Abstract
In El Haouaria region located in the Cap Bon peninsula (N.E. Tunisia), the hydrodynamic survey revealed a decrease in unconfined groundwater reserves of approximately 11% over the last 30 years due to the abstraction of water. Over that same time period, the groundwater table displayed a natural decrease of about 10 m in depth over an area of 230 km2. Hydrogeological investigations have shown that two aquifers which vertically integrate all of the permeable zones in the region can be distinguished: a shallow aquifer formed by filling the Quaternary and another deeper one in the Pliocene. These two aquifers are separated by a semi-permeable layer through which vertical exchange by seepage is possible. Different methods using geochemistry (Na+, Cl−, Br−) and stable isotopes (18O, 2H) are compared with the hydrodynamic information for identifying the main processes involved in the salinization and recharge. Forty groundwater wells were sampled to obtain additional information on the hydrochemical and isotopic characteristics of the groundwater defined in previous studies. Estimation of groundwater recharge in this semi-arid area is difficult due to the low amount and variability of recharge. Two analytical models, taking into account the long term decrease of the water table, were used to interpret 3H and 14C content in groundwater. The median annual renewal rate (recharge as a fraction of saturated aquifer volume) varies between 0.2 and 0.3%. For representative characteristics of the aquifer (30 m of saturated thickness, porosity 14%) this implies a recharge of 10 to 12 mm/yr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
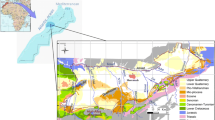
The El Haouaria coastal aquifer is a relatively small aquifer located in northeastern Tunisia, characterized by a semi-arid climate (Ben Hamouda et al., 2011) and located in the Cap Bon peninsula (Fig. 1). This study aims to integrate hydrogeological data with major ion geochemistry and the isotopic signatures of groundwater in order to identify the major hydrochemical processes responsible for groundwater mineralization. Environmental isotopes have been widely and extensively used over the past 60 years in groundwater investigations. The stable isotope compositions of oxygen and hydrogen, expressed as δ18O and δ2H values, respectively, are commonly used in regional groundwater studies to identify flow regimes and sources of recharge (Clark and Fritz, 1997; Mook 2001; Aggarwal et al. 2005). Radioactive isotopes (3H and 14C) can be used to estimate groundwater ages. The presence of detectable 3H is evidence for groundwater recharge after the period of nuclear bomb tests 1952–1964(Clark and Fritz 1997). 14C was used as a dating method to estimate the age of water from several hundred to about 35,000 years (Tamers 1975; Fontes and Garnier 1979; Clark and Fritz 1997; Geyh 2000).
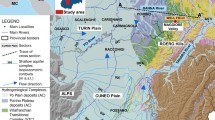
Here we measured stable isotopes and major ion chemistry (Na+, Cl−, Br−) in 40 groundwater samples from the El Haouaria aquifer. Much of the available water in the plain is used for irrigation to further agricultural development of the region. The El Haouaria plain is 12 km wide and 16 km long and is exposed to the sea on the east and west sides. The geologic map of the Cap-Bon Peninsula (Fig. 2) shows that the North and the South are bordered by clay Miocene outcrops with intercalations of sandstone. The surface area of the quaternary water table is 230 km2. Water resources in this region rely heavily on its rainfall potential and the atmosphere’s evaporation potential, which takes back the majority of the precipitation. The El Haouaria water table benefits from wet conditions with an average rainfall of 568 mm/year, a natural depression which forms a basin for collecting run-off water, and a sandy, very permeable dunal mantle that ensures the best conditions for rain infiltration. The average annual temperature varies from 17 to 19 °C. The wettest months are November and March, and the driest are July and August. Potential evapotranspiration calculated using Riou method (Riou 1980) exceeds 1100 mm/year. Few previous studies have been done on the isotopic characterization of the El Haouaria aquifer (Ennabli 1980; Lassoued 1982; Ben Hamouda 2008; Ben Hamouda et al. 2015; Tarhouni et al. 2015). Because of the low amount of recharge and variability in recharge conditions, the estimation of groundwater recharge and associated hydrodynamic functioning have been difficult to estimate. This study aims to integrate hydrogeological data with established isotopic and geochemical tools for the assessment of groundwater salinity and to estimate the groundwater recharge and renewal rates of the aquifer. This was accomplished using radioactive isotopes tritium and carbon 14.
Local geology and hydrogeology
The El Haouaria plain forms a depression (graben) facing East-West and is bordered by the sea on both sides, with outcrops of the Oum Douil formation in the north and south. The study area located in the Cap Bon peninsula is a geographical entity that constitutes the extension towards the NE of Tunisian Atlas (Khomsi et al. 2009, 2012, 2016). The El Haouaria plain settled on a Miocene-age synclinal structure (Fig. 3) Several geological studies have been carried out in the region (Khomsi et al. 2019a, b). The structure of the El Haouaria region results from orogenic movements dating mainly from the end of the Miocene, these movements generated folds of orthogonal directions. The region is located at the intersection of two directions. We distinguish tectonic units corresponding to these two directions and tectonic accidents mainly faults (Lassoued, 1982). The tectonic units follow two directions: the so-called “Tunisian” direction and the so-called “Sicilian” one because it is the direction of the large faults of the canal of Sicily.
The reservoir of the El Haouaria groundwater is composed of an overburden of quaternary sediment 50 m deep and a dunal cap under which rests a large Pliocene deposit 200 m deep making up the central filling of this depression. According to the geological cross sections done on boreholes in the region (Fig. 4a and b), the quaternary deposits are made up of intercalations of sandy layers and clay with travertin and tuff located at the summit.
a Bloc Diagram of El Haouaria region (Ben Hamouda et al., 2015). b Lithostratigraphic cross-section SW-NE
These two aquifer levels are separated by a semi-permeable layer of clay 10 to 15 m thick, which fills the deep aquifer located in the Pliocene deposits, as opposed to the groundwater table made up of quaternary sediments. Hydrogeological investigations have shown that two aquifers, which vertically integrate all of the permeable zones, can be distinguished: one groundwater table formed during the quaternary and a deeper aquifer formed during the Pliocene. These two aquifers are separated by a semi-permeable layer through which vertical exchange by seepage is possible (Fig. 4a and b).
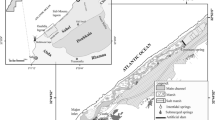
Our hydrogeological study showed that interconnection exists between the groundwater towards the deep aquifer at Dar Chichou and another one in the opposite direction at Gareât El Haouaria (a salty lake located in the center of the plain), where the deep aquifer supplies the groundwater. Examining the changes in piezometry from 1972 to 2004 revealed a continual decrease, which varied from 1 to more than 14 m in some places (Fig. 5).
Compared to 1972 values, an average decrease of 3.38 m was observed in 2001 across the El Haouaria plain. This decrease lessened to 1.4 m in 2006, such that there was a decrease of 11% since 2001 and 4.6% since 2006 for the total depth of the groundwater estimated at 30 m. These two aquifers have more than 200 m of depth in the plain characterized by an active fault of Azmour located in the southern part of the study area. For the quaternary unconfined aquifer, transmissivity varies widely from 8.10−4 to 17.10−4 m2/s and the storage Coefficient from 14.10−2 to 45.10−2. For the deep confined aquifer, the transmissivity varies from 10 .10–.4 m2/s to 87 .10–.4 m2/s and the storage coefficient from 1.10−4 to 9.10−4(Ben Hamouda 2008). Depth of the groundwater table varies across the region. The piezometric map established in 2006 shows that the aquifer is characterized by a groundwater divide (Fig. 6).
In the southern part of the aquifer, the groundwater flows towards the Garet El Haouaria (salty lake), located in the center of the plain, then flows towards the sea, located to the East and West. Groundwater flow coming from the north is also flowing towards the sea located in the East and West.
Material and methods
Previous chemical and isotopic data were taken from (Ben Hamouda, 2008). For this study, geographical position, depth to water table, electric conductivity, pH and temperature were measured with forty (40) Quaternary and Pliocene aquifer wells, and piezometers in 2002 and 2003 (Fig. 1). Samples were also collected for identification of major elements (Na+, Cl−, Ca2+, Mg2+, Br−), stable and radioactive isotopes (2H, 18O, 13C, 3H, 14C) and represented in Table 1. Samples were taken from piezometers after purging. Boreholes and dug wells did not need to be purged because at the time of sampling water was being extracted from these wells. Table 1 provides the main results from the spring of 2001 (end of the rainy period and beginning of heavy agricultural pumping) as well as the Br− and Cl− contents in 2003.
Chemical analysis of the water samples was performed at the National Centre for Nuclear Science and Technologies: Isotope Hydrology Laboratory, (Sidi Thabet, Tunisia). Major cation (Ca, Mg, Na, and K) concentrations were analyzed in filtered samples using an Analytic Jena atomic absorption spectrometer with a furnace AAS Vario 6 and anion (Cl, SO4 and NO3) concentrations were analyzed in filtered samples using a Dionex DX 120 ion chromatograph equipped with an AG14 and an AS14 Ion Pac columns and an AS-40 auto-sampler. Bromide concentrations were analyzed using a Dionex DX 120 ion chromatographer equipped with AG14 and AS14 Ion Pac columns at the Avignon University, France. The charge balance between major anions and cations is better than ±5%.
Isotopic analysis of the water samples (2H and 18O) were performed at the Institute of Groundwater Ecology-GSF Research Centre (Neuherberg/Germany) and supported by IAEA through TC project TUN/8/015 and TUN/8/017. Stable isotopes of oxygen and hydrogen were determined by isotope ratio mass spectrometry in a Finnigan MAT Gas Bench and analyzed using continuous flow on a Finnigan MAT 252 mass spectrometer. The δ18O values in samples were analyzed via equilibration with CO2 at 25 °C for 24 h (Epstein and Mayeda, 1953) and for the δ2H values via reaction with Cr at 850 °C (Coleman et al. 1982). Both δ18O and δ2H values were determined relative to internal standards that were calibrated using IAEA SMOW standards. Data were normalized following Coplen (1988) and are expressed relative to V-SMOW. Samples were measured at least in duplicates and the precision of the analytical measures is ±0.1‰ for δ18O and ± 1‰ for δ2H. The results are reported as δ18O and δ2H, where δ = ((Rsample/Rstandard)-1)×1000. Tritium (3H) was measured by the IAEA at the Isotope Hydrology Laboratory, Vienna, Austria. Tritium content was measured by electrolytic enrichment and liquid scintillation spectrometry (Taylor, 1977). Tritium concentration is reported in Tritium Units (TU). One TU is defined as the isotope ratio 3H/1H = 10−18. Radiocarbon analyses were collected in the field by precipitating BaCO3. Radiocarbon content was determined at the Isotope Hydrology Unit of the CNSTN (Centre National des Sciences et Technologies Nucléaires, Tunisia), through benzene synthesis and liquid scintillation spectrometry (Fontes 1971). The measured 14C concentrations are expressed as percent of modern carbon (pMc).
Origin of groundwater salinity
The salinity of the groundwater is determined by the total dissolved solids (TDS) and ranges from 3 to 8 g/l (the highest values are measured in the shallow aquifer). The chemical composition of groundwater plotted in a piper diagram (Fig. 7) shows a trend of water classified under a Na Cl SO4 Ca water type.
The strong correlation (r2 = 0.94) (Fig. 8) in the Na+/Cl− relationship suggests that these two elements have the same origin. Even for samples taken far from the sea, the molar relationship Na+/Cl− does not differ very significantly from that of the mediterranean sea (0.86) (Jones et al. 1999; Bouchaou et al. 2008; De Montety et al. 2008; Vengosh 2014; Moussa et al. 2017). The geographical location of the study area (peninsula) gives several hypotheses on the origin of salinity. First, the predominance of sodium and chloride can be explained by the proximity to the sea, via the spray caused by prevailing winds from the sea contributing to chloride and sodium concentrations (Ferchichi et al. 2017). Secondly, the development of agriculture and corresponding irrigation, in the El Haouaria region, will increase the concentration of salts. This is accentuated by evaporation of irrigated water which will increase soil salinity, and therefore, the contribution of these salts to the aquifer through the excess of water irrigation. Finally, the excessive use of fertilizers enriched in Na and Cl in this region will result in a systematic and additional presence of Na and Cl in the groundwater. The processes of dissolution, precipitation and cation-exchange are actively taking place within the groundwater system. Results indicate that groundwater is oversaturated with regarding several mineral phases (calcite, aragonite, and dolomite). However, most of the groundwater is unsaturated with respect to gypsum and anhydrite.
The Br-/Cl- relationship helps to identify the possibility of seawater intrusion (Jones et al. 1999; Ben Hamouda et al. 2013; Vengosh 2014,) since it is relatively constant (1.5 × 10−3) in the seawater considering the extremely long residence time of oceanic masses.Because of this, particular attention to the Br/Cl ratio (Fig. 9) was paid to sampling points near the Mediterranean Sea on both the east and west side of the El Haouaria plain. The water samples whose Br/Cl ratios are lower than the marine molar ratio (around 0.5 × 10−3) point to a different mineralization origin. Alternatively, samples whose molar Br/Cl ratios are above the sea water dilution line are not affected by seawater intrusion. In this case, the salinization of the groundwater would be expected to result from the ionic concentrations increasing due to both the evaporation of recharge waters and the effects of interactions between the groundwater and the geological formations.
Stable isotopes
Stable isotopes of hydrogen and oxygen are very suitable tracers for hydrogeological investigations because they are part of the water molecule and follow their behavior through the hydrological cycle and they are conservative in most low-temperature environments (Fontes 1976; Clark and Fritz 1997, Zuber et al. 2000; Kamel 2013, Moussa et al. 2017). The Deuterium - Oxygen 18 diagram shows two groups (Fig. 10). In the first group (18O between −5.4 and − 4.43 ‰ V-SMOW), points are located between the global meteoric water line (GMWL) and the local meteoric water line of Tunis-Carthage station (δ2H = 8δ18O + 12.4) (Celle-Jeanton et al. 2001). The location of these water points, often near rivers, is compatible with recharge from recent rain storms. The second group is mainly composed of deep Pliocene groundwater whose 18O contents vary between −5.69 and − 4.74 ‰, thus less than −4.41 ‰, the weighted average of rain in Tunis-Carthage. The quaternary waters of this group could result from a mixture of new and older waters (Ben Hamouda 2008). There is no indication of presence of water enriched in 18O and 2H falling on the mixing line with seawater. For the other points situated below the mixing line with seawater, they are under the influence of evaporation. A separate study of ionic relationships and stable isotopes is not able to identify the origin of the water precisely (Negrel et Petelet-Giraud2011). The combination of these two parameters is more explicit, especially the Cl− Vs. 18O graph (Fig. 11) that places the samples between different end-members. The first end-member, around 10 mg/l for Cl− and 4.41‰ V-SMOW for δ18O, is defined from the rainwater samples at Tunis-Carthage located about 40 km east of the study area and at practically the same altitude. The second end-member is represented by Mediterranean waters, having a δ18O content around (0‰) and a Cl− content of about 19,500 mg/l. The third end-member is the water from the Pliocene aquifer, with lower mineralization than the Quaternary water and more depleted 18O content. The other Quaternary points have Cl− levels varying between 300 and 2000 mg/l and δ18O levels from −5.5 to −4.0 ‰ V-SMOW. Their sometimesheavy mineralization cannot be understood from only one rainwater–seawater dipole.
Radioactive isotopes and conceptual model of recharge
Tritium (3H) is an intrinsic tracer for water molecules with a half-life of 12.43 years. It comes solely from the atmosphere and was introduced into the groundwater through recharged waters with Tritium levels identical to those of rainwater, immediately passing to the unsaturated zone. Carbon 14 (14C), unlike 3H, is a dissolved tracer and has a longer half-life, estimated at 5730 years. Similarly to 3H, 14C is immediately introduced into the groundwater through the unsaturated zone. The measured activity of 14C, like that of Tritium, could be considered representative of the water’s residence time in the aquifer (Favreau et al., 2000b; Ben Hamouda 2008; Guo et al. 2019). The conceptual model estimates renewal rates using the definition of recharge processes and aquifer boundaries along with incorporating constraints from hydrodynamic and geochemical approaches (Favreau et al., 2002a, b). In the El Haouaria plain, groundwater is recharged quickly across the land and provides the aquifer with water which has almost the same isotopic values as the atmosphere. The relatively low depth of the groundwater (30 m on average) suggests that groundwater infiltrates during recharge, which is consistent with the geochemical characteristics, showing a lack of vertical stratification of the aquifer water. Despite obvious simplistic characteristics, a simple model of vertical mixing seems to be the best adapted to estimate the natural rate of groundwater renewal:
Where: Ani = Activity in the aquifer for the year i, Rr = Annual renewal rate, Pe = period of radioisotope (14C or 3H) and Api = activity in the rain for the year i.
The model presumes homogenous isotopic features throughout the depth of the groundwater and constant storage, meaning, the output of water is exactly compensated by the infiltration of rainwater.
Since the climate has not changed significantly in the last 4000 years (Favreau et al., 2002a, b), the equation describing groundwater is considered stable over this period until 1980. After this date, by taking into consideration the piezometric drop in groundwater shown by hydrodynamic analysis (Ben Hamouda 2008), the same model can be written for a variation of the water supply:
Where: Hn represents the relative decrease (a dimensional value) of the groundwater supply in year n, compared to the initial supply of the aquifer in 1972, before the beginning of the long-term piezometric decrease.
Atmospheric history in tritium and carbon 14
During the 1950s and 1960s, nuclear testing significantly contributed to the amount of Tritium and Carbon 14 in the atmosphere (IAEA, WMO, 2015). In Tunisia, Tritium has been sampled in rainwater regularly since 1968. However, there have been no samples of 14C levels in the atmosphere. The input functions of the model (“Api” in both equations) were put together from stations near the study area (Nydal and Lovseth, 1996), (Fig. 12).
Since 1950, the input function for Tritium has been put together using the annual weighted rainwater values from the stations in the GNIP network of the IAEA such as the stations in Alexandria, Alger, Vienna, Tunis and Ottawa (1953–97) (Mook, 2001). The natural level at the beginning of the 1950s was estimated at nearly 5 TU (Kaufman and Libby, 1954). The recreated history of tritium (Fig. 12) is similar to that put together by Favreau in 2000.
For the input function of 14C, annual values from the African stations in Dakar, 1963–68, N’djamena, 1966–76, Tenerife, 1963–90 and from stations in the northern hemisphere from (Favreau, 2000) was used. Very little difference in 14C activity could be seen between each of these stations, similar to the hemispheric homogeneity of CO2 in the atmosphere.
Renewal rate and estimating recharge deduced from tritium levels
The model used in this study, takes into consideration the radioactive decrease in tritium and represents the annual change in the isotopic composition according to past volume and activity of the aquifer and infiltrated rainwater up to the sampling date of 1960. The annual renewal rate can be calculated for each representative elementary volume presumed homogenous from a few square kilometers above ground to a depth equal to the wet depth of the aquifer. Due to their smallness, whether or not horizontal transfers are taken into account does not significantly change the results (Fig. 13). To reduce the difficulties associated with a selective approach, the aquifer is considered based on its average characteristics in order to calculate the representative recharge rate in the study area. According to the average saturated thickness of the aquifer in 2001 (30 m) and the average (4.7 TU) and median (4.4 TU) values of Tritium, the median groundwater renewal rate is calculated at around 0.2% per year (Fig. 13).
Previous measurements of tritium were taken from the El Haouaria quaternary aquifer. Values varied from 13 to 32 TU (Lassoued 1982), their median was 20.5 TU for an average of 21.4 TU and a standard error of 5.6 TU. These results gave a median renewal rate of around 0.3% per year in 1980 (Fig. 12). Determining the renewal rate of the aquifer thus made it possible to calculate the recharge rate from the following equation: R = T x P x Rr (Cartwright et al. 2007), Where: T represents Thickness of aquifer in m and P the porosity in %.
Presuming a saturated depth of 30 m for the El Haouaria plain and a possible porosity of 14%, median recharge would be around 12 mm/year.
Renewal rate and estimating recharge deduced from carbon 14 levels
The carbon 14 measurements in the groundwater (10 in all), previously made in 1982, showed activity from 72 to 109 pMc (Lassoued 1982). Their median was 95 pMc with an average of 94 pMc and a standard error of 10 pMc. The new recent analyses supplement these measurements. The median 14C activity measured on the El Haouaria plain was 74 pMc with an average of 70 pMc and standard error of 9 pMc.
The renewal rate calculated for 1980 is nearly 0.2%. Focusing the calculations for the new data, the renewal rate is 0.04%, with a median value of activity of 74 pMc and an average of 73 pMc (Fig. 14). This median value thus leads to an annual recharge of around 3 mm/year, using the same hypotheses for calculating with tritium.
Discussion
In the El Haouaria aquifer, irrigation development that induces leaching of soils with dissolution of evaporate rocks (gypsum and halite minerals) was identified as the main source of mineralization contributing the qualitative degradation as the salinity of the groundwater. The stable isotopic (18O, 2H) results highlight the recharge of the shallow aquifer from current rainwater. There is no indication of presence of water enriched in 18O and 2H with the exception of evaporated water, and results do not show mixing with seawater. However, special attention has been given to the problem of possible future seawater intrusion into the wells due to their proximity to the Mediterranean Sea. Despite the relatively high uncertainty for Tritium, the similarity of the results can be considered a reciprocal validation of these two radioisotopes, which are very different in both nature and half-life. The differences between the results obtained for the calculation of the renewal rate from the model using tritium (Rr varies between 0.2 and 0.3 mm/year) and 14C (Rr varies between 0.04 and 0.2%) can be explained by the fact that:
First, the groundwater use in 1980 was not significant; therefore, was nearly equal to recharge in terms of volume. Currently, the abstraction has greatly expanded with irrigation becoming more frequent. Both of these factors encourage the mixing of old and new water through upward seepage following demand for deep water. The mixed water could come from returned irrigation water. These two factors explain the difference between the results in calculations of renewal rates from 14C between 1980 (Tr = 0.2%) and 2001 (Tr = 0.04%).
Secondly, a low ratio of old water, which is rich in dissolved inorganic carbon, compared to new water predominantly influences the final concentration of 14C in the mixture. This hypothesis is confirmed since a comparison of the calcium concentrations in the aquifer water in 1980 and 2001 showed a significant increase of Ca2+ levels over time. This increase in calcium could indicate a growing fraction of water oversaturated in calcium carbonate minerals due to higher residence time in the aquifer.
Thirdly, tritium, which is an intrinsic tracer for water molecules, its concentration depends solely on the ratio of volumes of water making up the mixture. Use of this radioisotope to determine renewal time is thus more reliable with respect to 14C. The 11% piezometric decrease of the El Haouaria aquifer since 1972 has not led to a change in the calculated renewal rate. In fact, the results obtained by the second model which uses water supply variation (piezometric decrease) in Eq. (2) did not provide any notable difference compared to the results obtained without a decrease by using eq. (1). For the El Haouaria above ground aquifer, calculating the renewal rate from the 3H model before the groundwater level started to decrease shows a probable range of 0.2 to 0.3%. In terms of water residence time in the aquifer, these values are equal to average flow-through times between 300 and 500 years. This is consistent with stable isotopes which indicate a mixture of recent water and water that is older than 4000 years BP. An attempt was also made to quantify the recharge of this region using other approaches. Piezometric observations made for the last 30 years or so in the Cap Bon show that the quaternary groundwater in El Haouaria aquifer has decreased (11%) but that this decrease is not generalized to all of the water wells. The quantification of the Cap Bon recharge was estimated at 36 mm/year by the water balance method (Ennabli 1980), 32 mm/year by Jemai, (Jemai 1998)18 mm/year by Paniconi (Paniconi et al. 2001) and 10 to 12 mm/year by the isotopic method. The recharge figures are less than those of 32 to 36 mm/year estimated by the water balance. The difference between the first method of estimating the annual recharge and the other methods could be primarily explained by the difference in the temporal observation scale: the water balance is strictly annual. Piezometric movement is the result of annual fluctuation and medium-term change. The isotopes are the result of a mixture occurring over several decades during which increased use, periods of drought and damage to vegetation cover greatly perturbed the balance of resources in underground water. However, the results are quite consistent.
Conclusion
In the El Haouaria aquifer, the water salinization seems to be acquired by dissolution of minerals in the aquifer system especially halite and gypsum. The stable isotopic (18O, 2H) and tritium results highlight the recharge of the shallow aquifer from current rainwater. Indeed, all of the water analyzed was high in tritium, thus indicating a recent infiltration of rainwater.
The interconnection between the deep and shallow aquifer was confirmed by stable isotopes (18O, 2H) and tritium which showed the presence of leakage from shallow towards the deep aquifer at Dar Chichou and in the opposite direction at Gareat El Haouaria. This is consistent with the lithology of the study area which shows no impermeable separations between the aquifer levels.
Concerning seawater intrusion, with the exception of evaporated water, the isotopic data confirmed the absence of enriched water similar to seawater, however special attention has been given to the problem of possible future seawater intrusion into the wells due to their proximity to the Mediterranean Sea.
The conceptual model using radioactive isotopes (3H and 14C) are interpreted in terms of renewal rate; the median was 0.2 to 0.3%, respectively, which means a recharge of about 12 mm/yr, which is consistent with other methods of estimating recharge. This weak infiltration is compatible with the stable isotope contents (18O and 2H) in groundwater, which show a mixing of old and recent waters. In addition to a better understanding of the local hydrogeological conditions, this study holds a methodological interest since it compares different approaches traditionally used to identify seawater intrusion in coastal aquifersand shows that a solid interpretation cannot be based on only one method, whether it be hydrodynamic or geochemical.
References
Aggarwal P, Froehlich K, Gonfiantini R, Gat JR (2005) Isotope hydrology: a historical perspective from the IAEA. In: Aggarwal PK, Gat JR, Froehlich KF (eds) Isotopes in the water cycle. Springer, Dordrecht. https://doi.org/10.1007/1-4020-3023-1_1
Ben Hamouda MF (2008) Hydrogeological, Geochimical and Isotopic Approach of the Coastal Aquifers systems Of Cap Bon: Case of the Eastern coastal and El Haouaria aquifer, Tunisia, 2008. PhD in Agronomic Sciences of the National Agronomic Institute of Tunisia [in french].
Ben Hamouda MF, Tarhouni J, Leduc C et al (2011) Understanding the origin of salinization of the Plio-quaternary eastern coastal aquifer of cap bon (Tunisia) using geochemical and isotope investigations. Environ Earth Sci 63:889–901. https://doi.org/10.1007/s12665-010-0758-1
Ben Hamouda MF, Carreira P, Marques JM, Egenkamp H (2013) Geochemical and isotopic investigations to study the origin of mineralization of the coastal aquifer of Sousse, Tunisia. Procedia Earth Planet Sci 7:61–64. https://doi.org/10.1016/j.proeps.2013.03.106
Ben Hamouda MF, Kondash AJ, Lauer N, Mejri L, Tarhouni J, Vengosh A (2015) Assessment of groundwater salinity mechanisms in the coastal aquifer of El Haouaria, northern Tunisia. Procedia Earth Planet Sci 13:194–198. https://doi.org/10.1016/j.proeps.2015.07.046
Bouchaou L, Michelot JL, Vengosh A, Hsissou Y, Qurtobi M, Gaye CB, Bullen TD, Zuppi GM (2008) Application of multiple isotopic and geochemical tracers for investigation of recharge, salinization, and residence time of water in the Souss–Massa aquifer, southwest of Morocco. J Hydrol 352:267–287. https://doi.org/10.1016/j.jhydrol.2008.01.022
Cartwright I, Weaver TR, Stone D, Reid M (2007) Constraining modern and historical recharge from bore hydrographs, 3H, 14C, and chloride concentrations: applications to dual-porosity aquifers in dryland salinity areas, Murray Basin, Australia. J Hydrol 332:69–92. https://doi.org/10.1016/j.jhydrol.2006.06.034
Celle-Jeanton H, Zouari K, Travi Y, Daoud A (2001) Isotopic characterisation of the precipitation in Tunisia. Variations of the stable isotope compositions of rainfall events related to the origin of air masses. CR Acad Sc Series IIA - Earth Planet Sci 333:625–631. https://doi.org/10.1016/S1251-8050(01)01671-8
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Lewis Publishers, New York 328 p
Coleman ML, Shepherd TJ, Durham JJ, Rouse JE, Moore GR (1982) Reduction of water with zinc for hydrogen isotope analysis. Anal Chem 54:993–995. https://doi.org/10.1021/ac00243a035
Coplen TB (1988) Normalization of oxygen and hydrogen isotope data. Chem Geol 72:293–297. https://doi.org/10.1016/0168-9622(88)90042-5
De Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhône delta (southern France). Appl Geochem 23:2337–2349. https://doi.org/10.1016/j.apgeochem.2008.03.011
Ennabli M (1980) Hydrogeologic study of the aquifers of the North-East of Tunisia for an integrated management of the water resources. PhD, University of Nice, France [in French].
Epstein S, Mayeda TK (1953) Variations of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224. https://doi.org/10.1016/0016-7037(53)90051-9
Favreau G (2000) Caractérisation et modélisation d'une nappe phréatique en hausse au Sahel: Dynamique et géochimie de la depression piézomktrique naturelle du kori de Dantiandou (sud-ouest du Niger). Ph.D, thesis, Department of Earth Sciences, University of Paris-Sud, Orsay, France.
Favreau G, Leduc C, Marlin C, Dray M, Taupin JD, Massault M, Le Gal La Salle C, Babic M (2002a) Estimate of recharge of a rising water table in semiarid Niger from 3H and 14C modeling. Groundwater. 40:144–151
Favreau G, Leduc C, Marlin C, Guéro A (2002b) Une dépression piézométrique naturelle en hausse au Sahel (Sud-Ouest du Niger). CR Geoscience 334:395–401. https://doi.org/10.1016/S1631-0713(02)01763-7
Ferchichi H, Farhat B, Ben Hamouda MF, Ben Mammou A (2017) Understanding groundwater chemistry in Mediterranean semi-arid system using multivariate statistics techniques and GIS methods: case of Manouba aquifer (northeastern Tunisia). Arab J Geosci 10:530. https://doi.org/10.1007/s12517-017-3314-4
Fontes JC (1971) Un ensemble destiné à la mesure de l’activité du radiocarbone naturel par scintillation liquide [a unit intended for the measurement of the activity of natural radiocarbon by liquid scintillation]. Rev Geogr Phys Géol Dyn 13(1):67–86
Fontes JC (1976) Isotopes du milieu et cycle des eaux naturelles. La Houille Blanche 3:4:205–221. https://doi.org/10.1051/lhb/1976010
Fontes JC, Garnier JM (1979) Determination of the initial 14C activity of the total dissolved carbon; a review of the existing models and a new approach. Water Resour Res 15:399–413. https://doi.org/10.1029/WR015i002p00399
Geyh MA (2000) An overview of 14C analysis in the study of groundwater. Radiocarbon. 42:99–114. https://doi.org/10.1017/S0033822200053078
Guo C, Shi J, Zhang Z et al (2019) Using tritium and radiocarbon to determine groundwater age and delineate the flow regime in the Taiyuan Basin, China. Arab J Geosci 12:185. https://doi.org/10.1007/s12517-019-4371-7
IAEA, WMO, (2015) Global Network for Isotopes in Precipitation. URL: https://www.iaea.org/services/networks/gnip Global Network of Isotopes in Precipitation (GNIP) | IAEA
Jemai S (1998) State evolution of the Korba aquifer: Hydrogeochemical study and numerical modelling, Master of Science, National Agronomic Institute of Tunisia. [in French]
Jones BF, Vengosh A, Rosenthal E, Yechieli Y (1999) Geochemical investigations. In: Bear J et al (eds) Seawater intrusion in coastal aquifers. Kluwer Academic Publisher, Dordrecht, pp 51–71. https://doi.org/10.1007/978-94-017-2969-7_3
Kamel S (2013) Salinisation origin and hydrogeochemical behaviour of the Djerid oasis water table aquifer (southern Tunisia). Arab J Geosci 6:2103–2117. https://doi.org/10.1007/s12517-011-0502-5
Kaufman S, Libby WF (1954) The natural distribution of tritium. Phys Rev 93:1337–1344. https://doi.org/10.1103/PhysRev.93.1337
Khomsi S, Ben Jemia MG, Frizon de Lamotte D, Maherssi C, Echihi O, Mezni R (2009) An overview of the late cretaceous–Eocene positive inversions and oligo-Miocene subsidence events in the foreland of the Tunisian atlas: structural style and implications for the tectonic agenda of the Maghrebian atlas system. Tectonophysics 475:38–582. https://doi.org/10.1016/j.tecto.2009.02.027
Khomsi S, Echihi O, Slimani N (2012) Structural control on the deep hydrogeological and geothermal aquifers related to the fractured Campanian-Miocene reservoirs of North-Eastern Tunisia for eland constrained by subsurface data. C R Géoscience 344:247–265. https://doi.org/10.1016/j.crte.2012.02.002
Khomsi S, Frizon de Lamotte D, Bédir M, Echihi O (2016) The late Eocene and Late Miocene fronts of the Atlas Belt in eastern Maghreb: integration in the geodynamic evolution of the Mediterranean domain. Arab J Geosci 9:650–670. https://doi.org/10.1007/s12517-016-2609-1
Khomsi S, Roure F, Khelil M, Mezni R, Echihi O (2019a) A review of the crustal architecture and related pre-saltoil/gas objectives of the eastern Maghreb atlas and tell: need for deep seismic reflection profiling. Tectonophysics 766:232–248. https://doi.org/10.1016/j.tecto.2019.06.009
Khomsi S., Roure F., Brahim N.B., Maherssi C., Arab M., Khelil M. (2019b) Structural Styles, Petroleum Habitat and Traps in the Pelagian-Sirt Basins, Northern Africa: An Overview and Future Exploration Developments. In: Rossetti F. et al. (eds) The Structural Geology Contribution to the Africa-Eurasia Geology: Basement and Reservoir Structure, Ore Mineralization and Tectonic Modelling. CAJG 2018. Advances in Science, Technology& Innovation (IEREK Interdisciplinary Series for Sustainable Development). Springer, Cham. https://doi.org/10.1007/978-3-030-01455-1_33
Lassoued S (1982) Hydrogéologie et géochimie isotopique de la plaine d’El Haouaria (Tunisie septentrionale). Thèse de doctorat de 3ème cycle. Université de Paris-Sud, France. 193p
Mook WG (2001) Environmental isotopes in the hydrological cycle, Principles and applications, 5 volumes, Unesco, IHP, Technical documents in hydrology, N° 39
Moussa AB, Salem SBH, Zouari K et al. (2017) Hydrochemical and stable isotopic investigation of groundwater quality and its sustainability for irrigation in the Hammamet-Nabeul basin, northeastern Tunisia. Arab J Geosci 10, 446 https://doi.org/10.1007/s12517-017-3233-4
Negrel P, Petelet-Giraud E (2011) Isotopes in groundwater as indicators of climate changes. Trends Anal Chem 30:1279–1290. https://doi.org/10.1016/j.trac.2011.06.001
Nydal R, Lovseth, K (1996)Carbon- measurements in atmospheric CO, from northern and southern hemisphere sites, 1962–1993. Oak Ridge National Laboratory NDP-057. https://doi.org/10.2172/461185
Paniconi C, Khlaifi I, Lecca G, Giacomelli A, Tarhouni J (2001) Modeling and analysis of seawater intrusion in the coastal aquifer of eastern cap-bon, Tunisia. Transp Porous Media 43(1):3–28. https://doi.org/10.1023/A:1010600921912
Riou C (1980) A simple empiric formula for estimating potential evapotranspiration in Tunisia. Cah. O.R.S.T.O.M., sér. Hydrol., vol. XVII, 2:129–137 [in french]
Tamers MA (1975) Validity of radiocarbon dates on groundwater. Geophys Surv 2:217–239. https://doi.org/10.1007/BF01447909
Tarhouni J, Ben Hamouda MF, Kondash AJ, Mejri L, Vengosh A (2015) Modeling the recharge and the renewal rate based on 3H and 14C isotopes in the coastal aquifer of El Haouaria, northern Tunisia. Procedia Earth Planet Sci 13:199–202. https://doi.org/10.1016/j.proeps.2015.07.047
Taylor CB (1977) Tritium enrichment of environmental waters by electrolysis: Development of cathodes exhibiting high isotopic separation and precise measurement of tritium enrichment factors. In: Proc. of the Int. Conf. of Low-Radioactivity Measurements and Applications. Slovenski Pedagogicke Nakladatelstvo, Bratislava, Slovakia, p. 131–140
Vengosh A (2014) Salinization and saline environments, reference module in earth systems and environmental sciences. In: Treatise on geochemistry, vol 11, 2nd edn, pp 325–378. https://doi.org/10.1016/B978-0-08-095975-7.00909-8
Zuber A, Weise SM, Osenbruck K, Pajnowska H, Grabczak J (2000) Age and recharge pattern of water in the Oligocene of the Mazovian basin (Poland) as indicated by environmental tracers. J Hydrol 233:174–188. https://doi.org/10.1016/S0022-1694(00)00224-9
Acknowledgements
This study was funded by the IAEA Technical Cooperation Project TUN/8/015 and TUN/8/017 “Assessment of Marine Intrusion in Cap Bon’s Coastal Aquifer Systems.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
This article is part of the Topical Collection on Geology of North Africa and Mediterranean regions
Rights and permissions
About this article
Cite this article
Ben Hamouda, M.F. Study of the groundwater salinity and modeling the recharge and the renewal rate based on stable and radioactive isotopes in El Haouaria aquifer, Tunisia. Arab J Geosci 14, 2246 (2021). https://doi.org/10.1007/s12517-021-08465-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-08465-6