Abstract
The present study aims to appraise the spatial and temporal dynamics of the coastal waters of the Mumbai coast in India. Four sampling stations were monitored based on the pollution and anthropogenic stress in the coastal area where two stations were fixed near to the coast (off Aksa and Juhu beach), while the other two were away from the coast. Sampling periodicity was monthly for the ten water quality parameters such as SST, pH, salinity, dissolved oxygen levels, alkalinity, chlorophyll a patterns, and nutrient levels such as ammonia, nitrite, nitrate, and phosphate. Overall SST, pH, alkalinity, and phosphorus showed significant variation when the data across seasons were analysed, whereas chlorophyll a and phosphorus showed significant variation across stations in the two-way univariate ANOVA model. SST and pH, and DO and pCO2 had a substantial negative correlation, but SST and salinity, SST and pCO2, pH and DO, alkalinity and pCO2, nitrate and phosphate, and phosphate and pCO2 had a significant positive correlation. Resource-rich coastal areas are continuously threatened globally due to developmental pressure that inevitably affects the environment, especially around megacities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal marine habitats are regarded as the most economically viable and environmentally beneficial areas on the globe accounting for over 40% of the ecosystem goods and services (Costanza et al. 1997). Water is one of the vital components in this ecosystem. The quality of water can be assessed by physicochemical and microbiological characteristics (Trivedi et al. 2009). The seawater characteristics change with reference to spatial and temporal variations affecting both the human and aquatic ecosystem (Gupta et al. 2009). The primary cause of coastal pollution is population growth, as well as poor waste management, which results in excessive waste entering the waters (Goldberg 1995). As a result, seawater quality monitoring and observations are required for proper water quality management (Udoinyang and Ukpatu 2015). Physical–chemical characteristics are an important model for estimating and monitoring changes in any coastal water body. Water quality characteristics such as SST, pH, salinity, dissolved oxygen, and nutrients greatly influence the water environment. Several studies have documented the prospective nature and effects of global changes on the coastal environment (Karthikeyan et al. 2014; Boesch et al. 2000). However, it is not possible to determine the dominating environmental factors and the comparative importance of selected components in the diverse natural habitat. This is especially evident in estuarine and coastal waters which are dynamic ecosystems where complex interaction between numerous factors is present. Further such changes are impacted by anthropogenic activities and can be indicative of the specific pollution pressure. Physical, chemical, and biological processes have an impact on the marine environment.
The open ocean is more stable than nearshore waters, where interactions with the terrestrial zone are more successful at causing changes in physicochemical properties. In the biochemistry of a water body, physical characteristics have a significant impact. Physical variations can have a major effect on the experimental system’s water quality, which can modify the geographical and temporal distribution of nutrients and/or biological populations. It has an impact on their range, diversity, and feeding patterns (Imam and Balarabe 2012). Changes in the physical ocean process can also have an impact on weather patterns and climate variability. The open ocean is known to be the main sink for anthropogenic CO2 (Sabine et al. 2004); nevertheless, human-made CO2 has affected water carbonate chemistry since preindustrial times and is likely to continue to do so in the coming years (Orr et al. 2005). Coastal ecosystems can also be acidified by carbon dioxide fluxes in the atmosphere (Miller et al. 2009; Feely et al. 2010) and/or upwelling of CO2-rich deep water (Feely et al. 2008). Probably due to a combination of processes such as climate change, watershed geology, and acid deposition, water buffering capacities can be affected by the melting of sea ice and mineral weathering. There are some recent evidences of coastal zones with seasonally low pH and/or elevated pCO2 conditions (Feely et al. 2010; Cai et al. 2011; Sunda and Cai 2012; Duarte et al. 2013; Melzner et al. 2013), while others have documented that some coastal regions have experienced progressively declining pH levels in recent decades (Waldbusser et al. 2011).
Because of the nonlinear nature of environmental data, spatiotemporal changes in water quality can be difficult to interpret; statistical methodologies are applied to provide comprehensive and accurate water quality analysis (Dixon and Chiswell 1996). Monitoring of coastal water quality is key to assess degradation, and derivation of data can be an informative tool for coastal management and policies. Mumbai coastal waters are subjected to several stresses from domestic, industrial, and marine transport activities along the coastal waters. Thus, the present study aims at the understanding of spatiotemporal dynamics of the coastal waters of the North Mumbai coast.
Material and methods
Study site
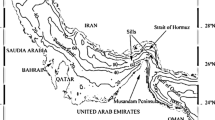
A sampling procedure was carried along the four stations in Mumbai to study the physicochemical analysis of coastal waters. The sampling stations were selected based on the stress factors like pollution and sewage discharges. Stations 1 and 2 (Fig. 1) are in the line of the mouth of Manori creek off Aksa beach, while stations 3 and 4 are away from the mouth and approximately 5–6 km to the south of the Versova creek. Stations 1 and 4 are located in inshore water, while stations 2 and 3 are in the offshore region at a depth beyond 50 m. Therefore, the sites give a comparison between stations located in inshore and offshore as well as between (station 1 and station 2) stations directly led by organic input from the creek and those which are a few kilometres away from the influx line of creek (station 3 and station 4). Station locations were recorded using a GPS data logger which are given below and are represented in Fig. 1.
-
Station 1: 19° 10′ 14.28″ N lat 72° 45′ 53.48″ E long (off Aksa beach)
-
Station 2: 19° 09′ 50.00″ N lat 72° 44′ 19.00″ E long
-
Station 3: 19° 04′ 10.40″ N lat 72° 45′ 48.45″ E long
-
Station 4: 19° 05′ 38.00″ N lat 72° 47′ 51.00″ E long (off Juhu beach)
Sampling
Monthly sampling was carried from September 2017 to May 2018 for 9 months using M.F.V Narmada-CIFE (Marine Fishing Vessel Narmada-Central Institute of Fisheries Education). The sampling period can be categorized into three seasons, post-monsoon (September 2017 to November 2017), winter (December 2017 to February 2018), and pre-monsoon (March 2018 to May 2018). Samplings could not be taken up due to the closed season ban in the month of June, July, and August. This study characterized the patterns of sea surface temperature (SST), pH, salinity, dissolved oxygen (DO) levels, alkalinity, chlorophyll a (Chl a) patterns, and nutrient levels such as ammonia, nitrite, nitrate, and phosphate. Physical parameters of seawater like SST, pH, and salinity were measured directly in the field using a calibrated mercury thermometer, OAKTON pH EcoTestr, and ATAGO S/Mill-E refractometer respectively. Water sampling and preservation were carried out in accordance with APHA (2005) guidelines. All the analyses were done in triplicate, and data quality was assured through standardization. Water samples were fixed for DO and analysed titrimetrically by Winkler’s iodometric method (APHA 2005). Samples were collected for laboratory analysis of alkalinity, nutrients, and Chl a and analysed, as per guidelines in APHA (2005) with the help of standard techniques and procedures. The alkalinity of the samples was determined by titrating them with a standard solution of strong acid (H2SO4) using phenolphthalein and methyl orange as indicators as recommended by APHA (2005). As specified in APHA, chlorophyll a was extracted using the acetone extraction procedure and evaluated using spectrophotometric determination (2005). Standard procedures were used to examine the nutrient parameters (ammonia, nitrate, nitrite, and phosphate) (APHA 2005). The phenate method was used to estimate ammonia–nitrogen, the colorimetric method in a spectrophotometer was used to determine nitrite-nitrogen using N(1-naphthyl)-ethylenediamine dihydrochloride and sulphanilamide solution, the UV spectrophotometric monitoring method was used to determine nitrate-nitrogen, and the ascorbic acid method was used to determine phosphate-phosphorus.
The pCO2 is the CO2 gas-phase pressure at which the dissolved CO2 is in equilibrium. The CO2SYS programme was used to estimate the partial pressure of carbon dioxide in water (pCO2) fluctuations in μatm for the Mumbai waters (Lewis and Wallace 1998). Using the CO2SYS programme, the pCO2 was calculated using measured salinity, temperature, pH, and alkalinity. Several researchers have found the relevant equilibrium constants, K1 and K2, that define CO2 speciation in seawater at various temperatures and salinities. According to Mehrbach et al. (1973) and refit by Dickson and Millero (1987), the dissociation constants K1 and K2 for carbonic acid were employed. The CO2SYS programme calculates the other two parameters using two of the four measurable CO2 system parameters: alkalinity, total inorganic CO2 (TCO2), pH, and either fugacity (fCO2) or partial pressure of CO2 (pCO2) at a set of input conditions (temperature and pressure) and a set of output conditions chosen by the user. The total carbonate, carbonate, and bicarbonate content of TCO2 is equal to the sum of dissolved CO2, carbonate, and bicarbonate. TCO2 and alkalinity are aqueous solution properties that are unaffected by temperature or pressure, whereas temperature and pressure influence fCO2, pCO2, and pH. We can define the other two parameters using any two of these factors (excluding the combination of fCO2 and pCO2), as well as additional water properties such as salinity, at a given temperature and pressure (Yuan 2006).
Statistical analyses
Two-way ANOVA was used to examine the change in the different environmental factors between seasons and at all stations. A two-way univariate ANOVA model with interaction (y = mu + station + season + station*season + error) was fitted to evaluate different responses. A post hoc analysis (Duncan’s multiple range) was conducted to test the clustering (significant differences in the means) of levels of stations and seasons. This was utilized to figure out how environmental variables varied across different stations and seasons. A p-value of 0.05 was judged significant in the ANOVA. In correlation analysis, the correlation coefficient is used to determine how closely two variables are linked (Healey 1999). The correlation coefficient indicates the strength of the relationship. The stronger the link, the higher the correlation. Spearman’s correlation coefficient was done to understand the correlation between environmental parameters in SPSS 21.0. Scatter plots were utilized to highlight the correlations between various environmental factors assessed during the sampling period. Since pCO2 is a derivative of pH, temperature, and alkalinity using CO2SYS software, it was not included in the ANOVA and has been presented respectively.
Results and discussion
The least squares (LS) means with standard errors of water quality parameters at four stations are highlighted in Table 1, and three different seasons are listed in Table 2. The distribution of physicochemical variations observed at four stations during the three seasons is illustrated in Fig. 2.
Distribution of different parameters across stations 1, 2, 3, and 4 in three different seasons {post-monsoon (September 2017 to November 2017), winter (December 2017 to February 2018), and pre-monsoon (March 2018 to May 2018)}, in the order temperature, pH, salinity, dissolved oxygen, chlorophyll a, alkalinity, ammonia, nitrite, nitrate, and phosphate
A similar trend emerged in the time distribution of sea surface temperature (SST) at all locations. Higher values were connected with the pre-monsoon season (28 to 31 °C) and the lowest with the winter season (25 to 29 °C), which could be associated with the solar radiation that the region experienced over the summer. During the study period, average temperatures at the sites ranged from 25 to 31 °C, demonstrating a seasonal pattern with low variation between stations. pH, a critical parameter in the carbonate system, was very variable. The pH levels varied between stations, ranging from 7.4 to 8.3. The lower pH values were associated with the summer (March to May), whereas higher pH values were reported during the post-monsoon (September to November). pH is governed by the buffering action of carbonic acid and is an important factor in maintaining the bicarbonate and carbonate system (Martin 1970). But comparatively lower average values of pH in the inshore areas indicate the pollution influx in these areas. Nearshore coastal waters had the most significant pH variations between seasons (Narayanan et al. 2016). However, Shetye et al. (2020) observed a pH change in the Eastern Arabian Sea along the Goa Coast from 7.96 to 7.46 between seasons. The variation in salinity value ranged from 32 to 39 ppt with no significant variation across stations and seasons. Similarly, DO levels also did not show significant variations across stations and seasons, and the DO concentrations varied between 2 and 9.6 mg/l. But an exceptionally lesser DO level in a range of 2 to 3 mg/l was evident in some winter months. Substantial reduction in DO was a common feature in the regions receiving organic waste. The existence of aquatic life including fishery is intimately linked with the availability of DO. Thus, a DO level of less than 4 mg/l showed a polluted environment which is majorly in the inshore areas. The effect is diluted further in the open shore areas due to the large assimilative capacity of the sea.
SST, pH, alkalinity, and phosphate were shown to have considerable seasonal modulation (p-value 0.05) across sampling periods, demonstrating seasonal variations. The atmospheric conditions appeared to have a significant impact on seasonal fluctuations. The levels of chlorophyll a and phosphorus differed significantly amongst the stations. There is no interaction effect noted, and the ANOVA table is represented in Table 3 as mean square values of different parameters measured across the stations and the seasons.
In general, Chl a concentration ranged between 3.2 and 6.1 mg/l; higher Chl a values were associated with station 3(offshore area). The areas (stations 3 and 4) had greater surface Chl a than the other two stations where the pollutants are probably pushed or accumulated due to less movement of water. Stations 1 and 2 may be affected by tidal movement in and out of the creek not allowing pollutants to become resident. The high chlorophyll a levels reported are likely related to the stratified water column, shallow mixing, and low sediment loads implying effective nutrient use in the study area (Pattanaik et al. 2020). Sporadic peaks in Chl a patterns were also observed during some sampling occasions at specific stations. The average alkalinity values ranged between 125 and 364 mg/l in the course of the study period. The greater values were linked to the post-monsoon season which showed a significant variation across the seasons. pH and alkalinity are high in post-monsoon, and they are related also. The abundance of household trash and the lack of usual tidal movement, which might have washed and neutralized soluble compounds, could have enhanced alkalinity values measured during the post-monsoon period. Alabaster and Lloyd (1980) documented a seasonal effect, with higher alkalinity levels during the wet season than the summer months. This may be one of the reasons for higher alkalinity in the post-monsoon season. Through the whole study period, the mean pCO2 ranged from 206.60 to 1844.40 atm. The pCO2 (water) level increases when the pH level decreases which was evident in the study. Kortzinger et al (1997) investigated CO2 emissions from the Arabian Sea during the SW monsoon, finding that there was a pCO2 supersaturation state during this season, resulting in high emissions to the atmosphere. In the lower thermocline, it was discovered that organic matter renewal can raise the pCO2 value by 1000 atm (Sarma et al. 1998). During the SW monsoon, seasonal fluctuations in pCO2 resulted in high readings of 520–685 atm and low values of roughly 266 atm along India’s southwest coast (Sarma et al. 2000).
Among nutrients, only phosphate showed significant variations across stations, and other nutrients such as ammonia, nitrite, and nitrate values did not show any significant variations across stations and seasons. The average values of ammonia, nitrite, and nitrate ranged between 0.28 and 1.31 ppm, 0.01 and 0.12 ppm, and 1.36 and 2.99 ppm, respectively. On most occasions, nitrate, ammonia, and phosphate nutrients were more prevalent in the water column than nitrite, with varying seasonal maxima. The discharge of sewage containing huge levels of organic substances, which produce massive amounts of ammonia, resulted in a higher concentration of ammonia. Particulate phosphorus has been linked to a variety of solid components, including detritus and particulate organic materials (Deborde et al. 2007). In the inshore stations (stations 1 and 4), phosphate levels were high compared to the offshore stations. High microbial breathing rates absorb oxygen quicker than they are replaced by horizontal or vertical ventilation, exposing eutrophic coastal areas receiving considerable volumes of organic material to hypoxia during summer months (Rabalais et al. 2002). Gowda et al. (2002) observed that the peak periods of Chl a coincided with the lower concentration of nitrate-nitrogen and phosphate-phosphorus. Phosphorus in post-monsoon is higher indicating flush from land towards the end of the monsoon still affecting the load.
Spearman’s correlation coefficient (R) showed significant positive and negative correlations between the physicochemical parameters (Table 4). A significant negative correlation was observed between SST and pH (R = − 0.339) and DO and pCO2 (R = − 0.364), whereas a significant positive correlation was evident between the parameters SST and salinity (R = 0.340), SST and pCO2 (R = 0.408), pH and DO (R = 0.660), alkalinity and pCO2 (R = 0.466), nitrate and phosphate (R = 0.439), and phosphate and pCO2 (R = 0.347). Numerous research on the link between pH and DO have discovered significant positive linear associations (Luis et al. 2010; Zhang et al. 2009). The rapid consumption of carbon dioxide increases the pH value.
Scatter plots (Fig. 3) were made to showcase the trends based on the correlation matrix. Several scatter correlations were investigated to better define the physical and biological influences on the pCO2 variation in the water column with time. We discovered that alkalinity (125 to 364 mg/l) was primarily related with pH 7.4 to 8.30, implying that coastal water played a role in this study. These were likewise linked to a significant level of salinity and average chlorophyll a levels (3.2 and 6.1 mg/l, respectively). Coastal acidification is a seasonal phenomenon characterized by a high degree of temporal and spatial regularity in DO levels (Cai et al. 2011). Furthermore, pCO2 had a substantial negative relationship with DO (R2 = 0.1455) and a significant positive relationship with SST, alkalinity, and phosphate levels. Noriega and Araujo (2014) discovered a strong negative relation between DO and pCO2, implying that extensive organic material decomposition in the water or the soil might have resulted in a fall in pH and an increase in pCO2 values.
Conclusion
The Arabian Sea to the west and numerous tidal inlets encircle Mumbai, a metropolitan city on India’s west coast. Continuous accumulation leads to the concentration of waste material in the coastal and marine ecosystem affecting the marine habitat. The ever increasing demand for coastal resources leads to persistent growth of population and indiscriminate exploitation of coastal resources that in turn triggers irreversible degradation of the coastal environment. The present study was attempted to gain a holistic insight into the spatiotemporal dynamics of north Mumbai coastal waters. The study indicates that temperature, pH, alkalinity, and phosphate significantly varied when the data across seasons were analysed, whereas chlorophyll a and phosphate showed significant variation across stations. Importantly, Chl a was found to be higher in areas expected to have more resident time for the pollutant, and phosphate was higher closer to the coast. This study also indicated that such a study coupled with hydrographical measurements of depth, currents, eddies, and sedimentation pattern will help to predict season-wise change even on a local scale.
References
Alabaster JS, Lloyd R (1980) Water quality criteria for freshwater fish. 1st Edition. Butterworths, London, pp 283. https://doi.org/10.1002/iroh.19810660329
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Boesch DF, Field JC, Scavia D (eds) (2000) The potential consequences of climate variability and change on coastal areas and marine resources: report of the coastal areas and marine resources Sector Team, U.S. Series No.# 21. NOAA Coastal Ocean Program, Silver Spring MD. pp 163
Cai WJ, Hu XP, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, Chou WC, Zhai WD, Hollibaugh JT, Wang YC, Zhao PS, Guo XH, Gundersen K, Dai MH, Gong GC (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci 4:766–770. https://doi.org/10.1038/ngeo1297
Costanza R, d’Arge R, De Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’neill RV, Paruelo J, Raskin RG (1997) The value of the world’s ecosystem services and natural capital. Nature 387(6630):253–260. https://doi.org/10.1038/387253a0
Deborde J, Anschutz P, Chaillou G, Etcheber H, Commarieu MV, Lecroart P, Abril G (2007) The dynamics of phosphorus in turbid estuarine systems: example of the Gironde estuary (France). Limnol Oceanogr 52(2):862–872. https://doi.org/10.4319/lo.2007.52.2.0862
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res Part A. Oceanogr Rese Pap 34(10):1733–1743. https://doi.org/10.1016/0198-0149(87)90021-5
Dixon W, Chiswell B (1996) Review of aquatic monitoring program design. Water Res 30(9):1935–1948. https://doi.org/10.1016/0043-1354(96)00087-5
Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36(2):221–236. https://doi.org/10.1007/s12237-013-9594-3
Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B (2008) Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320(5882):1490–1492. https://doi.org/10.1126/science.1155676
Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C (2010) The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar Coast Shelf Sci 88(4):442–449. https://doi.org/10.1016/j.ecss.2010.05.004
Goldberg ED (1995) Emerging problems in the coastal zone for the twenty-first century. Mar Pollut Bull 31(4–12):152–158. https://doi.org/10.1016/0025-326x(95)00102-s
Gowda G, Gupta TRC, Rajesh KM, Mendon RM (2002) Primary productivity in relation to chlorophyll a and phytoplankton in Gurupur estuary. J Mar Biol Assoc India 44(1 & 2):14–21
Gupta I, Dhage S, Kumar R (2009) Study of variations in water quality of Mumbai coast through multivariate analysis techniques. Indian J Mar Sci 38:170–177
Healey JF (1999) Statistics: a tool for social research, 5th edn. Wadsworth Publishing Company, Belmont
Imam TS, Balarabe ML (2012) Impact of physicochemical factors on zooplankton species richness and abundance in Bompai-Jakara catchment basin, Kano State, Northern Nigeria. Bayero J Pure Appl Sci 5(2):34–40. https://doi.org/10.4314/bajopas.v5i2.6
Karthikeyan K, Lekameera R, Mehta PN, Thivakaran GA (1999) Water and sediment quality characteristics near an industrial vicinity, vadinar, Gulf of Kachchh, Gujarat, India. J Internet Bank Commer 4(2):219–226
Kortzinger A, Duinker JC, Mintrop L (1997) Strong CO2 emissions from the Arabian Sea during south-west monsoon. Geophys Res Lett 24(14):1763–1766
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Oak Ridge. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy
Luis MB, Sidinei MT, Priscilla C (2010) Limnological effects of Egeria najas Planchon (Hydrocharita-ceae) in the arms of Itaipu Reservoir (Brazil, Paraguay). Limnology 11(1):39–47. https://doi.org/10.1007/s10201-009-0286-4
Martin DF (1970) Marine chemistry. Marcel Dekker Inc., New York, 1: pp 283–287. https://doi.org/10.4319/lo.1968.13.4.0726
Mehrbach C, Culberson CH, Hawley JE, Pytkowicx RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure 1. Limnol Oceanogr 18(6):897–907. https://doi.org/10.4319/lo.1973.18.6.0897
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160(8):1875–1888. https://doi.org/10.1007/s00227-012-1954-1
Miller AW, Reynolds AC, Sobrino C, Riedel GF (2009) Shellfish face uncertain future in high CO 2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS ONE 4(5):e5661. https://doi.org/10.1371/journal.pone.0005661
Narayanan RM, Sharmila KJ, Dharanirajan K (2016) Evaluation of marine water quality–a case study between Cuddalore and pondicherry coast, India. Indian J GeoMar Sci 45(4):517–532
Noriega C, Araujo M (2014) Carbon dioxide emissions from estuaries of northern and northeastern Brazil. Sci Rep 4(1):1–9. https://doi.org/10.1038/srep06164
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437(7059):681–686. https://doi.org/10.1038/nature04095
Pattanaik S, Roy R, Sahoo RK, Choudhury SB, Panda CR, Satapathy DR, Majhi A, D’Costa PM, Sai MS (2020) Air-sea CO2 dynamics from tropical estuarine system Mahanadi, India. Reg Stud Mar Sci 36:101284
Rabalais NN, Turner RE, Wiseman WJ Jr (2002) Gulf of Mexico hypoxia, aka “the dead zone”. Annu Rev Ecol Syst 33(1):235–263. https://doi.org/10.1146/annurev.ecolsys.33.010802.150513
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CSL, Wallace DW, Tilbrook B, Millero FJ (2004) The oceanic sink for anthropogenic CO2. Science 305(5682):367–371. https://doi.org/10.1126/science.1097403
Sarma VVSS, Kumar MD, George MD (1998) The central and eastern Arabian Sea as a perennial source of atmospheric carbon dioxide. Tellus 50B:179–184
Sarma VVSS, Kumar MD, Gauns M, Madhupratap M (2000) Seasonal controls on surface pCO2 in the central and eastern Arabian Sea. Proc Indian Acad Sci (Earth Planet Sci) 109(4):471–479
Shetye SS, Naik H, Kurian S, Shenoy D, Kuniyil N, Fernandes M, Hussain A (2020) pH variability off Goa (Eastern Arabian Sea) and the response of sea urchin to ocean acidification scenarios. Mar Ecol 41(5). https://doi.org/10.1111/maec.v41.510.1111/maec.12614
Sunda WG, Cai WJ (2012) Eutrophication induced CO2-acidification of subsurface coastal waters: interactive effects of temperature, salinity, and atmospheric pCO2. Environ Sci Technol 46(19):10651–10659. https://doi.org/10.1021/es300626f
Trivedi P, Bajpai A, Thareja S (2009) Evaluation of water quality: physico–chemical characteristics of Ganga River at Kanpur by using correlation study. Nat Sci 1(6):91–94
Udoinyang E, Ukpatu J (2015) Application of principal component analysis (PCA) for the characterization of the water quality of Okoro River Estuary, South Eastern Nigeria. 25–32
Waldbusser GG, Voigt EP, Bergschneider H, Green MA, Newell RI (2011) Biocalcification in the eastern oyster (Crassostrea virginica) in relation to long-term trends in Chesapeake Bay pH. Estuaries Coasts 34(2):221–231. https://doi.org/10.1007/s12237-010-9307-0
Yuan S (2006) The development of the web based CO2SYS program. Graduate Student Theses, Dissertations, & Professional Papers. 977. https://scholarworks.umt.edu/etd/977
Zhang JY, Huang J, Yan F, Zhang ZQ (2009) Preliminary study on characters of dissolved oxygen and the relationship with pH in Meiliang Lake. J Fudan Univ 48(5):623–627
Acknowledgements
The first author acknowledges the fellowship received from ICAR-CIFE during her Ph.D. studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Soman, C., Lal, D.M., Haridas, H. et al. Spatial and temporal dynamics of water quality along coastal waters of Mumbai, India. Arab J Geosci 15, 208 (2022). https://doi.org/10.1007/s12517-021-09374-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-09374-4