Abstract
El-Mex Bay and Nubaria are two huge coastal embayments located west of Alexandria, Egypt. The bay is a popular fishing spot as well as a recreational location. It is one of the most extensively polluted locations on the Egyptian Mediterranean coast, receiving a massive amount of agricultural, industrial, and sewage waste from the nearby Lake Mariut via the El-Umoum and Nubaria Drains. The current study evaluated the geographical variations of plankton and water quality in the western side of Alexandria, namely Nubaria and El-Mex sites, using multivariate statistical analysis for eight sites seasonally during 2018. The samples were examined extensively for species identification and abundance measurement. The water is very alkaline and well-oxygenated in the summer. The results showed that the annual average of phytoplankton in Naubaria site was 577 ± 496 × 103 cells l−1, while the annual average of zooplankton was 54.1 ± 50.0 × 103 ind m−3. A total of 122 and 91 phytoplankton species were quantified from El-Mex and Naubaria sites, respectively. However, 96 and 98 zooplankton species were identified in El-Mex and Naubaria site, respectively. The most differentiating factor between two sites and between various locations were salinity. The multiple correlations, redundancy analysis (RDA), regression analysis, and canonical correspondence analysis (CCA) were performed, and both indicated that the water temperature, salinity, and SiO4 were the main limiting factors for the abundance of most plankton communities. Also, some plankton groups acted as stimulants or inhibitors for the flourishing of other groups. The correlations between both phytoplankton and zooplankton in terms of abundance, temperature, and salinity were dismantled due to the evolution of plenty of euryhaline and eurythermal species adapting to these hard conditions. Also, water quality index (WQI) values indicate that the water of studied locations was heavily polluted. So, periodical monitoring for these areas, treatment of wastes before being discharged in marine water, and using new technologies for tracking pollutants are highly recommended. Monitoring programmes for phytoplankton and zooplankton ecology as a bioindicator for pollution were highly recommended in the study. In addition, this study suggests the usefulness of multivariate statistical methods in the analysis of water quality and recommendations for environmental recovery and restoration are proposed for preservation of El-Mex Bay and Naubaria sites in order to facilitate development of environmental and tourist activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is essential for survival in both amount and quality. The majority of available water sources (untreated surface water or groundwater) have been contaminated with harmful faecal matter organisms as a result of anthropogenic activity (Hussain and Al-Fatlawi 2020). Water quality is a whole of physical, chemical, and biological properties of the water (EEA 1999). The variation in the physical and chemical processes that occur is reflected in the dynamics of the biological populations, particularly planktonic community (Marques et al. 2007; Ćosić-Flajsig et al. 2020), where the interaction between various environmental variables can either favour the growth or mortality of plankton, both spatially and seasonally (Abdul et al. 2016). Also, their abundance can be altered by spatio-temporal variations in hydrochemical parameters and physical forces in aquatic ecosystems (Bianchi et al. 2003).The rapid response of plankton to the environmental changes made it a vital indicator for assessing eutrophication, pollution, global warming, and environmental problems, in cases of long-term changes, so planktons are utilized to screen the health of the natural ecosystem in the environment (Trishala et al. 2016; Kerich 2020). Diversity and abundance of plankton was well correlated with the abiotic factors (temperature, pH, TDS, DO, BOD, nitrate, phosphate, hardness, alkalinity) of the aquatic environment. Pollution affected the occurrence of some species whereas, other species were found to be tolerant to the extreme conditions of the abiotic parameters in case of polluted bodies and, thus, working as potential biological indicators in water quality monitoring studies (Jakhar 2013). For example, the presence of some zooplankton indicated that there was a high level of anthropogenic activities in and around the water body (Abdul et al. 2016). Dirican et al. (2009) reported the prevalence of rotifera species such as Brachionus and Keratella as indicators of eutrophic condition in aquatic systems. So also, Padmanabha and Belaghi (2008) confirmed that the occurrence of species like Filinia longiseta, Brachionus forficula, and Brachionus angularis, and high level of some ostracods and cladoceras species such as Bosmina, Moina, and Macrothrix indicate high level organic pollution as a result of high organic matter deposit.
In Mallipattinam, southeast coast of India, Varadharajan and Soundarapandian (2013) reported that high diversity index was linked to the presence of the sea grass and mangrove in the environment. However, such high diversity might indicate larger food chain, inter-specific interaction, and stability among the estuarine zooplankton community. Yusuf (2020) reported observations in Nasarawa reservoir, Katsina State Nigeria, and the results show four phytoplankton classes, Bacillariophyta, Chlorophyta, Cyanophyta, and Desmidiaceae, have positive close relation with dissolved oxygen, pH, transparency, and total dissolved solids. The coastal marine area west of Alexandria is a dynamic ecotype which is influenced by receiving over feeding of nutrients as well as other harmful substances due to sewage and industrial drainage which affects the ecosystem and biodiversity. The wide range of salinity allowed fresh, brackish, and marine organisms to co-exist and they adapted together. Wastewater was channeled away from many factories to be discharged in El-Mex site. Furthermore, Lake Mariout that comes top of the list of the most polluted lakes in Egypt provides El-Mex with its contaminated water through Umum Drain (Heneash et al. 2021). Being a crucial fishery zone, El-Mex sustained wanton destruction of marine life. It is worth to mention that the volume of discharged water from Umum Drain increased three times from 1982 to 1995 (Abdel Aziz 2002). Accordingly, Zakaria et al. (2019) considered El-Mex Bay as an estuarine zone. Naubaria site receives its water mainly from agricultural lands that are fertilized by huge amounts of phosphorus and nitrogenous fertilizers with random methods through Naubaria Drain, in addition to various human activities which undoubtedly have a strong direct impact on their water quality, degradation, and instability of ecosystem (El-Naggar et al. 2017; Mona et al. 2019). These appalling conditions spawned the biodiversity and counts of phytoplankton and zooplankton to come to light. Little attention has been cast on the ecological and biological characteristics of Naubaria Drain after being connected to the Mediterranean Sea in 1986. The first work was done by Gharib and Dorgham (2003) about the phytoplankton, and they concluded that phytoplankton density was low due to the wide variation of water salinity. Numerous works were done in El-Mex site; for example, physical–chemical parameters were estimated by Dorgham et al. (2004) and Shreadah et al. (2014). Indeed, many investigations have discussed the influences of changes in salinity variations on the density and distribution of both phytoplankton and zooplankton in El-Mex Bay and Umum Drain such as Soliman (2006), Zakaria et al. (2007), and Hussein and Gharib. There is no doubt that the narrow changes in the environmental conditions usually caused the ecosystem stability. With the change of environmental factors, critical changes will be happening in the bio-composition of any water body. It is well known that a species range may be determined seasonally, spatially, or geographically by one or more of a number of limiting factors such as competition with other species, the abundance of suitable food, temperature, salinity, sheltered condition, or other hydrographic factors (Aboul Ezz et al. 2014). Zakaria et al. (2019) stated that the zooplankton populations have the potential to respond to and reflect event-scale and seasonal changes in environmental conditions. Zooplankton may serve as sentinel taxa that reflect changes in marine ecosystems by providing early indications of a biological response to climate variability (Mansour et al. 2020). Multivariate statistical techniques, for example redundancy analysis (RDA) and canonical correlation analysis (CCA), have been presented the assessment of water quality and determine spatiotemporal variations and possible sources of pollutants in water (Techniques 2020). In addition, several of the difficulties that are expected to remain in the future, such as increasing temperatures and rising sea levels, are expected to exacerbate the effects of climate change. As a result of rapid population growth and industrial development, untreated or poorly treated industrial waste, domestic sewage, industrial waste, and agricultural runoff have moved to and via Mariut Lake south of the city, and eventually been released into the sea. These areas have also received a significant amount of agricultural runoff through canals and drains, providing a new concern for Egypt’s water supply. Anthropogenic influences are more pronounced in this zone, as they are in other coastal zones along the Alexandria coast. These areas have also received a huge amount of agricultural runoff via canals and drains, posing a new challenge for Egypt’s water supplies. This paper aims to indicate the distribution variability of two plankton groups along two highly dynamic sites in the western part of Alexandria coast. This is with reference to the water quality status during different seasons and their impact on the planktonic presence and abundance. The aims of the present study were as follows: (1) to evaluate the water quality using some physicochemical variables, (2) to determine the species diversity of the zooplankton community, (3) to study the impact of water quality on the planktonic presence and abundance, (4) to investigate the relationships between zooplankton species and environmental factors, and (5) to assess the relations between plankton communities and ambient conditions using multivariate statistical techniques.
Materials and methods
Sampling site description
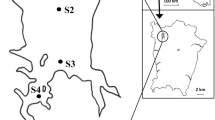
The study area has two sites: (I) Naubaria site that is located at the confluence of Naubaria Drain with Mediterranean Sea at 31° 09′ N and 29° 71′ E; the source of drainage water in this site is from Naubaria drain with 1.5–2-m depth and 20-m width. (II) El-Mex site is located at the discharging point of Umum Drain into El-Mex Bay in Mediterranean Sea and situated at 31° 10′ N and 29° 50′ E; the source of water is from El-Umum Drain with 3-km width and 10- depth. Representative eight samples were collected seasonally in year 2018 from both locations (three stations from El-Naubaria and five stations from El-Mex) as illustrated in Table 1 and Fig. 1. Mediterranean climate is found between the 30° and 45° latitudes. The Mediterranean Sea centrists the northern coastal line’s temperatures, making summers moderately hot and humid and winters moderately wet and mild, although sleet and hail common in and around the wettest cities, such as Alexandria. Mediterranean climate gets its name from the climate found around the Mediterranean Sea. Plants in Mediterranean climate must be able to survive long dry summers. Lakes in the Mediterranean climate zone experience high variation in rainfall and are vulnerable to changes in climate, land cover, and anthropogenically induced effects on water level and salinity.
Physicochemical parameters
Water temperatures were measured using a thermometer sensitive to 0.1 °C, pH values were gauged by a pocket pH meter (model 201/digital pH meter), water salinities were measured by a Beckman salinometer (Model NO.R.S.10), and dissolved oxygen concentrations were measured using Winkler methods. Dissolved inorganic nitrogen (DIN; nitrate, nitrite, ammonia), soluble reactive phosphorus (SRP), and reactive silicate (RS) were analysed according to standard methods outlined in APHA (1995).
Water quality index (WQI)
Water quality index (WQI) is a single number which can be calculated easily and used for overall description of the quality of water bodies. It was defined as a rating reflecting the composite influence of various water quality factors on the overall quality of water. Water quality classification is based on WQI value (Pesce and Wunderlin 2000): as when WQI value is > 300, the water quality is considered heavily polluted and unsuitable, 200–300 reflects very poor, and 100–200 is considered poor, while from 50 to 100 is good and < 50 is considered excellent. WQI was calculated by using the following equation (Horton 1965; Brown et al. 1970);
where n is the sum of factors and SI is the sub-index for all chemical factor which designed as:
where Wi is the relative weightiness of the chemical factor and Qi is the quality rating for all factor. Both are calculated as follows:
where Ci is the concentration of each chemical factor in all water sample in mg/L, and Si is the seawater standard for all chemical parameter in mg/L (Heneash et al. 2021).
Phyto- and zooplankton collection and identification
Phytoplankton samples were collected from different stations by the Niskin water sampler. The samples were fixed with 4% formalin, then poured into 1 dm3 cylinder container, left about 24 h for settlement, then siphoned out carefully to 100 ml to state counting and identification. Phytoplankton was determined by a counting chamber according to Utermöhl’s method (Utermöhl 1958). The phytoplankton species were identified according to Huber Pestalozzi (1938), Khunnah (1967), and Starmach (1983). Zooplankton samples were collected by filtering of 50 l of water through a 55 μm mesh standard plankton net. The collected samples were preserved directly in 4% formalin solution. Each sample was concentrated to 100 ml and a subsample of 5 ml was transferred into counting cell and each plankter was identified using a research binocular microscope and expressed as ind m−3. The identification of recorded zooplankton was done according to Edmondson (1959), Dussart (1969), and Biek (1972). Diversity (H′) stands for both phytoplankton and zooplankton that have occurred. They were rated by Shannon, Wiener, and Spearman’s yardsticks in terms of their visionary and granted correlations (Shannon and Wiener 1963).
Shannon index (H)
The most widely used index of heterogeneity was calculated by the following formula:
where N is the total number of individuals of all species and n is the number of individuals of a species.
The evenness index (E) was calculated according to the following formula (Pielou 1966):
where H is the Shannon index and S is the number of species.
Species richness (D) was calculated according to the following formula:
where S is the total number of species and N is the total number of individual in the sample.
Similarity index
The percentage of similarity between different sites was calculated by the following equation:
where S is the number of species common in both sites (A and B) and U is the number of species found in A and absent in B, and V is the number of species found in B and absent in A.
Multivariate statistical analysis
The Statistical Package Program (SPSS 8.0), Spearman Rank correlation analysis, the multiple correlation, and regression analysis were used for calculating the relation between different parameters. According to large amount of data redundancy and linearity which increase the amount of data calculation, therefore this present study used to select some multivariate indices (Hong and Abd El-Hamid 2020). Canonical correlation analysis (CCA) and redundancy analysis (RDA) were used to reproduce and explain the relationship among response variables besides explanatory variables by possible and indirect explanatory variables. Canonical correspondence analysis (CCA) was done using several computer software including MINITAB Release 18 and PAST PAleontological STatistics Ver. 3.25. RDA was done by the software Canoco 5.0 (Houston, TX, USA).
Results
Physical and chemical conditions at Naubaria and El-Mex sites
The average concentrations of different physiochemical parameters in the studied locations are illustrated in Table 2. Water temperature ranged between 19.56 ± 0.06 °C and 26 ± 0.38 °C in Naubaria for winter and summer seasons, respectively. Three different stations are characterized by the increase of salinity values; the ranges fluctuated between high (36.21–39.81‰) in St. 1 and slightly high (24.27–32.50‰ and 12.91–17.70‰) in St. 4 and St. 8, respectively, while the other stations have salinities ranged from 1.72 to 8.60‰. Values of pH are above its normal standard, as the maximum value is 8.68 at St. 1. The two sites showed poor aeration; the dissolved oxygen concentrations were low and fluctuated from 2.0 in autumn and 12.9 mg/l in summer. Dissolved inorganic nitrogen (DIN) value was usually higher in El-Mex than Naubaria and it had the ranges of nitrate (37.38–1189.5 µg/l), nitrite (12.51–392.58 µg/l), ammonia (6.0–2180 µg/l), phosphate (4.59–181.62 µg/l), and silicate (86–7006 µg/l). High values are usually recorded at stations near the impact of Naubaria and Umum Drains.
Composition and seasonal dynamics of phytoplankton community
In the two sites, the phytoplankton community was represented by 152 species. Out of them, 31 species were restricted to Nubaraia, while 62 species were confined to El-Mex, whereas 60 species were familiar in both sites. Few of them were perennial, while most of them were seasonal. The top 10 abundant taxa at stations of two sites and seasonal variations are illustrated in Table 3 and Figs. 2 and 3.
The dominant phytoplankton species at the Naubaria and El-Mex sites: (A) Chaetoceros constrictus. (B) Coscinodiscus centralis. (C) Coscinodiscus granulate. (D and E) Cyclotella meneghiniana. (F) Surirella striatula. (G) Skeletonema costatum. (H) Leptocylindrus danicus. (I) Synedra ulna. (J) Scenedesmus quadricauda. (K) Scenedesmus dimorphus. (L) Pediastrum simplex. (M and N) Pediastrum duplex. (O) Pleurosigma decorum. (P and Q) Euglena acus. (R) Prorocentrum cordatum. (S) Prorocentrum micans. (T) Scrippsiella trochoidea
Naubaria site
A total of 91 phytoplankton species were identified at Naubaria site. The most diverse group was diatoms (27 genera, 52 species); also it is the first group quantitatively (84.53%). Dinophyceae came second in importance with remarkably low numbers (6 genera, 17 species). Freshwater Chlorophyceae, euglenophyceae, cyanophyceae, and Charophyta contributed with thirteen, five, four, and one species, respectively. The most diverse station was St. 3 with 56 species; this station is strongly affected by freshwater and urban sewage. St. 1 had 45 species and it was unique because it harboured 24 species not recorded in the other stations, mostly marine and brackish species such as Asterionellopsis glacialis, Chaetoceros constrictus, Leptocylindrus minimus, Pseudonitzschia seriata, and Aulacoseira granulata. Cyclotella meneghiniana was the only species recorded at the three stations during the four seasons. High numbers of species were recorded in autumn (42), followed by 37, and 34 species were recorded in winter and spring, while summer (27) harboured the lowest number of species.
The lowest and highest Shannon diversity indices were 0.940 (St. 3, summer) and 2.620 (St. 3, winter), respectively. The lowest diversity of phytoplankton was observed in summer (1.091 ± 0.225) whereas highest values were recorded in winter (2.100 ± 0.604). Changes in diversity indices were obvious among stations and seasonal scales and phytoplankton diversity may be a more sensitive indicator of ecosystem response to regional variation and less extent phytoplankton abundance. This appeared from the insignificant correlation between the density of phytoplankton and species number (r = 0.403, p = 0.194). The correlation between phytoplankton density and diversity recorded no significant value (r = − 0.293, p = 0.355). An annual average of phytoplankton in Naubaria site was 577 ± 496 × 103 cells l−1. The highest values were observed in the autumn and the lowest was recorded in winter (Fig. 2). During winter, the mean phytoplankton count was 174 ± 109 × 103 cells l−1. Dinophyceae constituted 40.26% of the total counts; St. 2 harboured the main bulk (67.2%) with the dominance of Prorocentrum cordatum. Bacillariophyceae formed 38.34% of the total; Cyclotella meneghiniana and Skeletonema cf. costatum were the most abundant diatoms. During spring, the phytoplankton counts increased to 386.6 ± 306.8 × 103 cells l−1. The percentage of bacillariophyceae showed little increase to 51.44%, Chlorophyceae (23.85%), and Dinophyceae (21.55%). The diatom Lauderia annulata (32.47%) was the most abundant species, while the dinoflagellate Prorocentrum cordatum (14.08%) was the most frequent species. During summer, the phytoplankton mean was 622.2 ± 174.0 × 103 cells l−1. Bacillariophyceae increased to reach 95.3% of the total counts. St. 2 and St. 3 harboured the high diatom percentage with the most dominant of Cyclotella meneghiniana (38.57%), Nitzschia palea (21.43%), and Aulacoseira granulata (20.71%). Autumn had the highest phytoplankton densities (1126.7 ± 680.5 × 103 cellsl−1). Bacillariophyceae had the highest percentage (96.94%), while dinoflagellates dropped to 0.39%. Skeletonema cf. costatum was the leader forming 39.45% of the total abundance, followed by Melosira varians (17.26%). Freshwater chlorophyceae, euglenophyceae, and cyanophycea species achieved the lowest numbers and vanished from most stations.
El-Mex site
A total of 122 phytoplankton species were quantified from El-Mex site. Bacillariophyceae were the most diverse group (56 species) and the first quantitatively group (89.27%), followed by Dinoflagellates with a remarkably lowest number (22 species). Freshwater euglenophyceae, chlorophyceae, and cyanophyceae contributed with 20, 18, and 5 species, respectively. Charophyta was represented by one species. The most diversified stations were St. 8 and St. 4 (60 and 58 species, respectively) with 23 and ten species restricted to each, respectively. Bacillariophyceae were highly frequent in this site (> 85%) including Cyclotella meneghiniana (53.8%) and Skeletonema cf. costatum (19.4% of the total density). High numbers of species were recorded in winter (60), followed by spring (43) and summer (31), while autumn harboured the lowest number (35 species). The phytoplankton diversity index (H′) ranged between 0.612 and 2.786. The mean values were 2.146 ± 0.442 (winter), 1.081 ± 0.320 (spring), 1.307 ± 0.631 (summer), and 1.626 ± 0.755 (autumn). The correlation between phytoplankton counts and diversity was clearly negative (r = − 0.687, p < 0.05). The annual average of phytoplankton was 1230.1 ± 1199.5 × 103 cells l−1. The lowest phytoplankton count was observed in winter (306 ± 188 × 103cells l−1) while the highest was registered in spring (2112 ± 1748 × 103 cells l−1). Also, spatial fluctuation varied in abundance and in dominant species. During winter, Bacillariophyceae was the most dominant group (61.22%). Cyclotella meneghiniana formed the main bulk of the phytoplankton at St. 5, St. 6, and St. 7, whereas Skeletonema cf. costatum was dominant at St. 4 and Scenedesmus dimorphus was frequently noted at St. 6. Dinoflagellates were predominant (45.5%) at St. 4; also genus Euglena has considerable density during winter. During spring, Bacillariophytceae was the dominant group at all stations except for St. 4, where the monospecific bloom of euglenophycean Euglena acus was recorded. Cyclotella meneghiniana was the most dominant species at St. 5, St. 6, St. 7, and St. 8. During summer, the annual average of phytoplankton was 949 ± 486 × 103 cells l−1. Bacillariophyceae dominated the phytoplankton at all stations with a bloom dominated by Cyclotella meneghiniana. Chlorophyceae reached to maximum flourishing in summer and dominated by Scenedesmus quadricauda. Cyclotella striata appeared in considerable numbers at St. 5, Licmophora abbreviate and Nitzschia palea at St. 8, and Prorocentrum cordatum at St. 4. Throughout autumn, Bacillariophyceae prevailed at all stations (67.11–96.8%) and reached their highest percentage at St. 4 (91.7%) and St. 8 (96.8%). There was an increase in Euglenophyta counts at St. 6 and St. 7.
Composition and seasonal dynamics of zooplankton community
Table 4 and Figs. 3, 4, and 5 show the top 10 abundant taxa and the seasonal variations of zooplankton in the two investigated sites. In general, there are great differences in density and species diversity in zooplankton community among stations. One hundred four zooplankton species were recorded, 12 out of them specified to Naubaria site, 10 species to El-Mex site, and 86 species were common to both sites.
The dominant zooplankton species at the Naubaria and El-Mex sites; (A) Oithona nana, (B) Euterpina acutifrons, (C) Acanthocyclops vernalis, (D) Acanthocyclops americanus, (E) Halicyclops magniceps, (F) Acartia clausi, (G) Diaphanosoma excisum, (H) Free living nematodes, (I) Cytheridea papillosa, (J) Difflugia oblonga, (K) Lamellibranchia Veliger, (L) Centropyxis aculeata, (M) Favella ehrenbergii, (N) Brachionus plicatilis, (O) Brachionus urceolaris, (P) Brachionus quadridentatus
Naubaria site
In total, 98 holoplankton species were identified and other larval immature stages. Copepods were the main diverse group represented by 33 species belonging to 23 genera and four orders (Cyclopoida (15), Calanoida (8), Harpacticoida (7), and Poecilostomatoida (one species)). The adult copepods constituted 70.46% of the total counts. Cyclopoida (38.78% of the total copepods) was more abundant than the other orders. Protozoans formed 21 species (6 tintinnids, 8 foraminiferans, four Radiozoa, and 3 Amoebozoa). Rotifers constituted 14 species, Ostracoda 9 species, and Cladocera 5 species. Polychaeta and Mollusca were represented by three species for each. Cnidaria and Chordata were likewise with 2 species for each, while Amphipoda, Insecta, Cirripedia, and Nematoda were represented by only one species for each. A high diversity (79 species) was recorded at St. 3, followed by 67 species at St. 1 and 63 species at St. 2. Only 13 species out of 98 were registered as perennially appearing during the four seasons. The annual average of zooplankton abundance was 41.6 ± 39.6 × 103 ind m−3. Copepoda was the predominant component made up 41.5% of the total zooplankton. Nauplii and copepodites are the most vulnerable stages of the copepod life cycle; they made up 29.54 of the total copepods. The most dominant copepod species were Acanthocyclops americanus (7.78%), Acartia clausi (6.97%), and Oithona nana (6.13%) of the total copepods. Protozoa was the second group, forming 17.79% of the total zooplankton count and mostly represented by Amoebozoa, forming 40.05% of the total protozoans. Zooplankton diversity index (H′) ranged between 2.715 and 3.608. The mean values were 3.321 ± 0.254 (winter), 2.740 ± 0.035 (spring), 2.999 ± 0.191 (summer), and 2.910 ± 0.110 (autumn). The correlation between zooplankton counts and diversity was strongly positive (r = 0.728, p < 0.05). In winter, the zooplankton crop was largest than other seasons (82.1 ± 67.4 × 103 ind m−3). Copepods contributed 44.3% to the total zooplankton dominated by Acartia clausi (10.32%) and Acanthocyclops americanus (9.4% to the total copepods). Protozoans came the second making up 20.24% of the total zooplankton count. They were shown by Favella spp., mainly at St. 1, while Rotifera showed the lowest counts (6.32%). In spring, the average was 30.7 ± 17.6 × 103 ind m−3. It was the most productive season for copepods (52.6% of the total zooplankton) with an increase in their larval stages (55.4%). The most adult species were Halicyclops magniceps. During summer, the average of zooplankton was 24.0 ± 6.8 × 103 ind m−3. Rotifera was the leading element forming 32.0% of the total count and restricted to St. 2 and St. 3. Brachionus plicatilis, Brachionus urceolaris, and Filinia longiseta were the most dominant. Protozoans ranked second (27.27%) and represented by 13 species (four non-tintinnid ciliates, five tintinnids, and four foraminiferans) with a dominance of Favella ehrenbergii (50.0% of the total Protozoa). Copepods accounted for 20.2% of the total count. Oncychocamptus sp. and Euterpina acutifrons made up 11.02% for each of the total copepods. During autumn, the zooplankton community (29.3 ± 20.1 × 103 ind m−3) was dominated by copepods (40%) and rotifers (30%). The leading species was Brachionus plicatilis (18.3%), Brachionus urceolaris (8.9%), and Acanthocyclops spp. (4.5%).
El-Mex site
El-Mex site harboured 96 zooplankton species including larval stages of different groups. Copepods constituted 33 species and their developmental stages. The adult copepods constituted 77.93% of the total copepods; out of them Cyclopoida formed 39.23% of the total copepods. Protozoans formed 18 species (6 tintinnids species and 6 foraminiferans, 3 Radiozoans and 3 Amoebozoans). Rotifers constituted 16 species, Ostracoda 10 species, and Cladocera 5 species, Polychaeta 4 species, Mollusca 3 species, Chordata 2 species, and one species of each of other groups. The highest richness (60 species) was recorded at St. 6 and St. 8, followed by St. 5 (54 species), St. 7 (45), and St. 4 (44). It is notably clear that rotifers and protozoans disappeared from St. 4 during winter and rotifers vanished from St. 8 during summer. The annual average of zooplankton was 54.1 ± 50.0 × 103 ind m−3. Copepoda was by far the most dominant group making up 44.29% of the total zooplankton. Nauplii and copepodites formed collectively 22.07% of the total copepods. The most dominant species were Oithona nana (6.43%), Euterpina acutifrons (5.49%), and Acanthocyclops longuidoides (5.45% of the total copepods). Rotifera reaching 25.71% of the total zooplankton, with relatively high numbers in St. 8. Brachionus spp. formed 49.7% of the total Rotifera. Protozoans forming 9.86% of the total zooplankton densities were mostly shown by Amoebozoa (63.6% of the total protozoans). Centropyxis aculeate, Tintinnopsis spp., and Schmidingerella spp. showed high frequency at St. 8. Zooplankton diversity index (H′) was ranged between 1.748 and 3.578. The mean values were 3.101 ± 0.478 (winter), 2.108 ± 0.295 (spring), 2.621 ± 0.376 (summer), and 2.589 ± 0.187 (autumn). The correlation between zooplankton counts and diversity was not significant (r = 0.384, p = 0.094). In winter, the zooplankton crop reached an average of 78.8 ± 62.5 × 103 ind m−3. Copepods constituted 54% of the total zooplankton count and dominant with Acartia clausi and Oithona nana. Rotifera (19.5%) was dominated by Brachionus plicatilis and Asplanchna sp. Protozoa made up 10.7% and was mainly represented by Amoebozoa (71.2% of the total protozoa). Centropyxis aculeate and Difflugia sp. were highly dominant protozoans. Ostracoda (8.5%) was dominated by Cypridina mediterranea. During spring, the zooplankton community was denser than other seasons (108.9 ± 61.1 × 103 ind m−3). It was the most productive season for rotifers, where they were representing 63.5% of the total zooplankton and dominated by Brachionus calyciflorus (65.3% of the total rotifers). St. 6 and St. 7 showed high rotifers density. Copepods (22.6% of the total zooplankton count) were represented by 22 species. Oithona nana made up 10.4% of the total copepods, while copepod nauplii and copepodites were constituted as the most numerous metazoans (60% of the total copepods). Cladocera contributed 5.14% to the total community. In summer, the average zooplankton count was 32.1 ± 24.8 × 103 ind m−3. Rotifers were the most dominant and counted more than 57%. They were represented by 15 species. Brachionus plicatilis, Asplanchna sp., and Elosa sp. were the most dominant species. Rotifers were disappeared in St. 8. Copepoda (10.6%) was dominated by Acartia clause and Calocalanus pavo. Protozoa contributed 5.5% and represented by nine species (five tintinnids, two Radiozoa, and 1 for each of foraminifera and Amoebozoa) and was dominated by Favella ehrenbergii (33.2% of the total Protozoa). During autumn, the zooplankton community (26.2 ± 17.4 × 103 ind m−3) was dominated by copepods (25.6%), rotifers (24.7%), and protozoans (10.4%). Nauplii and copepodites formed collectively 55% of the total copepods and the dominance of oithonid Oithona nana (15.5% of the total copepods), as well as the rotifer Brachionus plicatilis (38.3%) and B. urceolaris (23.5% of the total rotifers) and the protozoans Centropyxis aculeata (47.1% of the total protozoans).
The relationship between phytoplankton community and physio-chemical characteristics
Naubaria site
Spearman correlation analysis showed that the significant correlations between phytoplankton counts and the environmental parameters were not in progress, but with phytoplankton groups; euglenophyceae were negatively correlated with temperature (r = − 0.628, p < 0.05). Chlorophyceae were positively correlated with phosphate and silicate (r = 0.661; r = 0.582, respectively, at p < 0.05). Cyanophyceae were positively correlated with phosphate (r = 0.619, p < 0.05). Also, dinoflagellates and euglenophyceae were positively correlated with silicate (r = 0.640 and r = 0.737 at p < 0.05). None of the other correlations between phytoplankton groups and environmental variables was statistically significant (p > 0.05). Despite salinity values showing high variations in Naubaria site (4.62–39.81 ppt), the correlations between salinity values and all the bio physicochemical variables proved no significant correlations.
El-Mex site
Spearman correlation between environmental parameters and phytoplankton groups had a strong positive common denominator between dinoflagellates and salinity (r = 0.758, p < 0.001) and a negative one with phosphate (r = − 0.473, p < 0.05). Euglenophyceae was negatively correlated with nitrite and ammonia (r = − 0.520 and r = − 0.543, respectively at p < 0.05) and positively correlated with pH (r = 0.682, p < 0.05).
The relationship between zooplankton community and physio-chemical characteristics
Naubaria site
The most abiotic parameters that best correlated with zooplankton groups were salinity and temperature. Salinity was positively correlated with Ostracoda and Foraminifera (r = 0.640 and r = 0.813, respectively at p < 0.05) and negatively correlated with Rotifera (r = − 0.811, p < 0.05). On the other hand, copepods showed a negative correlation with temperature (r = − 0.681, p < 0.05).
El-Mex site
The relationships between zooplankton and abiotic parameters showed that phosphate and silicate were the only parameters that correlated with total zooplankton counts (r = 0.588 and r = 0.543, respectively at p < 0.05). Copepoda was positively correlated with phosphate (r = 0.662) and silicate (r = 0.603) and negatively correlated with temperature (r = − 0.522). Rotifera was positively correlated with ammonia (r = 0.492, p < 0.001). Protozoa showed a strong positive correlation with phosphate (r = 0.726, p < 0.001) and silicate (r = 0.529, p < 0.05).
Multiple correlation and regression analysis
The correlation between physical and chemical parameters
The multiple correlation analysis between physical and chemical parameters revealed that temperature was the most parameter that correlated with others, where it was moderately positively dependent on dissolved oxygen and NO3 (r = 0.424 and 0.665, respectively), while it was negatively correlated with NH4 and SiO4 (r = − 0.460 and − 0.674, respectively). Whereas the pH was strongly positively affected by PO4 (r = 0.961), the dissolved oxygen was positively correlated with NO3 (r = 0.621) and electrical conductivity was positively correlated with NO2 (r = 0.881).
The correlation between plankton communities
The relationship between plankton communities explained that the phytoplankton abundance was positively correlated with the main dominant phytoplankton groups: Bacillariophycea and Chlorophyceae (r = 0.995 and 0.588, respectively). Also, Chlorophyceae was positively affected by Bacillariophycea (r = 0.528) and Charophyceae (r = 0.610). Accordingly, Cyanophyoceae had positive relation with Dinophyceae (r = 0.461).
Concerning zooplankton groups, the rotifers were the main common groups and rely on the cladocerans, and chordates (r = 0.822 and 0.394, respectively), while copepods were positively correlated with protozoans, ostracods, and decapods (r = 0.561, 0.373, and 0.353, respectively). Including copepods, planktonic protozoans were positively correlated with ostracods, Amphipods, nematodes, and molluscs (r = 0.594, 0.719, 0.581, and 0.799). Ostracods and nematodes positively relied on molluscs (r = 0.377 and 0.524). As a result of the correlations between zooplankton and phytoplankton communities, some zooplankton groups were depending on their counterpart of phytoplankton. Accordingly, copepod abundance was positively correlated with the abundance of Chlorophyceae and Charophyta (r = 0.585 and 0.390, respectively). On the other side, ostracods were positively relying on each of Bacillariophycea and Charophyta (r = 0.639 and 0.255, respectively). Ultimately, the total zooplankton density was not correlated with the total phytoplankton density.
The correlation between plankton communities and physicochemical parameters
The correlations between planktonic communities and physicochemical parameters showed that the total phytoplankton density was negatively relying only on temperature (r = − 0.422). In this context, the rotifers were negatively correlated with salinity (r = − 0.372), while copepods were affected by temperature and SiO4 (r = − 0.576 and 0.354, respectively). The dissolved oxygen and electrical conductivity do not have any impacts on all plankton communities, whereas pH and PO4 have the same effects on nematodes and polychaetes. Chlorophyceae was negatively correlated with the temperature and salinity (r = − 0.425 and − 0.411, respectively) and positively with SiO4 (r = 0.559). Also, NO3 has a positive effect on Euglenophyceae (r = 0.469), while the salinity and NH4 have positive effects on molluscs (r = 0.432 and 0.414, respectively) (Fig. 6). The multiple regression analysis between plankton communities and dominant groups with physicochemical parameters is exhibited in the following prediction regression equations:
By excluding the non-influential parameters that were not significantly correlated with plankton communities, the stepwise regression equations show the previous equations of prediction regression having some errors as follows;
Canonical correspondence analysis (CCA)
The confirmation on the relationship between environmental parameters and plankton communities was done by CCA. The analysis showed three different correlation categories on the two analysis axes of variation with a proportion of variance of 66.61% on axis 1 and 17.18% on axis 2 that indicates the most effective and influential variables (Fig. 7). The first category was that the relationship between measured physicochemical parameters revealed the close positive correlation between PO4 and pH on axis 1 without any relation between the two parameters with the temperature and salinity. While the temperature was in a positive relation with NO3 on axis 1, it was in negative relation with SiO4 and NH4 on axis 2. In this context, there is a close positive correlation between NO2 and EC and another between NO3 and SiO4 on axis 1. The second category was the relationship between plankton communities which explain that the gathering occurred between Copepoda and Protozoa in strong positive relationships that affected positively total zooplankton abundance. On the other side, the phytoplankton of Bacillariophycea and Chlorophyceae formed moderate positive gatherings that affected phytoplankton abundance. The relationship between phytoplankton and zooplankton has only appeared in the positive correlation between Chlorophyceae with the gathering of Copepoda and Protozoa. The last correlation category was the relationship between physicochemical parameters and plankton communities which showed the negative influence of water temperature on copepods, protozoans, and Chlorophyceae on axis 1. So, the water temperature was the main limiting factor for the abundance of copepods, protozoans, and Chlorophyceae. On the other hand, NH4 had positive impacts on copepods and protozoans. In other context, rotifers were mainly affected negatively by water salinity on axis 2. Bacillariophycea was not affected by any measured physicochemical parameters on both axes.
Redundancy analysis (RDA)
The result graph of RDA shows the explanatory variables and water quality in the same coordinate plane. The length of the arrowhead of the explanatory variable indicates the influence degree of the explanatory variable on the water quality, and a longer arrowhead shows a larger effect. The angle among the explanatory variable arrow and the water quality indicator arrow shows the correlation between them. When the angle is acute, they are positively correlated; when it is equal to 90 degrees, there is no correlation; when it is an obtuse angle, it is largely excluded in this study. RDA 1 had strong positive loadings on Rotifera, Copepods, Bacillariophyceae, and phytoplankton and Chlorophyceae beside Temp, DO, EC, NO3, NO2, and SiO4 in addition a negative loading on protozoans, Salinity, pH, DO, NH4+, PO4. A positive loading on bacillariophyceae, Temp, DO, pH, NO3 was observed, and in addition a negative loading on protozoans, phytoplankton, rotifera, copepods, chlorophyceae, salinity, EC, NO2, PO4, SiO4, DO, and NH4+. Positive and negative correlations in RDA 3–7 are represented in Table 5. Furthermore, Fig. 8 displays the relation between RDA 1 and RDA 2; the observed data showed that this model had low levels, as the results were spaced out and dispersed in the distribution of plankton in the winter season, while it was suitable for the distribution of organisms in the rest of the seasons. Also, the changes in relation to the organisms with the seasons were observed; from these changes, season has a great effect on organisms’ community as shown in Fig. 9.
Water quality index (WQI)
The data of WQI and water kind based on six factors are pH, NO2, NO3, PO4, NH3, and DO. The values of average water quality index of all sampling sites fluctuated from 440.12 to 969.37 (heavily polluted) as revealed in Table 6. The lowest value of aquatic WQI showed at site (1) through spring season which was poor water quality, while the maximum data was displayed at site (6) during summer season.
Discussion
Increased human activity and discharged sewage containing high levels of nutritional salts as well as other toxic compounds had a significant impact on the biotic factors of several sites along the Mediterranean Sea coast (Mona et al. 2019; Farrag et al. 2019; Shaban et al. 2020). Naubaria and El-Mex sites are just mere examples of these coastal sites. The temperature of seawater is very critical to all biological processes (Heneash 2015). Temperature and salinity are the main factors in controlling species distribution and abundance (Zakaria and El-Naggar 2019). High salinity oscillations were observed in the different stations of the two sites. St. 1 and St. 4 were much affected by Mediterranean Sea water. It ranged between 36.2 and 39.8 ppt for St. 1 and between 24.3 and 32.5 ppt for St. 4. There was a noticeable increase of salinity at St. 8, which is located inside Umum Drain (range: 12.91–17.7 ppt). Such increases were resulting from overuse of seawater in the cooling process of petroleum companies at the lifting water Mex plant. The salinity of other stations ranged between 1.72 and 8.6 ppt. Salinity decreased in El-Mex from 37 ppt (Nessim and Tadros 1986) to 35.3 ppt (Dorgham et al. 2004) and reached to 11.24 ppt in the current study, indicating the strong effect of Umum Drain water over the years. Tavsanoglu et al. (2015) showed that the changes in salinity will have a substantial impact on aquatic ecosystems, where it can result in osmotic stress on cells, uptake or loss of ions, and effects on the cellular ionic ratio (Redden and Rukminasari 2008). Dissolved oxygen is considered one of the maximum important factors controlling the biota in the aquatic habitat. El-Mex site remains poorly oxygenated (average: 3.5 mg l−1); these values are below the threshold level of good oxygenation (< 4 mg l−1) that was mentioned by Huet (1973), while Naubaria site still had a better status for life to survive (average: 5.5 mg l−1). The low oxygen in El-Mex site may be attributed to the utilization of dissolved oxygen in the decomposition of organic matter processes (Dorgham et al. 2004). The decrease in oxygen supply in the water showed a negative effect on the aquatic life. The highest value occurs during winter season in which temperature decrease as it related to the vegetation production, which liberates remarkable amounts of oxygen into the water through photosynthesis (Alprol et al. 2021). A recent study during 2012–2013 showed that the Western Harbor (east of El-Mex Bay) was well-oxygenated all year (Heneash et al. 2015). Relatively high oxygen was observed during summer and autumn in Naubaria site which could be an indication of high phytoplankton counts. Increasing levels of nutrient within water bodies can cause eutrophication and an associated rapid growth and reproduction of plankton; nutrient concentrations were registered as a sign of eutrophication and they are 1.2–2.0 μM for NH4, 0.5–4.0 μM for NO3, and > 0.2–0.3 μM for PO4. According to these values, the two sites could be classified as hypereutrophic sites. Nutrient concentrations in El-Mex site were twofold of the values recorded in Naubaria site because the discharged waters of Umum Drain are more effective than the one of the Naubaria Drain (Dorgham et al. 2004). The average annual concentration of nitrate in Naubaria site was 223.5 μM; this value was twenty times the value recorded by Shams El-Din and Abel Halim (2008) in the same area. The increase in nitrate values is attributed to the discharge of sewage wastes, and increase in mineralization of organic matter (Heneash et al. 2021). DIN values (average: 1386 μM) have increased dramatically compared with the records by Nessim and Tadros (1986) and Dorgham et al. (2004) who registered 4.06 and 5.73 μM, respectively. The high values recorded in the two sites may be attributed to the rising effect of discharged wastes. Ammonia constituted the main bulk of DIN (NO2: NO3: NH4 is equal 1:3.3:7.8) in El-Mex and is equal to 1:4:10 in Naubaria. The high concentrations of ammonia may be due to rich organic matter which may be resulting from fishing and wastewater discharged through Umum Drain or maybe also a result from zooplankton excretion (Shreadah et al. 2014) has appeared from the positive relation with zooplankton numbers; all these factors created a pollution status. The ratios between nitrogen and phosphorus in the two sites fluctuated between 6.8 and 259. Chiaudani and Vighi (1978) indicated that the ratio of more than six means phosphorus is a limiting factor. As a result of the abnormal nutrient values, neither phosphorus nor nitrogen can be considered as a limiting factor in the two sites. Nitrogen and phosphorous are the nutrients most likely to remain deficient in the contaminated environment. SRP in the two sites was increased dramatically reaching an average of 28.15 and 93.86 μM, respectively. A high concentration might have resulted from the regeneration of the adsorbed phosphate from the bottom sediment and subsequent release in the water column by turbulence. Phosphorus was much lower in other works (1.18 μM by Dorgham et al. (2004); 0.84 μM by Nessim and Tadros (1986), and 0.46 μM by Zaghloul (1996).
Nutrient concentrations in Umum Drain registered high levels up to 28, 346, 42, and 22 µM for phosphate, silicate, ammonia, and nitrites, respectively. Phosphate had no correlation with salinity in Naubaria, possibly because the input was not from one source, but had a negative correlation in El-Mex which means that the discharging fresh water may be coming from a definite source. Heneash et al. (2015) showed that low concentrations of silicate in the south-eastern Mediterranean Sea, Egypt, during spring season were accompanied by dense bloom of euglenophyceae and not of bacillariophyceae; also, in summer season, silicate reached maximum levels in parallel with low bacillariophyceae counts. The total recorded taxa in the two sites were 152 for phytoplankton and 104 for zooplankton. Many of the recorded phytoplankton species are considered neritic or pelagic. Across the three last decades, a general decreasing trend in phytoplankton and zooplankton diversity was observed while an increasing trend in densities was registered in the two sites. Phytoplankton richness in Naubaria was reduced from 131 species (Gharib and Dorgham 2003) to 91 species in the present study, and the average counts increased from 7253 cells l−1 (Gharib and Dorgham 2003) to 577 × 103 cells l−1 (present study); these increases attributed to high availability of dissolved inorganic nitrogen (N) and phosphorus (P) by providing additional resources to support phytoplankton growth, and may also alter ecosystem structure (Burford, et al. 2014). For zooplankton, the richness decreased from 120 species (Abdel Aziz 2005) to 95 species (present study) and the average density increased from 31.3 × 103 ind m−3 (Abdel Aziz 2005) to 54.1 × 103 ind m−3 (present study). For El-Mex site, the phytoplankton diversity was decreased gradually from 162 species (Gharib 1998) to 152 species (Hussein and Gharib 2012) and 122 species in the present study, and the density increased from 940 × 103 cells l−1 (Gharib 1998) to 1230 × 103 cells l−1 (present study). Heneash et al. (2015) recorded 157 phytoplankton species in the Western Harbor (east of El-Mex Bay) while Khairy and Gharib (2017) recorded 141 species in El Dekhaila Harbor (west of El-Mex Bay). On the other hand, the zooplankton richness in El-Mex Bay decreased from 121 species (Hussein 1997) to 80 species (Soliman 2006) to reach 95 species in the current study, and the average density was 196 × 103 ind m−3 (Soliman 2006) decreased to 8918 ind m−3 (Abou Zaid et al. 2014) and reached to 54 × 103 ind m−3 in the present study. Heneash et al. (2015) recorded 106 species in the Western Harbor (east of El-Mex Bay) while Heneash (2015) recorded 75 species in El-Dekhaila Harbor (west of El-Mex Bay). The emergence of Chlorophyceae from dominant groups is abnormal recorded in the Mediterranean Sea water, which is accustomed to record Bacillariophyceae and Dinophyceae as dominant groups. Bacillariophyceae were the most dominant phytoplankton in the two sites (> 85%) with 52 and 56 species recorded in Naubaria and El-Mex, respectively. The most dominant species were Cyclotella meneghiniana and Skeletonema cf. costatum. The genus Cyclotella is an indicator of pollution with organic matter (Palmer 1969) and can acclimatize to exist to a wide range of salinities (2.53–39.81 ppt) with the maximum occurrence at 3.9 ppt (Table 7). Skeletonema cf. costatum flourished at temperature 22.5–23.2 °C, and salinity between 6.1 and 39.81 ppt and showed maximum persistence at 38.6 ppt (Table 7). The two species are euryhaline and eurythermal (Huang et al. 2004) and spread along the Egyptian Mediterranean waters (Ismael and Dorgham 2003) and so can be considered estuarine species. Many genera of marine origin prevailed in the phytoplankton at St. 1 and St. 4 even if in low numbers as Asterionellopsis, Chaetoceros, Coscinodiscus, Leptocylindrus, Licmophora, and Pseudo-nitzschia from bacillariophyceae and Protoperidinium and Prorocentrum from dinoflagellates. Marine phytoplankton and zooplankton species that adapted to high salinity cannot coexist in freshwater and freshwater species that survive in low salinity cannot tolerate seawater. But some marine or freshwater species can tolerate salinity fluctuations. In the present investigation, many species showed high salinity tolerance and can exist in an environment that is thoroughly different from its original one; this was clear from the absence of a homogenous community between stations in the same site. These species can be called estuarine species: from phytoplankton, Cyclotella meneghiniana, Lauderia annulata, Aulacoseira granulate, and Skeletonema cf. costatum (Table 7). The high persistence of the neritic, euryhaline, and eurythermal Skeletonema cf. costatum may be an indication of freshwater discharge and tolerance of a high degree of pollution (Gharib 2006) and can be regarded as a sigh of eutrophication (Mihnea 1985). Cyanophyceae has not been enumerated in the two sites because their growth is inhibited in salinities over 2 ppt (Moisander et al. 2002). The present results showed that zooplankton species, Brachionus plicatilis, Favella ehrenbergii, Centropyxis aculeata, Acartia clausi, and Oithona nana, can tolerate salinity gradient and exist in hard conditions (Table 7). Such changes in salinity levels control some species growth. Oithona nana is one of the most ubiquitous copepods that occurred along the coastal ecosystem (Zakaria et al. 2018a), while Acartia clausi is among dominant copepods on the Mediterranean coast (Zakaria et al. 2016). Rotifers species are considered the most sensitive bio-indicator to water quality (El-Naggar 2015). It was noticeable that no rotifers registered in St. 1 as its salinity ranged between 36.2 and 39.8 ppt, but rarely recorded at St. 4 (24.3–32.5 ppt) suggesting that rotifers were not delivered via marine water and are very sensitive to high water salinity (El-Damhougy et al. 2019). In the current study, the freshwater, euryhaline genera Brachionus can tolerate a wide range of salinity (Paturej and Gutkowska 2015; El-Naggar 2015) reaching 24 ppt (Table 7). A total of 33 copepods species as well as Nauplius larvae and Copepodite stages were identified in the two sites. Regardless of Nauplius larvae, the top three highly abundance copepod species in the two sites were the cyclopoids Acanthocyclops americanus, Oithona nana, and the calanoid Acartia clausi. Previously, they represented by only 22 species in El-Mex Bay (Soliman 2006) and 50 species by Abou Zaid et al. (2014) with the same dominant species. The copepod richness in El-Mex site is high compared to nineteen species recorded by Heneash (2015) in El Dekhaila Harbor and thirteen species by Abdel Aziz (2002) in the Western Harbor. The present study is unanimously accepted, copepods were higher than the protozoan density in nutrient-rich estuarine sites and an opposite relation was noticed at the open water, and the numbers of marine protozoans were significantly decreased with salinity gradient; besides it did not show any response to the high nutrients. Protozoa were represented by 21 species with averages of 7323 ind m−3 in Naubaria and 5336 ind m−3 in El-Mex site. Zakaria et al. (2018b) identified 116 protozoan species in the southeastern Mediterranean with an average of 45.3 ind m−3. Many studies have revealed that phytoplankton population and nutrient concentrations are proportional. However, in many studies, increased fertilizer inputs have not resulted in a direct link between phytoplankton abundance and nutrient levels. The impact of increased nutrient availability on phytoplankton community structure remains unknown. But many investigations have proven a negative effect on phytoplankton diversity by increasing nutrient input (Wang et al. 2006), while species richness is increasing with nutrient availability because in line with the species–energy theory (SET) in addition to expect the appearance of blooming harmful algae and the water condition gets worse (Nhu et al. 2019). The following observation was achieved through the present results where there was no significant correlation between phytoplankton and DIN in the two sites. So, phytoplankton in stressed coastal sites are controlled by many factors; some are environmental parameters and others are incomprehensible.
Temperature and salinity are the most environmental factors determining the structure of both phytoplankton and zooplankton assemblages (El-Naggar et al. 2019); this may be achieved in a steadily stable system, but with the highly dynamic one, multiple factors may be responsible for the abundance and the community structure of the plankton rather than environmental parameters. This observation is resulting from the absent or weak correlation between plankton abundances and temperature or salinity and also stemming from the presence of many euryhaline species in the present study that can be adapted to salinity gradients but their optimal growth exists at a certain salinity or salinity range (Table 7). Some groups were controlled by salinity variations, for instance, copepods, Radiozoa, and Amoebozoa favouring lower salinity and dinoflagellates favouring high salinity, while the temperature affected the abundance of copepods and euglenophyceae. The relationship analysis between plankton groups and nutrients explained that the responses of these parameters were different in the two sites. In Naubaria site, a positive correlation was observed between phosphate and each of chlorophytes and cyanophytes. Silicate showed a positive correlation with green, euglenophycean and dinoflagellates. For El-Mex site, total zooplankton counts were positively correlated with phosphate and silicate. Some groups were controlled by nutrient variations; for instance, copepods favouring higher phosphate and silicate; dinoflagellates flourish well in low phosphate, but Protozoa preferred water with high phosphate. Rotifers were positively correlated with ammonia in addition ammonia and nitrite had a negative correlation with euglenophyceae. Lifelong monitoring studies are necessary to dissect the relationship between phytoplankton, zooplankton, and abiotic parameters. CCA and RDA results displayed that industrial wastewater discharge and agricultural fertilizer consumption were the chief pollution sources for nutrients, while population density, wastewater discharge, and urbanization were related to organic pollution. Our results mostly aligned with Abdul et al. (2016) who reported that, according to CCA, the species variation patterns were significantly related to the environmental heterogeneity patterns. The quality of water in even sampling sites moves towards very poor and heavily contaminated environments. This may be owing to municipal pollutants which are pumped directly into the sampling sites by a pipeline from sewage, Dekhaila harbour and western harbour, beside industrial drains, leading to the seawater around the sampling sites becoming unsuitable for maritime life as a result of continuous pollution (Alprol et al. 2021).
Conclusion
Due to the discharge of untreated domestic, industrial, and agricultural effluents, as well as the effect of ships movements from and to the harbours, El-Mex Bay and the Naubaria drain are under stress. As a result, the water in this bay is eutrophic and very different from that in the open sea. Similarly, when compared to other coastal zones around the Alexandria coast, anthropogenic effects were minimal. Furthermore, during the study period, no severe nutrient concentrations were found, and no harmful algal blooms were reported. In this study, water qualities of sampling sites move towards very poor and heavily polluted due to the two sites being affected by different sources of wastewaters. In addition, many phytoplankton and zooplankton species have demonstrated high salt tolerance and may thrive in environments other than their native habitat, earning them the title of estuarine species. Differences in nutrient contents are linked to discharged wastewaters and agricultural waste, both of which may be thrown indirectly or directly into sampling sites as a result of environmental stress and yet have an impact on public health. There was a considerable positive association between zooplankton numbers and diversity (r = 0.728, p 0.05). Ninety-six zooplankton species were found at the El-Mex location, including larval stages of various groups. There were 33 species of copepod; in addition, the connection between zooplankton numbers and diversity (r = 0.384, p = 0.094) was not significant. Also, the multivariate indices show the source of pollution which CCA and RDA confirm that industrial wastewater discharge and agricultural fertilizer consumption were the chief pollution sources for nutrients. Finally, it can be concluded that multivariate analysis should be provided for more details about correlation among water quality and plankton species. The zooplankton crop was larger in winter than in other seasons. For this, long-term studies are important to provide a good insight into plankton ecology in these areas. So, some recommendations include continuous monitoring studies necessary to explain the relationship between phytoplankton, zooplankton, and abiotic parameters. In addition, water quality will continue deteriorating due to salt water intrusion; soil transport is still in progress. Also, water treatment plants and sewage systems are necessary for protection and upgrading of health conditions.
Data availability
The datasets generated during and/or analysed through the current study are available from all authors.
References
Abdel Aziz NE (2002) Impact of water circlulation and discharge wastes on zooplankton dynamics in the Western Harbour of Alexandria, Egypt. Egypt J Aquat Biol Fish 6(1):1–21. https://doi.org/10.21608/ejabf.2002.1725
Abdel Aziz NE (2005) Short term variations of zooplankton community in the West Naubaria Canal, Alexandria, Egypt. Egypt J Aquat Res 31(1):119–133
Abdul WO, Adekoya EO, Ademolu KO, Omoniyi IT, Odulate DO, Akindokun TE, Olajide AE (2016) The effects of environmental parameters on zooplankton assemblages in tropical coastal estuary, South-west, Nigeria. Egypt J Aquat Res 42(3):281–287. https://doi.org/10.1016/j.ejar.2016.05.005
Aboul Ezz SM, Heneash AM, Gharib SM (2014) Variability of spatial and temporal distribution of zooplankton communities at Matrouh beaches, south-eastern Mediterranean Sea, Egypt. Egypt J Aquat Res 40(3):283–290. https://doi.org/10.1016/j.ejar.2014.10.002
Abou Zaid MM, El Raey M, Aboul Ezz SM, Abdel Aziz NE, Abo-Taleb HA (2014) Diversity of Copepoda in a stressed eutrophic bay (El-Mex Bay), Alexandria, Egypt. Egypt J Aquat Res 40:143–162. https://doi.org/10.1016/j.ejar.2014.05.001
Alprol AE, Heneash AM, Soliman AM, Ashour M, Alsanie WF, Gaber A, Mansour AT (2021) Assessment of water quality, eutrophication, and zooplankton community in Lake Burullus, Egypt. Divers 13:268. https://doi.org/10.3390/d13060268
APHA (American Public Health Association) (1995). Standard Methods, 19th ed. Washington, D.C. 1015 pp
Bianchi F, Acri F, Aubry FB, Berton A, Boldrin A, Camatti E, Comaschi A (2003) Can plankton communities be considered as bio-indicators of water quality in the Lagoon of Venice? Mar Pollut Bull 46(8):964–971. https://doi.org/10.1016/s0025-326x(03)00111-5
Biek H (1972) Ciliated Protozoa. World Health Organization, Genoa, p 198
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) Water quality index-do we dare? Water Sew Works 117(10):339–343
Burford MA, Davis TW, Orr PT, Sinha R, Willis A, Neilan BA (2014) Nutrient-related changes in the toxicity of field blooms of the Cyanobacterium, Cylindrospermopsis Raciborskii. FEMS Microbiol Ecol 89(1):135–148. https://doi.org/10.1111/1574-6941.12341
Ćosić-Flajsig G, Vučković I, Karleuša B (2020) An innovative holistic approach to an e-flow assessment model. Civ Eng J 6:2188–2202. https://doi.org/10.28991/cej-2020-03091611
Chiaudani G, Vighi M (1978) Metodologia standard di saggio algare per 10 studio della contaminazione della acque marine. Quaderni Instit Ricersa Sulle Acque 39:120
Dirican S, Haldun M, Suleyman C (2009) Some physicochemical characteristics and Rotifers of Camligoze Dam lake, Susehri, Sivas, Turkey. J Anim Vet Adv 8(4):715–719. https://medwelljournals.com/abstract/?doi=javaa.2009.715.719
Dorgham MM, Abdel-Aziz NE, El-Deeb KZ, Okbah MA (2004) Eutrophication problems in the Western Harbour of Alexandria, Egypt. Oceanologia 46:25–44
Dussart B (1969) Les Copépodes des eauxcontinentales. Tome II, Cyclopoïdeset Biologie quantitative. BoubéeetCie Edit. Paris, 292 p
Edmondson WT (1959) Fresh water Biology 2nd Edition, Johon Wiley and Sons, New York 20: 1248pp
EEA (European Environmental Agency) (1999) Nutrients in European Ecosystems. Environmental Assessment Report No. 4. Copenhagen: European Environmental Agency, 155 p
El-Damhougy KA, El-Naggar HA, Aly-Eldeen MA, Abdella MH (2019) Zooplankton groups in Lake Timsah, Suez Canal, Egypt. Egypt J Aquat Biol Fish 23(2):303–316. https://doi.org/10.21608/EJABF.2019.31461
El-Naggar HA (2015) The rotifers as a bioindicators for water pollution in the Nile Delta (Planktonic Rotifers distribution in the aquatic habitats of the Nile Delta, Egypt). A book, LAP LAMBERT Academic Publishing GmbH & Co. KG, ISBN 978-3-659-76103-4, 131 pp
El-Naggar HA, El-Gayar EE, Mohamed ENE, Mona MH (2017) Intertidal Macro-benthos diversity and their relation with tourism activities at Blue Hole Diving Site, Dahab, South Sinai, Egypt. SYLWAN 161(11):227–251. https://doi.org/10.13140/RG.2.2.17950.84804.
El-Naggar HA, Khalaf Allah HMM, Masood MF, Shaban WM, Bashar MAE (2019) Food and feeding habits of some Nile River fish and their relationship to the availability of natural food resources, Egypt. J Aquat Res 45(3):273–280. https://doi.org/10.1016/j.ejar.2019.08.004
Farrag MMS, El-Naggar HA, Abou-Mahmoud MMA, Alabssawy AN, Ahmed HO, Abo-Taleb HA, Kostas K (2019) Marine biodiversity patterns off Alexandria area, southeastern Mediterranean Sea, Egypt. Environ Monit Assess 191:367. https://doi.org/10.1007/s10661-019-7471-7
Gharib SM (1998) Phytoplan community structure in Mex Bay, Alexandria, Egypt. Egypt J Aquat Biol Fish 2(3):31–194. https://doi.org/10.21608/EJABF.1998.1632
Gharib SM (2006) Effect of freshwater flow on the succession and abundance of phytoplankton in Rosetta Estuary, Egypt. Int J Oceans Oceanogr 1(1):19
Gharib SM, Dorgham MM (2003) Weekly variations of phytoplankton community in the west Naubaria Canal. J Egypt Acad Soc Environ Dev 4(3):201–218
Heneash AMM (2015) Zooplankton composition and distribution in a stressed environment (El Dekhaila Harbour), South-Eastern Mediterranean Sea, Egypt.Int J Adv Res Biol Sci 2(11):39–51.http://s-o-i.org/1.15/ijarbs-2-11-7
Heneash AMM, Tadrose HRZ, Hussein MMA, Hamdona SK, Abdel Aziz NA, Gharib SM (2015) Potential effects of abiotic factors on the abundance and distribution of the plankton in the western harbour, south-eastern Mediterranean Sea, Egypt. Oceanologia 57:61–70. https://doi.org/10.1016/j.oceano.2014.09.003
Heneash AM, Alprol AE, El-hamid HTA, Khater M, Damhogy KAEl, (2021) Assessment of water pollution induced by anthropogenic activities on zooplankton community in Mariout Lake using statistical simulation. Arab J Geosci 14:641. https://doi.org/10.1007/s12517-021-06977-9
Huang L, Jian W, Song X, Huang X, Liu E, Qian P, Yin K, Wu M (2004) Spedes diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Mar Pollut Bull 49:30–39. https://doi.org/10.1007/s11434-013-5823-1
Huber Pestalozzi C (1938) Des phytoplankton des SuessWassers, I. Teule, Die Binnengewasser-Stuttgart, 342 pp
Huet M (1973) Text book of fish culture. Breeding and cultivation of fish, Fish. News Books Ltd, Surrey, 436
Hussein MM (1997) Zooplankton community structure in the offshore neritic area of Alexandria waters, Egypt. Bull Nat Inst Ocean Fish 23:241–265
Hussein NR, Gharib SM (2012) Studies on spatio-temporal dynamics of phytoplankton in El-Umum drain in west of Alexandria. Egypt J Environ Biol 33(1):101–105
Hong G, Abd El-Hamid HT (2020) Hyperspectral imaging using multivariate analysis for simulation and prediction of agricultural crops in Ningxia, China. Comput Electron Agric 172(05):105355. https://doi.org/10.1016/j.compag.2020.105355
Horton RK (1965) An index number system for rating water quality”. J Water Pollu Cont Fed 37(3):300–305
Hussain TS, Al-Fatlawi AH (2020) Remove chemical contaminants from potable water by household water treatment system. Civ Eng J 6:1534–1546. https://doi.org/10.28991/cej-2020-03091565
Ismael AA, Dorgham MM (2003) Ecological indices as a tool for assessing pollution in El-Dekhaila Harbour (Alexandria, Egypt). Oceanologia 45(1):121–131
Jakhar P (2013) Role of phytoplankton and zooplankton as health indicators of aquatic ecosystem: a review. Int J Innov Stud 2(12):489–500
Khairy HM, Gharib SM (2017) Factors regulating composition and abundance of phytoplankton in El Dekhaila Harbor, South-Eastern Mediterranean Sea, Egypt. Asian J Biol Sci 10(2):27–37. https://doi.org/10.3923/ajbs.2017.27.37
Khunnah MC (1967) Chlorococcales. The Indian Council of Agricultural Research, New Delhi (p. 363). Kanput: JobPress Privare Ltd
Kerich EC (2020) Households drinking water sources and treatment methods options in a regional irrigation scheme. J Human, Earth, Futur 1:10–19
Mansour AF, El-Naggar NA, El-Naggar HA, Zakaria HY, Abo-Senna FM (2020) Temporal and spatial variations of zooplankton distribution in the Eastern Harbor, Alexandria, Egypt. Egypt J Aquat Biol Fish 24(4):421–435. https://doi.org/10.21608/EJABF.2020.100475
Marques SC, Azeiteiro UM, Martinho F, Pardal MA (2007) Climate variability and planktonic communities: the effect of an extreme event (severe drought) in a southern European Estuary. Estuar Coast Shelf Sci 73:725–734. https://doi.org/10.1016/j.ecss.2007.03.010
Mihnea PE (1985) Effect of pollution on phytoplankton species. Rapp Comm Int Mer Medit 2(9):85–88
Moisander PH, Hench JL, Kononen K, Paerl HW (2002) Small-scale shear effects on heterocystous cyanobacteria. Limnol Oceanogr 47:108–119. https://doi.org/10.4319/lo.2002.47.1.0108
Mona MH, El-Naggar HA, El-Gayar EE, Masood MF, Mohamed ENE (2019) Effect of human activities on biodiversity in Nabq Protected Area, South Sinai, Egypt. Egypt J Aquat Res 45:33–43. https://doi.org/10.1016/j.ejar.2018.12.001
Nessim RB, Tadros AB (1986) Distribution of nutrient salts in the water and pore water of the Western Harbour of Alexandria. Bull Natl Inst Ocean Fish 12:165–174
Nhu YDT, Hoang NT, Lieu PK, Harada H, Brion N, Hieu DV, Olde Venterink H (2019) Effects of nutrient supply and nutrient ratio on diversity–productivity relationships of phytoplankton in the Cau Hai lagoon, Vietnam. Ecol Evol 9(10):5950–5962. https://doi.org/10.1002/ece3.5178
Padmanabha B, Belaghi SL (2008) Ostracods as indicators of pollution in Lake Mysore. J Environ Biol 29(3):415–418
Paturej E, Gutkowska A (2015) The effect of salinity levels on the structure of zooplankton communities. Arch Biol Sci 67(2):483–492. https://doi.org/10.2298/ABS140910012P
Pielou EC (1966) Shannon’s formula as a measure of species diversity, Its Use and Misuse. Am Nat 100:463–465
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Córdoba City (Argentina) on Suquı́a River. Water res34(11):2915–2926.https://doi.org/10.1016/S0043-1354(00)00036-1
Redden AM, Rukminasari N (2008) Effects of increases in salinity on phytoplankton in the Broadwater of the Myall Lakes, NSW, Australia. Hydrobiology 608(1):87–97. https://doi.org/10.1007/s10750-008-9376-2
Shannon CE, Wiener W (1963) The mathematical theory of communication Urbana, IL: Univ. of Illinois 127 p
Shreadah MA, Masoud MS, Khattab AM, El Zokm GM (2014) Impacts of different drains on the seawater quality of El-Mex Bay (Alexandria, Egypt). J Ecol Nat Environ 8(8):287–303. https://doi.org/10.5897/JENE2014.0465
Shaban W, Abdel-Gaid SE, El-Naggar HA, Bashar MA, Masood MF, Salem ES, Alabssawy AN (2020) Effects of recreational diving and snorkeling on the distribution and abundance of surgeonfishes in the Egyptian Red Sea northern islands. Egypt J Aquat Res 46(3):251–257. https://doi.org/10.1016/j.ejar.2020.08.010
Shams El-Din NG, Abel Halim AM (2008) Changes in phytoplankton community structure at three touristic sites at western Alexandria Beach. Egypt J Aquat Biol Fish 12(4):85–118. https://doi.org/10.21608/ejabf.2008.2007
Said TO (2015) Major ions anomalies and contamination status by trace metals in sediments from two hot spots along the Mediterranean Coast of Egypt. Environ Monit Assess 187:280. https://doi.org/10.1007/s10661-015-4420
Soliman AM (2006) Impact of Umum Drain on zooplankton community structure in Max Bay, Alexandria, Egypt. Int J Oceans Oceanogr 1(1):121–138
Starmach K (1983) Euglenophyta—Eugleniny. Vol. 3.Panstwowe Wydawnictwo Naukowe (in Polish), Warszawa, Poland
Tavsanoglu UN, Maleki R, Akbulut N (2015) Effects of salinity on the zooplankton community structure in two maar lakes and one freshwater lake in the Konya closed basin, Turkey. Ekoloji 24(94):25–32. https://doi.org/10.5053/ekoloji.2015.944
Techniques MS (2020) Spatiotemporal analysis of water quality using multivariate statistical techniques and the water quality identification index for the Qinhuai River Basin, East China. Water 12(2764):2764. https://doi.org/10.3390/w12102764
Trishala K, Parmar DR, Agrawal YK (2016) Bioindicators: the natural indicator of environmental pollution. Front Life Sci 9(2):110–118.https://doi.org/10.1080/21553769.2016.1162753.
Varadharajan D, Soundarapandian P (2013) Zooplankton abundance and diversity from Pointcalimere to Manamelkudi, South East Coast of India. J Earth Sci Clim Change 4(5):151–161. https://doi.org/10.4172/2157-7617.1000151
Wang Z, Qi Y, Chen J, Xu N, Yang Y (2006) Phytoplankton abundance, community structure and nutrients in cultural areas of Daya Bay, South China Sea. J Mar Syst 62:85–94. https://doi.org/10.1016/J.JMARSYS.2006.04.008
Yusuf ZH (2020) Phytoplankton as bioindicators of water quality in Nasarawa reservoir, Katsina State Nigeria. Acta Limnol Bras 32(1). https://doi.org/10.1590/s2179-975x3319.
Zaghloul FA (1996) Further studies on the assessment of eutrophication in Alexandria harbours, Egypt. Bull Fac Sci Alex Univ 36(1):281–294
Zakaria HY, Radwan AA, Said MA (2007) Influence of salinity variations on zooplankton community in El-Mex Bay, Alexandria, Egypt. Egypt J Aqua Res 33(3):52–67
Zakaria HY, Hassan AM, El-Naggar HA, Abou-Senna FM (2016) Abundance, distribution, diversity and zoogeography of epipelagic copepods off the Egyptian Coast (Mediterranean Sea). Egypt J Aqua Res 42(4):459–473
Zakaria HY, Hassan AM, El-Naggar HA, Abou-Senna FM (2018a) Biomass determination based on the individual volume of the dominant copepod species in the Western Egyptian Mediterranean Coast. Egypt J Aqua Res 44:89–99
Zakaria HY, Hassan AM, El-Naggar HA, Abou-Senna FM (2018b) Planktonic protozoan population in the Southeastern Mediterranean off Egypt. Egypt J Aqua Res 44:101–107
Zakaria HY, El-Naggar HA (2019) Long-term variations of zooplankton community in Lake Edku, Egypt. Egypt JAqua Biol Fish 23(4):215–226. https://doi.org/10.21608/ejabf.2019.53997
Zakaria HY, El-Kafrawy SB, El-Naggar HA (2019) Remote sensing technique for assessment of zooplankton community in Lake Mariout, Egypt. Egypt J Aquat Biol Fish 23(3):599–609. https://doi.org/10.21608/EJABF.2019.57142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors.
Additional information
Communicated by Broder J. Merkel.
Responsible Editor: Broder J. Merkel
Rights and permissions
About this article
Cite this article
Heneash, A.M., Alprol, A.E., El-Naggar, H.A. et al. Multivariate analysis of plankton variability and water pollution in two highly dynamic sites, southeastern Mediterranean (Egyptian coast). Arab J Geosci 15, 330 (2022). https://doi.org/10.1007/s12517-022-09595-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-09595-1