improve the quality of food crops grown in the area.
A total of 21 soil and sediment samples were collected from seven locations along the main seasonal river (Khor Abuhabel, 3 samples) and the main pesticide store (4 samples). The result indicated that insecticide residues were detected in all soil samples analyzed. The analysis revealed the presence of heptachlor, malathion, and dimethoate in all samples, with no detectable level of p.p-DDT in all samples. Organophosphate compounds represent the group with the highest level detected, followed by organochlorines and pyrethroids. The highest level detected corresponds to the dimethoate (8.752 ppm) while the lowest level detected corresponds to heptachlor (0.505 ppm). The absence of p.p-DDT may be explained by the possible environmental conversions and this is in line with Mulla (1960) who mentioned that DDT was lost during the first 24 h after addition to soil, Lichtenstein and Schulz (1960) reported that high temperature enhanced the rate of pesticide disappearance from soil by increasing the rate of degradation. Abbadi and Elzorgani (1981) reported that irrespective of a large amount of DDT used in Gezira since 1949, the residue of DDT was 0.26 ppm in cotton and 0.24 ppm in wheat soil.
The analysis of Khor Abuhabel sediment samples showed the presence of OCs and OPs, while the level of pyrethroids was below the detection limit. Total OCs and OPs were 1.972 ppm and 9.525 ppm respectively. This seasonal river originates from the Nub Mountains and flows through the cotton schemes in the area. Therefore, the presence of pesticide residues in the river sediments may be explained by possible leaching from pesticide-polluted soil by runoff water which finally settles and accumulates in the river sediments (Matsumura 1985; Okoya et al. 2013; Masia et al. 2013). The mobility and persistence of pesticides are controlled by their adsorption into soil particles and that was affected by organic matter, clay, soil minerals, soil pH, and temperature (Vryzas et al. 2007). Besides the detected pesticides being hydrophobic, they present a tendency to accumulate in sediment (Masia et al. 2013). The absence of DDT and deltamethrin residues can be explained by their possible conversion by environmental factors (Matsumura 1985; Quantick 1985).
Generally, organophosphates were found at relatively higher levels than OCs. This may be explained by the ongoing use of OPs in the area as claimed by the interviewed agricultural inspectors in the area. Further, organochlorine pesticides are well known as universal contaminants of aquatic environments and are characterized by long environmental persistence, bioaccumulation along the food chain, and in fat bodies of higher animals (Matsumura 1985; Osama and Mohamed 1984; Harris and Mailes 1975; Croll, 1969; Lerch et al. 2009; Saha et al. 2021). Therefore, the presence of their residues in the soil and sediment even after their banning or restriction is expected based on the previous history of their heavy use and long environmental persistence. Heptachlor was the most frequent contaminant detected in soil, water, and blood samples investigated in previous studies from Sudan (Elmahi 1996, Elbashir 1998; Abdelbagi et al. 2000, 2003; Elbashir et al. 2015; Abdelbagi et al. 2015; Nesser et al. 2016; 2020; Mohamed et al. 2021). Endosulfan and its metabolite endosulfan sulfate were detected in the current study. It is the only organochlorine pesticide permitted for use in Sudan where its use constitutes at least 50% of the annual spray regime in cotton until 8 years ago (Abdelbagi 2006; Abdelbagi et al. 2000, 2003). However, endosulfan does not have long environmental persistence as a parent compound, but instead, it forms a persistent metabolite, endosulfan sulfate (Tomlin 2003). Considerable levels of endosulfan residues were detected in the sediments of both wet and dry seasons (Harriet et al. 2012; Okoya et al. 2013). The pyrethroids were detected at low levels and in few samples; these compounds were commonly used in Sudan for pest control in cotton and vegetable as well as in the public health sector. They are known for their short environmental persistence, and therefore the absence or the reduced levels of their residues are expected (Matsumura 1985; Tomlin 2003). Many environmental factors may contribute to the rapid disappearance of pyrethroids in environmental samples (Quantick 1985).
It is worth mentioning that the use of most OCs (heptachlor) in Sudan was banned and/or severely restricted (DDT) since the early 1990s. This may explain the absence of DDT residues in the analyzed samples. On the other hand, heptachlor, although severely restricted in the early 1990s and banned after 2000, was found at a measurable level in many locations. Heptachlor is known for long environmental persistence (Matsumura 1985; Quantick, 1985; Harriet et al. 2012; Vera et al. 2019); therefore, its presence in soil samples is expected.
The analysis of soil of main pesticide store showed the presence of OCs and OPs at total levels of 1.599 ppm and 20.990 ppm, respectively. This finding is in line with Alexander (1972) and Babiker (1998).
The level detected was not high, which may indicate that the degree of pollution with pesticide residues in this area is not so serious.
Conclusion
The rain-fed cotton production area of western Sudan is known for heavy pesticide application. Other food crops are also grown within the crop rotation in the area. The very limited and old studies in this area indicated the presence of measurable levels of pesticide residues in soil, crops, and human blood samples in the area. Therefore, the current study aimed to investigate the residue levels of some commonly used pesticides in sediment samples from Khor Abuhabel (the main seasonal stream in the area) and the soil surrounding the main pesticide stores in the area. Sampling, extraction, and GLC analysis were done following the AOAC method. Detectable levels of heptachlor, malathion, and dimethoate were found, although their levels were not high. The highest level detected corresponds to dimethoate while the lowest level detected belongs to heptachlor. The residue levels in the soil of the pesticides almost double the level in Khor Abuhabel sediment.
The current study recommends regular monitoring of pesticide contaminations in soils, sediments, and crops grown in the area. Further, the authorities should act immediately towards the relocation of the pesticide store to a place far from the catches of seasonal streams to avoid contamination by runoff water or leaching of eroded contaminated soil. Proper management of pesticide stores and safe disposal of obsolete stocks are of equal significance and should be adopted. Adoption of IPM program in cotton and other crops and enforcement of Pesticide and Pest Control Act of 1994 and relevant Environmental Protection laws shall be followed to reduce the environmental contamination and reduce the risk to humans, animals, and the environment.
Data availability
Data is presented in the manuscript and available as:
References
Abbadi KH, Elzorgani GA (1981) Residues of DDT in soil of the Sudan Gezira. Egypt: 1st international congress for soil pollution and protection from pesticides residues, Zagazig University, 22–28 August 1981.
Abdelbagi AO, Elmahi AM, Osman DG (2000) Chlorinated hydrocarbon insecticides residues in the Sudanese soil of limited or pesticide use. Arab J Plant Prot 18:35–39
Abdelbagi AO, Elmahi AM, Osman DG (2003) Organochlorine insecticides residues in Sudanese soil of intensive pesticide use and in surface soil of Qurashi pesticide store. UK J Agric Sci 11:59–68
Abdelbagi AO, Osman DG, Mohamed AA, Omer S, Ali FM, Saad A, Mukhtar N (2006) The manual of pesticide use in the Sudan SSMO(ed) Khartoum University press. Sudan (Arabic Ref).
Abdelbagi AO, Elbashir AB, Hammad AMA, Elzorgani GA, Laing MD (2015) Organochlorine levels inhuman blood from residents in areas of limited pesticide use in Sudan. Toxicol Environ Chem 97(2):266–273. https://doi.org/10.1080/02772248.2015.1031669
Abdelbagi AO (2006) Pesticide use and management in the Sudan. National Workshop on Insecticide Resistance and Its Management, Khartoum: Federal Ministry of Health and WHO, January 29–31, 2006.
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2(1):1–12. https://doi.org/10.2478/v10102-009-0001-7
Alexander M (1972) Environmental toxicology of pesticides. F. Matsumura, G.M. Boush, and T. Misato, editors. Academic Press, New York . pp 637.
Association of Official Analytical Chemists (AOAC). (2019) Official methods of analysis, Agricultural Chemical; Contaminants; Drugs 21th ed. Volume 1, pp 684, Washington, USA.
AOAC-International (1996) Official Method of Analysis of the Association of Official Analytical Chemists. AOAC International, Arlington., USA.
Babiker MY (1998) Level and movement of some pesticides in Qurashi store area, Hasahisa Province. Thesis, Faculty of Agriculture, University of Khartoum, Central Sudan. M. Sc
Burger J, Mol F, Gerowitt B (2008) The ‘necessary extent’ of pesticide use—thoughts about a key term in German pesticide policy. Crop Prot 27:343–351
Croll PT (1969) Organochlorine pesticides in British water. Water Treat Examination 18:255–274
Damalas CA (2011) Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8(5):1402–1419. https://doi.org/10.3390/ijerph8051402
Elbashir AM (1998) Organochlorine insecticides levels in human blood samples collected from residents in areas of limited and intensive insecticides use in the Sudan. Thesis, Faculty of Agriculture, University of Khartoum, M. Sc
Elbashir AB, Abdelbagi AO, Hammad AM, Elzorgani GA, Laing MD (2015) Levels of organochlorine pesticides in the blood of people living in areas of intensive pesticide use in Sudan. Environ Monit Assess 187(3):1–10. https://doi.org/10.1007/s10661-015-4269-0
Elmahi MA (1996) The distribution of some chlorinated hydrocarbon pesticide residues in the Sudanese soils. Thesis, Faculty of Agriculture, University of Khartoum, M. Sc
FAO, ITPS (2017) Global assessment of the impact of plant protection products on soil functions and soil ecosystems FAO, Rome, Italy, pp 30.
Harriet KM, Sampson MA, Naa-DP LM, Sarah BA, Anita OT, Paul F (2012) Determination of organochlorine pesticide residue in sediment and water from the Densu River Basin. Ghana Chemosphere 86(3):286–292. https://doi.org/10.1016/j.chemosphere.2011.10.031
Harris CR, Mailes JR (1975) Pesticide residue in the Great Lake region of Canada. Res Rev 57:27–79
Kreutzweiser DP, Thompson DG, Scarr TA (2009) Imidacloprid in leaves from systemically treated trees may inhibit litter breakdown by nontarget invertebrates. Ecotoxicol Environ Saf 72:1053–1057
Lerch TZ, Dignac MF, Nunan N, Barriuso E, Mariotti A (2009) Ageing processes and soil microbial community effects on the biodegradation of soil 13C–2,4-D nonextractable residues. Environ Pollut 157:2985–2993
Lichtenstein EP, Shulz KR (1960) Epoxidation of aldrin and heptachlor in soil as influenced by autoclaving moisture and soil type. J Econ Entomol 53:192
Mahmoud I, Imadi SR, Gul A, Shazadi K, Hakeem KR (2016) Effects of pesticides on environment. In: Hakeem K., Akhtar M., Abdullah S. (eds) Plant, Soil and Microbes. Springer, Cham. https://doi.org/10.1007/978-3-319-27455-3_13
Masiá A, Campo J, Vázquez-Roig P, Blasco C, Picó Y (2013) Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J Hazard Mater. 263 P: 95–104. https://doi.org/10.1016/j.jhazmat.2013.09.035. Epub 2013 Sep 21. PMID: 24140087.
Matsumura F (1985) Toxicology of insecticides. Plenum Press, New York, p 406
Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, Sharma MP, Yadav GS, Jhariya MK, Jangir CK, Pathan SI, Dokulilova T, Pecina V, Marfo TD (2020) Impact of agrochemicals on soil microbiota and management: a review. Land 9:34. https://doi.org/10.3390/land9020034
Mohamed AO, Abdelbagi AO, Abdalla MA, Ishag AESA, Hammad AMA, Hur J-H (2021) Insecticide residues in cotton, sorghum and fallow soil from the Nuba Mountains Cotton Corporation of South Kordofan State. Sudan J Health Pollut 30(210608):2021
Mohamed OA (2013) Insecticide residues in soil of South Kordofan. Sudan: Ph.D. Dissertation, Faculty of Natural Resources & Environmental Studies, University of Kordofan,
Mulla MS (1960) Loss of chlorinated hydrocarbon insecticides from the soil surface in the field. J Econ Entomol 53:785–788
Nesser GAA, Abdelbagi AO, Hammad AMA, Tagelseed M, Laing MD (2016) Levels of pesticides residues in the White Nile water in the Sudan. Environ Monit Assess 188:374. https://doi.org/10.1007/s10661-016-5367-3
Nesser GAIA, Abdelbagi AO, Ishag AESA, Hammad AMA, Tagelseed M (2020) Seasonal levels of pesticide residues in the main and the Blue Nile waters in Sudan. Arab J Geosci. 13:994. https://doi.org/10.1007/s12517-020-05969-5
Nuba Mountains Cotton Corporation (NMCC) (2008) Annual report, NMCC, Ministry of Agriculture, Sudan
Okoya AA, Ogunfowokan AO, Asubiojo OI, Torto N (2013) Organochlorine pesticide residues in sediments and waters from cocoa producing areas of Ondo State, Southwestern Nigeria. ISRN Soil Science 2013:1–12. https://doi.org/10.1155/2013/131647
Osama AA, Mohamed B (1984) Organochlorine residues in fish from the River Nile. Egypt Bull Environ Contam Toxicol 33:246–252
Oudejans JH (1994) Agro-pesticides: properties and functions in integrated crop protection. United Nations Economic and social commission for Asia and the Pacific. Bangkok, Thailand. pp vii+329.
Quantick HR (1985) Aviation in crop protection population and insect control. London, Collins, pp 428.
Saha A, Ghosh RK, Bhaduri D (2021) Pesticide pollution in soils and sediment in India: status, impact and countermeasures. In: Rakshit A, Singh S, Abhilash P, Biswas A (eds) Soil science: fundamentals to recent advances. Springer, Singapore. https://doi.org/10.1007/978-981-16-0917-6_41
Spencer WF, Cliatn MM (1969) Vapor density of dieldrin. Environ Scien Technol 3:670–674
Tomlin CDS (2003) The pesticide manual, 12th edn. University of London, British Crop Protection Council. U.K.Tropical Medicine
Vera S, Hans GJM, Paul Z, Marc T, Coen JR, Violette G (2019) Pesticide residues in European agricultural soils – a hidden reality unfolded. Sci Total Environ 653:1532–1545. https://doi.org/10.1016/j.scitotenv.2018.10.441
Vryzas Z (2018) Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Current Opinion in Environ Sci Health 4:5–9. https://doi.org/10.1016/j.coesh.2018.03.001
Vryzas Z, Papadopoulou-Mourkidou E, Soulios G, Prodromou K (2007) Kinetics and adsorption of metolachlor and atrazine and the conversion products (deethylatrazine, deisopropylatrazine, hydroxyatrazine) in the soil profile of a river basin. Eur J Soil Sci 58:1186–1199
Acknowledgements
The financial support made available by Ministry of Higher Education and Scientific Research, Sudan, is highly acknowledged.
Funding
The Ministry of Higher Education and Scientific Research, Sudan, supported this research.
Author information
Authors and Affiliations
Contributions
Azhari is the team leader who proposed the work and designed the experiments. Amna, Abd Elaziz, and Ahmed executed the experiments and analyzed the results. Azhari, Amna, Abd Elaziz, and Jang wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
This paper was selected from the 3rd Conference of the Arabian Journal of Geosciences (CAJG), Tunisia 2020
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamed, A.O., Abdelbagi, A.O., Ishag, A.E.S.A. et al. Insecticide residues in Khor Abuhabel sediments and soil of South Kordofan State, Sudan. Arab J Geosci 15, 499 (2022). https://doi.org/10.1007/s12517-022-09769-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-09769-x