Abstract
This present study was aimed at investigating the effects of mining activities on groundwater quality of Enyigba-Ameri, Nigeria. Assessment of the impacts of mining activities on trace metal pollution in water was carried out using modified trace metal pollution index (m-TPI) and modified ecological risk index (m-RI). Water samples were collected during the pre-mining (PRM) and post-mining (POM) and analyzed for their physicochemical and trace element concentrations. The computed m-TPI for the PRM has a mean positive index (PI) value of 0.950 and a negative index (NI) mean value of −0.081. The m-TPI for POM has a mean PI value of 126.002 and NI mean value of −0.001. The m-RI for Pb+Zn ranged from 7.769 to 175.090 at the POM, and the m-RI for Cd+Zn for the PRM ranged from 8.772 to 47.349. The m-PI results revealed that all the water samples during the PRM were good for drinking and domestic uses but were all of “unacceptable quality” during the POM. Also, the results showed that 12.5%, 50%, 25%, and 12.5% of the water samples were of very high, high, moderate, and low Cd+Zn ecological risk respectively during the PRM. But during the POM, 50%, 12.5%, 12.5%, and 25% of the samples belong to very high, high, moderate, and low Pb+Zn ecological risk respectively. The decrease in the quality of water from the PRM to the PRM suggests anthropogenic pollution of mining activities. Therefore, legislation against indiscriminate waste discharges is recommended to reduce the adverse effects on the water resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining of Sphalerite (ZnS) and galena (PbS) for Pb-Znin Enyigba-Ameri area started in the year 1900 by the natives as artisan miners. The mined Pb-Zn was used as cosmetics and fishing net weights (Offodile 1980). Commercial mining in the study area began in 1925 when a British company started underground and open-cast mining of sphalerite and galena at the Enyigba and Ameri fields respectively. In the year 1945, mining activities were taken over by a German company which used open-cast method, and the PbS and ZnS were beneficiated at the site by differential floatation using xanthate collector. However, active commercial mining and artisan mining by the natives ceased at the onset of the Nigerian Civil War in 1967. Commercial mining resumed in 2011 by a Chinese company using open-cast mining technique. In addition to the Chinese company, several local companies have been licensed to mine in the area. Usually, untreated mine water are introduced directly into the nearby surface water bodies, and abandoned mine pits are left without any treatment and no land recovery processes carried out. These pits have long become cesspools.
Trace metals could be released into groundwater through geogenic sources and anthropogenic sources (Adaikpoh et al. 2005; Karbassi et al. 2007; Reza and Singh 2010; Houria et al. 2020). In the natural state, trace metal contamination is usually at low concentrations (except in some special cases) and could be released from weathering and dissolution of minerals of the host rocks. Anthropogenic contamination usually results to very high metal concentrations in groundwater and the environment (Reiners et al. 1975; Charles et al. 1994; Karbassi et al. 2008; Obianyo 2019) and are derived from sources such as mining, industrial effluents, indiscriminate use of fertilizer and pesticides laden with trace metals in agricultural fields, and municipal solid and liquid wastes (Ammann et al. 2002; Nouri et al. 2008). Groundwater quality can be degraded through excessive abstraction and contamination that emanates from discharge of untreated mine water (Abou-Zakhem and Hafez 2015; Hussain and Al-Fatlwai 2020). Most trace metals pose threats to human body, ecosystem, and the environment because they are non-biodegradable and accumulate in the human system causing damage to the various internal organs and human systems in general (Lee et al. 2007; Lohani et al. 2008; Guiamel and Lee 2020). Only few (such as Cu, Se, and Zn) at low concentrations are known to be of nutritional importance to plants and animal growth and development (Kar et al. 2008; Suthar and Singh 2008; Aktar et al. 2010; Nkansah et al. 2019).
Mining of lead and zinc ores in the Enyigba-Ameri area is carried out using the open-cast mining method. The area is characterized by active mine pits and abandoned mine pits which have since become artificial ponds and pits. The artificial ponds and pits during the peak dry season serve as sources of drinking water and water for domestic and agricultural purposes for the teaming population. Trace metals from tailings emanating from mining activities of the area are thought to have some adverse environmental impact especially on groundwater and the ecosystem. The impacts of mining activities on groundwater resources can be assessed by the use of reference materials and enrichment calculation methods (Salomons and Förstner 1984; Abrahim and Parker 2008). Several researchers have reported the use of reference materials in evaluating the effects of anthropogenic activities on groundwater quality of the Enyigba-Ameri area (Okogbue and Ukpai 2013; Opoke and Osayande 2018; Okolo et al. 2018; Aloke et al. 2019; Omonona et al. 2019; Iroha et al. 2020) and very few reports are available on the use of enrichment methods (Nnabo 2015; Obasi et al. 2018). All the researchers who have used the existing enrichment calculation methods (pollution indices) for the evaluation of the impacts of mining activities on groundwater of the Enyigba area have done so without considering the limitations that are associated with these calculation methods. In the present study, one modified pollution index and a newly proposed modified ecological index that take care of the limitations of the exciting pollution indices are introduced, which is the gap this research intends to cover. In addition to this, this research intends to use two-time groundwater sampling campaigns or programs instead of a one-time sampling campaign which all the previous works have used. The present study, therefore, is aimed to (i) identify the sources of major cations and anions in groundwater, and (ii) evaluate the impacts of mining activities on trace metal concentrations in groundwater Enyigba-Ameri area and assess the risk associated with consumption of contaminated water using the modified trace metal pollution index and the newly proposed modified ecological risk index. The findings of this research are hoped to help the different environmental stakeholders that are concerned with water quality management and land use planning.
General description of area
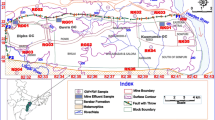
The Enyigba-Ameri Pb-Zn metallogenic hub is situated in the rural communities of the present day Ebonyi State, southeastern Nigeria (latitude 6° 07′ N–6° 14′ N and longitudes 8° 06′ E–8° 12′ E) and cover a total area of 33.06 km2 (Figure 1).Enyigba-Ameri is located southeast of the Abakaliki city at about a distance of 16 km away. The area has a population of 8000 inhabitants (2006 census) with majority of the inhabitants engaged in farming and mining. Agricultural activities in the area involved growing of vegetables (pumpkin) near streams and food crops (rice, cassava, and maize) in different farmlands scattered all over the area. During the dry season when the surface water (streams) levels drop, mine water are used for irrigation purposes.
Enyigba-Ameri area lies in an area of moderate relief that range from 125 to 250 m above mean sea level (Inyang 1975). Ebonyi River and its ephemeral tributaries drain the area. The average annual precipitation and temperature varies from 250 to 2125 mm and from 27 to 28 °C respectively.
Geologically, the area is underlain by two formations, the Tertiary to Quaternary alluvium and the Cretaceous Abakaliki Shale. The alluvium consists of loose gravel, sand, and clay. The Abakaliki Shale consists of shale, with lenses of limestone and sandstone. A type section of the Abakaliki Formation located in Enyigba mine is shown in Figure 2. In this section, sandstone overlies the basement rock unconformably. The sandstones are very hard. The sandstone bed is usually gray in color and thin ranging from 5 to 50 mm thick. Sandstone beds are rich in gastropods and molluscs. Generally, there are two units of shale rocks in the Abakaliki Formations. The basal shale unit (Unit E) is black in color and very compact; it is calcareous and carbonaceous in nature possessing high organic content, and it is very fissile. This shale unit is thinly laminated and fractured in places. In this unit, the shale trend in the NE-SW direction and a dip direction in NW-SE with a dip amount ranging from 4 to 32°. The second shale unit (Unit F) overlies the basal unit. This younger shale unit varied from grayish brown to pinkish brown in color. It is thinly laminated, fissile, and calcareous. Unit F generally trend in the NE-SW direction and dips in NW-SE direction with dip amount ranging from 18 to 52°. Abakaliki Shale is known to be very rich in ammonite fauna. Common ammonite species found in the shale are Diploceras quadratum, Hysteroceras binum, and Elobiceras lobitoense (Nwajide, 20..). Also, Abakaliki Shale is rich in clay minerals, and the shale is rich mainly in chlorite, illite, and kaolinite. Aguman (1989) observed that illite and chlorite increase with depth of burial in the Abakaliki Shale. Diagenetic conversion of kaolinite to illite and chloride may explain the enrichment of chlorite and illite with depth (Dunoyer de Segonzac et al. 1967). The lateritic top soils constitute the uppermost rock unit. The lateritic soil is highly ferruginized. A type section of the Abakaliki Formation with a very thick limestone bed located at a quarry 16 km west of Abakaliki town is shown in Figure 3. The limestone lithology of the Abakaliki Formation is black and micritic, and is composed of grapestones, infralimeclasts, and brown algae remains (Abatan et al. 1993). The Abakaliki Shale has an average thickness of 500 m, and it strongly folded and fractured in places. The overburden layers consist of lateritic soil and weathered shale and which vary in thickness from 18.5 to 20.0 m. The shale mineralogical composition includes clay minerals, quartz, chert, feldspars, carbonates, and iron oxides. The Pb-Zn ores (galena, sphalerite, and chalcopyrite) are located as veins within the black Albian shale. The depths to the NE-SW trending veins that bear the Pb-Zn ores usually range from 8.0 to 15.0 m from the ground surface (Obiora et al. 2016). Siderite and calcite are associated gangues of the main ores. The southern Benue Trough during the Cretaceous experienced intense volcanic activities. The presence of baked shales, pillow lava structures, and pyroclastics in Abakaliki area are evidences of intense magmatic activities in the southern Benuede Trough. Groundwater is usually abstracted from fractured shale which acts as aquifers, and the aquifers occur at depth ranging from 45 to 68 m. The fracturing of the shale rock reduces with depth, and it is not regional in extent. In the study area, there is no major perennial river that flows within the area; the area is drained by ephemeral streams which serve as tributary to the perennial Ebonyi River located in Ebonyi State. The groundwater is usually recharged via precipitation.
Materials and methods
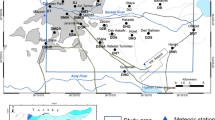
Groundwater samples were collected using a steel bailer from eight locations scattered across the study area from boreholes that have not failed over the past three decades used by the locals for drinking and domestic purposes. Eight groundwater samples were collected in 2010 during the pre-mining period (PRM) from 8 different boreholes, and eight groundwater samples from the same boreholes were collected in 2016 during the post mining period (POM), an interval of 7 years.
Prior sampling, each aquifer was pumped for a considerable time to allow groundwater from the aquifer to be sampled. To avoid cross contamination, each sterile high-density polyethylene bottles was first rinsed with the water to be sampled. Water sample collected were filtered using 0.45-μm filter and preserved by acidifying with ultrapure nitric acid (pH< 2). The groundwater samples collected were analyzed for their physicochemical characteristics and trace metal contents. Transient parameters (temperature; electrical conductivity, EC; total dissolved solids, TDS; pH) were measured in situ at the time of sample collection using Hanna (H19828 USA) multi-parameter digital meter. All the laboratory analyses were conducted at the Chemistry Department, Water Supply and Sanitation Project, Ibadan, Nigeria. The analytical procedures used in the determination of the major ions and trace metal concentrations are in accordance with the APHA (2005). Reagent blank determinations were used to correct the instrument readings. The general preliminary assessment of the groundwater quality was carried out with respect to WHO (2011) standard. The comparative study of the water quality of the pre-mining period (PRM) with the post-mining time (POM) was carried out using modified water quality indices namely, modified pollution index (m-PI), modified trace metal pollution index (m-TPI), and modified ecological risk factor (m-ERF).
The weighted arithmetic indices, m-TPI, and m-ERF, were employed in this study because of their suitability in assessing water quality. The choice of weighted arithmetic method is premised on the following edge it has over the other methods: application of quality parameters that can be turned into mathematical equation that give rating and grading to the water sources, policy makers, and citizens can easily use this index for communication of overall water quality, assurance about suitability of water for human consumption, and different parameters that can be used with their composition that is important for assessment and management of water quality (Phadatare and Gwande 2016).
Two pollution indices, trace metal pollution index (Mohan et al. 1996) and potential ecological risk index (Hakanson 1980), based on trace metal contents, have been widely used for groundwater quality monitoring and characterization purposes (ref) but they are not without some short comings.
Two of the defects of the TPI technique are imbedded in the calculation of the sub-index (Qi). The first defect involves the use of absolute difference between measured concentration and permissible guideline value{Mi(−)Ii}. When absolute difference between Mi and Ii is used (instead of the real value) in estimating TPI, the value of TPI may be high even when Ii is greater thanMi. The second defect is associated with the expression, Si − Ii of Eq. 2. It has been observed that not all organization saddled with the responsibility of providing guideline values for trace metals provide both Si and Ii stipulated values. For examples, the Standard Organization of Nigeria (SON 2017) and the World Health Organization (WHO 2011) did not provide values for both Si andIi, thereby making SON and WHO standards not suitable for calculating TPI using Eqs. 1 and 2. It is because of these flaws that are associated with the use of TPI for evaluating water quality that Chaturvedi et al. 2018 introduced a modified trace metal pollution index (m-TPI) to cater for the defects associated with the original trace metal pollution index, and provide a better index for evaluating the quality of water for drinking and domestic purposes. This new index is calculated as follows (Chaturvedi et al. 2018):
where, m − TPIi is the modified trace metal pollution index; φi is the relative weightage factor and it is defined by the expression:
where Wi is estimated from the relationship:
The proportionality constant is taken as 1 for all the trace meters in this present study.
The sub-index, αi for all the trace metals is defined as follows
The WHO (2011) standard guideline values for trace metals were used as the highest desirable concentration (Ii).
It should be noted that the numerator in Eq. 2 is a modulus while that of Eq. 7 is not. Therefore, αi will be positive if Mi>Ii, αi will be zero when Mi=Ii, and αi will be negative when Mi<Ii, implying that the sample passes the quality test for the given trace metal.
In this new index, every water sample is defined by two separate indices, a positive index (PI) and a negative index (NI) of m − TPIi. In this modified index, all PIs and Nis of each water sample are added separately but not together to obtain two composite numbers for PI and NI. Based on the two indices of m − TPIi, water quality may be classified as follows: Excellent (−1≤NI≤0 and PI=0); Very good to Good (−1<NI≤0 and 0<PI≤UL); and Unacceptable (NI≤0 and PI>UL), where UL is the upper limit of the positive index.
In this present study, we proposed a new index known as modified potential ecological risk index (m-RI) for evaluating aggregate ecological risk caused by trace metal contamination. This modified index has two advantages over the original ecological risk index (RI) approach. Firstly, it takes into consideration the synergistic effects of different trace metals of any water sample which RI does not have. Secondly, it calculates the risks associated with an ecosystem using a source-specific factor derived from multivariate analysis (factor analysis). The modified ecological risk index (m-RI) thus has the ability to estimate the synergetic effects of two or more contaminants emanating from the same source(s). Modified ecological risk index (m-RI) can be calculated as follows:
where ϑn is the reciprocal of the derived eigenvalue of trace metal concentrations only, Mi is the measured concentration of trace metal i. Ii is the highest desirable limit of the trace metal i, and it is equivalent to the WHO (2011) maximum acceptable concentrations of trace metals. Ti is the toxic-response factor of the trace metal i and n is the number of the trace metals emanating from the same sources. The toxic response factor (Ti) for Cd, Pb, and Zn are 30, 5, and 1 respectively (Hakanson, 1980; Xu et al. 2008). The classification scheme for the proposed index was based on equal count (quantile) of the index values and this was carried out using the Q-GIS software. The scheme is as follows: very low risk (1.5–3.0); low risk (3.0–9.0); moderate risk (9.0–22.8); high risk (22.8–42.2); and very high risk (42.2–175.0). The eigenvalue is obtained from principal component analysis (PCA). PCA is a statistical technique that transforms a large number of variables (parameters) in a data set into a smaller number of rational and unrelated components. The defined components consist of linear weighted combinations of the initial parameters. The number of components extracted was based on the number of components that have Eigenvalues greater or equal to one. Usually, the first extracted component is always found to have the highest eigenvalue value and also explains the maximum variation in the original data set. The second component will always have lesser eigenvalue and explains a lesser variation in the original data set than the first component, and the third component will also have a lesser Eigenvalue than the first and second components and so on. The various statistical analyses used in the present study were conducted using Statgraphic Centurion XI. The stage-by-stage processes used for this study are presented in the methodology flowchart (Figure 4).
Results and discussion
Aquifer characteristics and groundwater flow
The boreholes sampled were drilled to depths ranging from 32.1 to 85.0 m with an average depth value of 48.4 m using rotary method. The boreholes were well screened and cased using PVC screens and PVC casing respectively. The aquifer thicknesses ranged from 19 to 60.8 m. The aquifer parameters—transmissivity, hydraulic conductivity, and specific yield varied from 0.117 to 1.295 m2/day, 0.006 to 0.014 m/day, and 1.22 × 10−3 to 1.8 × 10−3 respectively. Static water level (SWL) ranged from 4.5 to 13.8 m during the PRM and from 4.8 to 14.5 m during the POM. The SWL results revealed a decrease in SWL (an appreciation in the depth to water level in a well) of some of the boreholes during the POM, which may be attributed to increase in recharge emanating from mining wastewater released into the environment. From the static water level data measured from the wells and the elevations above the mean sea level of the well location, the hydraulic heads of all the sampled boreholes were estimated. The hydraulic head maps for the PRM and POM periods are presented in Figure 5. The figure revealed that groundwater flows from the northwestern and northeastern sections into the southwestern and southeastern sections. Figure 5 also revealed that groundwater flow direction pattern during the PRM and POM is the similar. Groundwater samples S2, S4, S6, and S8 referred to diverging points, and samples S1, S3, S5, and S7 are converging points. The hydrogeological cross section across A and A line of section of the study area is presented in Figure 6.The cross section shows that S4 and S8 are recharging zone while S5 and S3 are in the discharging zone.
Physicochemical parameters and major ion characteristics
The descriptive statistics of physicochemical parameters, major ions, and trace metals are presented in Tables 1 and 2. ANOVA tests carried out revealed that there is significant difference between the means of K and NO3 during the PRM and POM periods (Table 3). NO3 showed disparity in mean, variance, and median during the two time periods (Table 3).The means of K concentrations during the PRM and POM are 22.23 mg/l and 7.87 mg/l respectively, and the means of the concentrations of NO3 during the POM are 54.96 mg/l and 0.95 mg/l respectively. The sources of NO3 and K may be attributed to application of chemical fertilizers; details of the sources of ions are discussed in later sections. During the PRM period, agricultural activity was the predominant activity but there was a decline in agricultural activities during the POM period as attention was shifted to mining activities. The ionic concentrations of the major ions during PRM and POM periods are in the order of abundance\( :{\mathrm{Ca}}^{2+}>{\mathrm{HCO}}_3^{-}>{\mathrm{NO}}_3^{-}>{\mathrm{K}}^{+}>{\mathrm{Mg}}^{2+}>{\mathrm{Na}}^{+}>{\mathrm{Cl}}^{-}{\mathrm{SO}}_4^{2-} \) and \( {\mathrm{HCO}}_3^{-}>{\mathrm{Ca}}^{2+}>{\mathrm{Mg}}^{2+}>{\mathrm{Cl}}^{-}>{\mathrm{Na}}^{+}>{\mathrm{SO}}_4^{2-}>{\mathrm{K}}^{+}>{\mathrm{NO}}_3^{-} \)respectively.
Significant difference was also observed between the means of Fe (p value −0.038), Cd (p value −0.000), and Pb (p value −0.015) of the PRM and POM (Table 3). The significant differences in the measured means may be attributed to the effect of mining activities that was thought to release these trace metals. The concentrations of trace metals during the PRM and POM decreased in the order of Zn>Fe>Pb>Cd and Zn>Pb>Fe>Cd respectively.
Sources of ions and water classification
Gibbs diagram (Gibbs 1970), a plot based on ratio of (Cl/(Cl+HCO3) as a function of TDS (Figure 7), revealed that the major mechanism controlling the geochemistry of groundwater of the area is geology: water-rock interactions. To decipher the sources of the dissolved ions, different ionic ratio plots were created. The plot of the sum of Ca2+ and Mg2+ (Ca+Mg) against total cations (TZ+) (Figure 8a) showed that majority of the data plotted along the equilibrium line ([Ca+Mg] = [TZ+]), and the existence of high ratio of Ca+Mg over TZ+ points to the possibility of substantial contribution of Ca and Mg to the total cations. The plot of the ratio Ca+Mg and HCO3 (Figure 8b) showed that Ca, Mg, and HCO3 must have been produced from interactions of minerals rich in Ca and Mg with water as all the samples plotted above the theoretical line ([Ca]:0.5[HCO3]) of calcite and plagioclase dissolution (Omonona et al. 2019). Calcium, Mg, and HCO3 in water of the study area may be attributed to the dissolution of calcite and weathering of feldspar minerals in shale which underlies the area. However, a plot of Ca versus Mg (Figure 8c) revealed that all the samples occurred above the [Ca2+]:2[Mg2+] line, which points to the fact that silicate weathering affects the groundwater chemistry far more than calcite dissolution process.
The source of salinity in the area is revealed by plotting Na versus Cl (Figure 8d). A molar ratio of Na/Cl equals to one indicates halite dissolution while a ratio greater than one showed that Na ions were released into water mainly by silicate weathering process. Since none of the data point plotted on the halite dissolution line ([Na]:[Cl]) ruled out the possibility of Na ions being released by halite dissolution process. Na ion concentration in water of the area may be attributed to silicate weathering process.
Pearson’s correlation model shows how two or more variables are related (Adams et al. 2001). A correlation coefficient of any two variables determines how one parameter (variable) predicts the other by measuring the strength of linear relationships between two variables (Omonona et al. 2014). Any two variables with a p value less than 0.05 and a positive correlation coefficient will always have similar sources. Ca, Mg, Na, Cl, SO4, and HCO3 have positive correlation coefficient and p value <0.05 with TDS, and this shows that these ions were released from the same chemical source and through similar chemical process. K and NO3 have a p value less than 0.05 and positive correlation coefficient value, indicating that these ions were released from the same sources and through similar chemical processes. (Table 4) Both NO3 and K have p values > 0.05 and negative correlation coefficients with most of the major ions (Ca, Mg, Na, Cl, SO4, HCO3), indicating that NO3 and K were released from sources different from those of Ca, Mg, Na, Cl, SO4, and HCO3. It was also observed that the correlation coefficient values between the Fe-K, Pb-NO3, Pb-K, and Zn-K were negative, and their p values were less than 0.05, indicating that the Fe and K; Pb, NO3, and K; and Zn and K were released from different sources and through different chemical processes. While the major ions were thought to be released from weathering of host rock minerals, NO3 and K were thought to be released from application of chemical fertilizers and other associated agricultural activities.
Piper diagram gives details of the different hydrochemical facies present in the groundwater of the area. Piper diagram is a plot of the milliequivalent percentage of anions: HCO3+CO3, Cl, and SO4 on the anion-triangle and the cations: Ca, Mg, and Na+K on the cation-triangle. The cation and anion plotted points are further projected into the central diamond field that defines the different hydrochemical facies. Hydrochemical data from the study area plotted in four of the six subdivisions of the diamond-shaped fields (Figure 9), illustrating four water types namely, Ca-HCO3 water type, Ca-Mg-Cl-SO4 water type, Ca-Mg-HCO3 water type, and Na-K-Cl-SO4 water type. The bicarbonate (Ca-HCO3) water type is characterized by temporary water hardness; ten groundwater samples (four PRM samples (1, 2, 5, and 8) and six POM samples (1, 2, 3, 4, 5, 6,)) belong to the Ca-HCO3 hydrochemical facies.
Ca-Mg-Cl-SO4 water type (two PRM samples (3 and 4) and two POM samples (7 and 8)) and Ca-Mg-HCO3 water type (one PRM sample (7)) are named mixed water types as no major ion is dominant over the other. This water type is formed when fresh water undergoes simple dissolution or mixing (Ravikumar et al. 2015). Na-K-Cl-SO4 water type (one PRM sample (5)) indicates the dominance of alkali metals over alkaline earth metals and strong acidic anions over weak anions.
Saturation index (SI) is employed in understanding the mineral dissolution dynamics in groundwater system. SI values of minerals predict the pattern of water and mineral chemical equilibrium and rock-water reactions (Zhang et al. 2020). A mineral in groundwater will be in an unsaturated state with the surrounding environment if the mineral’s SI value is less than zero; it would be in a supersaturated state if mineral has SI greater than zero, and the mineral phase will be in an equilibrium state if the mineral SI value is equal to zero. For a mineral with SI value less than zero, the mineral will continuously be dissolved by the groundwater; the mineral will precipitate if the SI value is greater than zero; and no dissolution or precipitation will take place for a mineral with SI value equals to zero. The SI of a mineral can be calculated using the equation (Omonona et al. 2014): \( \mathrm{SI}=\log \left(\frac{K_{\mathrm{IAP}}}{K_{\mathrm{SP}}}\right) \) (where KIAP is the ion activity product for a mineral equilibrium reaction and KSP is KSP which is the solubility product of the mineral). The SI values of selected minerals (calcite, dolomite, gypsum, and halite) in groundwater were estimated using the PHREEQC software. The results of the SI of the selected minerals based on the hydrochemical data are presented in Table 5.
The values of the SI of calcite (SIcalcite) ranged from −3.08 to 0.09 and −1.36 to −0.45 for the PRM period and POM period respectively, and this shows that calcite mineral varied from unsaturation to supersaturation state during the two time periods. The values of SI of dolomite (SIdolomite) ranged from −7.33 to −0.16 during the PRM period and varied from −2.98 to 1.26 during the POM period, indicating that dolomite continuously weathered during the PRM period and varies gradually from undergoing dissolution and precipitation during the POM period. The values of SI of halite (SIhalite) and SI of gypsum (SIgypsum) during the PRM and POM periods are less than zero, indicating that halite and gypsum will continuously weather in groundwater during the PRM period and POM period.
Trace metal concentration
The trace metal concentrations in groundwater samples from the area and the maximum allowable concentration limits (MCL) are presented in Table 1. The concentration of Zn was 100% below the MCL in drinking water at the PRM time but 87.5% during the POM period. The concentration of Fe above the MCL in drinking water was found in 50% of the water sampled during the PRM and in 87.5% of the water sampled during the POM period. The Cd concentration in all the locations were higher than the MCL of 5 μg/l during the POM while only 50% of the locations had Cd concentration above the MCL in drinking water at the PRM time. During the PRM period, Pb concentration was above the MCL value of 15 μg/l in only 12.5% of the locations studied while at the POM period, all the locations have Pb concentrations above the MCL in drinking water. The positive correlation coefficient values and p values less than 0.05 between Fe and Cd, Fe and Pb, Fe and Zn, Cd and Pb, Cd and Zn, and Pb and Zn revealed that the sources of these trace metals may be attributed the mining activities in the area. The elevated concentrations of Pb in water of the area may be attributed to discharges from metal waste tailings acid mine drainage that is rich in metals. Obiora et al. 2016 also attributed high concentration of Pb in water sources of Enyigba area to elemental contributions from acid mine waters. In the present study, the effects of acid mine drainage on the groundwater are not well pronounced because of dilution that results from precipitation and influx from nearby tributaries. However, the effects of acid mine drainage are obviously observed on the streams and the vegetation around the immediate vicinities of the discharge of the acidic waste waters. Cu concentrations in water were determined during the PRM only; there is no data for Cu concentrations during the POM. The concentrations of Cu ranged from 0.198 to 0.967 with a mean of 0.684. All the water sampled has Cu concentrations below the MCL value (2.0 mg/l). The concentration of Cu in water of the area may be attributed to weathering of chalcopyrite mineral. The variation of trace metals between the two time periods (PRM and POM) of measurements showed that there are statistical differences in the means of Fe, Cd, and Pb (Table 3). The analysis of variance also revealed that there are statistical differences in the standard deviations of Cd, Pb, and Zn and statistical differences in the medians of Fe, Cd, and Pb (Table 3). One of the most significant factors that influence the mobility of metals in water is pH. Most metals are very mobile at low pH and become nearly immobile at high pH. A method of classification of water samples was developed by Ficklin et al. 1992 and modified by Caboi et al. 1999 which was based on the sum concentrations of metals as a function water pH. The sum of the trace metals Fe, Cd, Pb, and Zn was plotted in the Caboi diagram. From the diagram, all the groundwater plotted within the “near-neutral high acid” category (Figure 10). In the Enyigba-Ameri area, trace metal concentrations from groundwater compared well with mean concentrations of trace metals from other regions dominated by mining activities like Pradesh, India (Tiwari et al. 2013; Tadiboyina and Ptsrk 2016); Tripura, India (Brindha et al. 2020); Qinghai-Tibet Plateau, China (Wei et al. 2018); and Rosia Montana district, Romania (Florea et al. 2005). The POM trace metal concentrations of the Enyigba-Ameri area and those of the other regions were above the local guideline values and the WHO (2011) standards for drinking water. However, trace metal concentrations in groundwater from the coal mining regions of Dhanbad district, India, and Jerada Province, Morocco, were far lower than local guideline values and the WHO standards for drinking water (Battioui Mounia et al. 2013; Prasad and Mondal 2008).
Trace metal pollution
The results of the computed m-TPI (PI and NI) together with their descriptive statistics are presented in Table 6. The values of the positive index (PI) of the m-TPI ranged from 0.0172 to 2.043 with a mean value of 0.966 for the PRM and varied from 79.319 to 175.437 with a mean value of 126.277 for the POM period. The PRM values of the negative index (NI) of the m-TPI ranged from −0.369 to 0.000 with a mean value of −0.101, and the POM values of the negative index (NI) of the m-TPI varied from −0.005 to 0.000 with a mean value of −0.001. The m-TPI water quality rating scale revealed that water samples taken from Ameka 1, Ameka 2, Amegu 2, and Ishiagu 2 ranked as good and water samples taken from Amegu 1, Ameri 1, Ameri 2, and Ishiagu 1 ranked as good during the PRM period in the study area, and for the POM period, water samples taken from all the localities (Ameka 1, Ameka 2, Ameri 1, Ameri 2, Amegu 1, Am`egu 2, Ishiagu 1, and Ishiagu 2 ) ranked as unacceptable for drinking. The worst pollution status was recorded for samples taken from Ameka 1 (Well S1), Amegu 2 (Well S4), Ishiagu 1 (Well S7), and Ishiagu 2 (Well S8) (Table 6) during the POM period, and the high m-TPI values of these samples may be attributed to the effect of mining activities.
The effects of trace metals in groundwater on the ecosystem scenario of the study area were assessed using the modified ecological risk index (m-RI). Table 7 presents the results of the m-RI for the PRM and POM periods. During the PRM periods, two components were extracted from the PCA. The first component has an eigenvalue of 1.67, explains 41.93% of the variation of the total data set, and has high positive loading factors on Cd and Zn (0.681939, 0.545377). The second component has an eigenvalue of 1.16, explains 29.12% of the variation of the total data set, and has a high positive factor loading on Pb (0.65335) and a high negative loading factor on Fe (−0.624441). During the PRM period, the trace metals, Cd and Zn, were thought to be released from the same sources and via the same or similar processes. The main contributors of Cd and Zn could be agricultural activities and weathering of the bedrocks, and those of Pb and Fe may be weathering of bedrock minerals. During the POM period, only one component has eigenvalue greater than one, and so it was the only component that was extracted. This component explains 65.07% of the variation of the total data set and has high positive loading factors on Pb, Zn, and Fe. These trace metals could have been released from leaching of the ore minerals and mining activities.
During the PRM period, two categories of trace metals were identified Cd and Zn, and Pb and Fe. The calculated m-RI of Cd and Zn during the PRM period ranged from 8.77 and 47.35 with a mean value of 29.77. Since there is no toxic response set for Fe, only Pb was used in the estimation of m-RI for the second category of metals (Pb and Fe). The m-RI of Pb ranged from 1.49 to 5.36 with a mean value of 2.83. During the POM period, only one group of trace metals was identified and it comprises Pb, Zn, and Fe. The m-RI for Pb and Zn during the POM period ranged from 5.17 to 116.71 with a mean value of 46.08. Mean deviation and percentage deviation from the mean of m-RI (Pb), m-RI (Cd + Zn), and m-RI (Pb+Zn) values were calculated for all the sampling points for the PRM and POM periods (Table 8). Four sampling points (50%) of groundwater have lower m-RI (Pb) than the mean value; three sampling points (37.5%) of groundwater show lower m-RI (Cd+Zn) than the mean, and five sampling points (62.5%) of groundwater indicate lower m-RI (Pb+Zn) than the mean values. The 12 sampling points (Table 8) with negative percentage deviation from mean values reflect a better quality of groundwater with respect to trace metals (Prasad and Bose 2001).
Following the proposed classification, it was observed that during the PRM period, seven of the groundwater samples (Ameka 1, Amegu 1, Amegu 2, Ameri 1, Ameri 2, Ishiagu 1, and Ishiagu 2) belong to very low Pb ecological risk, and one groundwater sample (Ameka 2) belongs to low Pb ecological risk class. One groundwater sample (Ameri 2), two groundwater samples (Amegu 1 and Ameri 1), four groundwater samples (Ameka 1, Ameka 2, Ishiagu 1, and Ishiagu 2), and one groundwater sample (Amegu 2) belong to low Cu+Zn ecological risk, moderate Cu+Zn ecological risk, high Cu+Zn ecological risk, and very high Cu+Zn ecological risk respectively. During the POM period, one groundwater sample (Amegu 2), two groundwater samples (Ameka 2 and Ameri 1), and five groundwater samples (Ameka 1, Amegu 1, Ameri 2, Ishiagu 1, and Ishiagu 2) belong to (Pb+Zn) low ecological risk, moderate ecological risk, and very high ecological risk respectively.
The scheme is as follows: very low risk (1.5–3.0); low risk (3.0–9.0); moderate risk (9.0–22.8); high risk (22.8–42.2); and very high risk (42.2–175.0). Analysis shows that out of the 12 sampling points of groundwater that have negative percentage from the mean of m-RI, four groundwater samples (Amegu 1, Ameri 1, Ameri 2, and Ishiagu 2), two groundwater samples (Ameri 1 and Amegu 2), three groundwater samples (Amegu 1, Ameri 1, and Ameka 2), one groundwater sample (Ameri 1), and one groundwater sample (Amegu 1) belong to very low ecological risk, low ecological risk, moderate ecological risk, high ecological risk, and very high ecological risk respectively. Comparing the two time periods, it was observed that during the POM period, four of the eight samples (Ameka 1, Amegu 1, Ishiagu 1, and Ishiagu 2) belong to very high ecological risk while one of the eight samples (Amegu 2) during the PRM period belong to very high ecological risk. This showed that groundwater was more contaminated during the POM period than the PRM period.
The water from sampling points S1 (Ameka 1), S7 (Ishiagu 1), and S8 (Ishiagu 2) both have very high ecological risks and ranked as unacceptable for drinking, although S1 (Ameka 1) has low ecological risk but ranked unacceptable for drinking, there is to a large extent a resemblance between the two indices.
Conclusions
This study demonstrated that the modified pollution indices used as relevant tools for assessing the effects of mining activities on groundwater quality. In this study, hydrochemical data from Enyigba-Ameri area plotted in Gibbs diagram and cross plots, and in addition to saturation indices of selected minerals showed that rock weathering process, calcite and halite dissolution, and weathering of silicate minerals are the principal geochemical processes that affect the chemistry of groundwater of the area.
The present study revealed that the concentrations of the trace metals in groundwater of the area increased progressively from the PRM period to the POM period. Based on the results of the two indexing approaches (m-TPI and m-RI), the trace metal concentrations in the groundwater of the Enyigba-Ameri area are more polluted during the POM period compared to the PRM period. Groundwater during the PRM period mostly has low pollution status while the POM period has moderate pollution and high pollution status. Pollution of groundwater during the POM period can be the effect of mining activities.
These modified-pollution indices have shown to be effective for assessing the impact of mining activities on groundwater quality. The result of this study though typical for the present study area, may also be useful in assessing the adverse effects of mining activities on the quality of groundwater of other regions. We recommend that groundwater planning and development should be in areas outside the metallogenic nucleus as the cost of remediating contaminated aquifers may not be cost-effective.
References
Abou-Zakhem B, Hafez R (2015) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci 73:6591–6600. https://doi.org/10.1007/s12665-014-3882-5
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238. https://doi.org/10.1007/s10661-007-9678-2
Adaikpoh EO, Nwajei GE, Ogala JE (2005) Heavy metals concentrations in coal and sediments from river Ekulu in Enugu, Coal City of Nigeria. J Appl Sci Environ Manag 9(3):5–8. https://doi.org/10.4314/jasem.v9j3.17343
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241(1–2):91–103. https://doi.org/10.1016/S0022-1694(00)00470-X
Aktar MW, Paramasivam M, Ganguly M, Purkait S, Sengupta D (2010) Assessment and occurrence of various heavy metals in surface water of Ganga River around Kolkata: a study for toxicity and ecological impact. Environ Monit Assess 160(1–4):207–213. https://doi.org/10.1007/s10661-008-0688-5
Aloke C, Uzuegbu IE, Ogbu PN, Ugwuja EI, Oriya OF, Obasi IO (2019) Comparative assessment of heavy metals in drinking water sources from Enyigba community in Abakaliki Local Government Area, Ebonyi State, Nigeria. Afr J Environ Sci Technol 13(4):149–154. https://doi.org/10.5897/AJEST2018.2517
Ammann AA, Michalke B, Schramel P (2002) Speciation of heavy metals in environmental water by ion chromatography coupled to ICP-MS. Anal Bioanal Chem 372(3):448–452. https://doi.org/10.1007/s00216-001-1115-8
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Brindha K, Paul R, Walter J, Tn ML, Singh MK (2020) Trace metal contamination in groundwater and implications on human health: comprehensive assessment using hydrogeochemical and geostatistical methods. Environ Geochem Health 42:3819–3839. https://doi.org/10.1007/s10653-020-00637-9
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb-Zn Montevecchio-Ingurtosu mining district, Sardinia, Italy. Chron Rech Miniere 534:21–28
Charles TD, James KO, Akeiverfeldt (1994) Biogeochemistry of small catchments: a tool for environmental research. In: Moldan B, Cemy J (eds) Trace metals speciation and cycling, 13th edn. Wiley, UK
Ficklin WH, Plumee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water-rock interaction, vol 7. Balkema, Rotterdam, pp 381–384
Florea RM, Stoica AI, Baiulescu GE, Capota P (2005) Water pollution in gold mining industry: a case study in Rosia Montana district, Romania. Environ Geol 48:1132–1136. https://doi.org/10.1007/s00254-005-0054-7
Guiamel IG, Lee HS (2020) Watershed modeling of the Mindanao River Basin in the Philippines using the SWAT for water resources management. Civ Eng J 6(4):626–646. https://doi.org/10.28991/cej-2020-03091496
Houria B, Mahdi K, Zohra TF (2020) Hydrochemical characterisation of groundwater quality: Merdja Plain (Tebessa Town, Algeria). Civ Eng J 6(2):318–325. https://doi.org/10.28991/cej-2020-03091473
Hussain TS, Al-Fatlwai AH (2020) Remove chemical contaminants from potable water by household water treatment system. Civ Eng J 6(8):1534–1546. https://doi.org/10.28991/cej-2020-03091565
Inyang PBE (1975) Climate in Ofomata Gek. In: Nigeria in maps, Eastern State. Ethiope Pub. House, Benin City, pp 25–26
Iroha IR, Ude IU, Okoronkwo C, Ovia k, Okafor COO, Akuma SO (2020) Comparative assessment of physicochemical characteristics, metal levels and anion contents of water from different aquatic environments in Ebonyi State Biomed J Sci Tech Res 29(5):22834–22864.https://doi.org/10.26717/BJSTR.2020.29.004868
Kar D, Sur P, Mandal SK, Saha T, Kole RK (2008) Assessment of heavy metal pollution in surface water. Int J Environ Sci Technol 5(1):119–124. https://doi.org/10.1007/BF03326004
Karbassi AR, Nouri J, Ayaz GO (2007) Flocculation of trace metals during mixing of Talar river water with Caspian Seawater. Int J Environ Res 1(1):66–73
Karbassi AR, Monavari SM, Nabi Bidhendi GR, Nouri J, Nematpour K (2008) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147(13):107–116. https://doi.org/10.1007/s10661-007-0102-8
Lee CL, Li XD, Zhang G, Li J, Ding AJ, Wang T (2007) Heavy metals and Pb isotopic composition of aerosols in urban and suburban areas of Hong Kong and Guangzhou, South China evidence of the long-range transport of air contaminants. Environ Pollut 41(2):432–447. https://doi.org/10.1016/j.atmosenv.2006.07.035
Lohani MB, Singh S, Rupainwar DC, Dhar DN (2008) Seasonal variations of heavy metal contamination in river Gomti of Lucknow city region. Environ Monit Assess 147(1–3):253–263. https://doi.org/10.1007/s10661-007-0117-1
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31(2):283–289. https://doi.org/10.1080/10934529609376357
Mounia B, Mostapha B, Rachid H, Hassan B, Abdelhakim J, Mohamed S (2013) Impact of mining wastes on groundwater quality in the Province Jerada (Eastern Morocco). Int J Eng Sci Technol 5(8):1601–1615
Nkansah MA, Donkoh D, Akoto O, Ephraim JH (2019) Preliminary studies on the use of sawdust and peanut shell powder as adsorbents for phosphorus removal from water. Emerg Sci J 3(1):33–40
Nnabo PN (2015) Assessment of contamination of underground water sources in Enyigba Pb-Zn district, southeastern Nigeria using metal enrichment and pollution indices. Int J Therm Sci 4:46–57. https://doi.org/10.18483/ijSci.825
Nouri J, Mahvi AH, Jahed GR, Babaei AA (2008) Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environ Geol 55(6):1337–1343. https://doi.org/10.1007/s00254-007-1081-3
Obasi PN, Esom NE, Okolo CM, Edene EN (2018) Assessment of water pollution status in the mining area of Ameka, south eastern Nigeria using metal pollution index. Int J Sci Eng Sci 2(1):66–73
Obianyo JI (2019) Effect of salinity on evaporation and the water cycle. Emerg Sci J 3(4):255–262. https://doi.org/10.28991/esj-2019-01188
Obiora SC, Chukwu A, Toteu SF, Davies TC (2016) Assessment of heavy metal contamination in soils around lead (Pb)-Zinc (Zn) mining areas Enyigba, southeastern Nigeria. J Geol Soc India 87:453–462. https://doi.org/10.1007/s12594-016-0413-x
Offodile ME (1980) A mineral survey of the Cretaceous of the Benue Valley, Nigeria. Cretac Res 1(2):101-124
Okogbue CO, Ukpai SN (2013) Evaluation of trace element contents in groundwater in Abakaliki metropolis and around the abandoned mine sites in the southern part, Southeastern Nigeria. Environ Earth Sci 70:3351–3362. https://doi.org/10.1007/s12665-013-2401-4
Okolo CC, Oyedotun TDT Akamigbo FOR (2018) Open cast mining: threat to water quality in rural community of Enyigba in south-eastern Nigeria. Appl Water Sci 8(204):1–11. https://doi.org/10.1007/s13201-018-0849-9
Omonona OV, Onwuka OS, Okogbue CO (2014) Characterization of groundwater quality in three settlement areas of Enugu metropolis, southeastern Nigeria, using multivariate analysis. Environ Monit Assess 186:651–664. https://doi.org/10.1007/s10661-013-3405-y
Omonona OV, Amah JO, Olorunju SB, Waziri SH, Ekwe AC, Umar ND, SW Olofinlade (2019) Hydrochemical characteristics and quality assessment of groundwater from fractured Albian carbonaceous shale aquifers around Enyigba Ameri, southeastern Nigeria. Environ Monit Assess 191(125):1–22. https://doi.org/10.1007/s10661-019-7236-3
Opoke SU, Osayande AD (2018) Physicocheical, major anions and heavy metals in surface water and groundwater at Pb-Zn mining areas of Ebonyi State, Nigeria. J Appl Sci Environ Manag 22(7):1017–1020. https://doi.org/10.4314/jasem.v22i7.3
Phadatare SS, Gwande S (2016) Review paper on development of water quality index. Int J Eng Res Technol 5:765–767
Prasad B, Bose JM (2001) Evaluation of heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188. https://doi.org/10.1007/s002540100380
Prasad B, Mondal KK (2008) The impact of filling an abandoned opencast mine with fly ash on ground water quality: a case study. Mine Water Environ 27(1):40–45. https://doi.org/10.1007/s10230-007-0021-5
Ravikumar P, Somashekar RK, Prakash K (2015) A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Sci 80:31073–31077
Reiners WA, Marks RH, Vitousek PM (1975) Heavy metals in subalpine soils of New Hampshire. Oikos 26:264
Reza R, Singh G (2010) Assessment of heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7(4):785–792. https://doi.org/10.1007/BF03326187
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, Berlin Heidelberg Tokyo
Suthar S, Singh S (2008) Vermicomposting of domestic waste by using two epigeic earthworms (Perionyx excavatus and Perionyxsansibaricus). Int J Environ Sci Technol 5(1):99–106. https://doi.org/10.1007/BF03326002
Tadiboyina R, Ptsrk PR (2016) Trace analysis of heavy metals in ground waters of Vijayawada industrial area. Int J Environ Sci Educ 10(11):3215–3229
Tiwari RN, Mishra S, Pandey P (2013) Study of major and trace elements in groundwater of Birsinghpur area, Satna district Madhya Pradesh, India. Int J Water Res Environ Eng 5(7):380–386. https://doi.org/10.5897/IJWREE2012.0408
Wei W, Ma R, Sun Z, Zhou A, Bu J, Long X, Liu Y (2018) Effects of mining activities on the release of heavy metals (HMs) in a typical mountain headwater region, the Qinghai-Tibet Plateau in China, Int J Environ Res Public Health 15:1–19. https://doi.org/10.3390/ijerph15091987
WHO (2011) Guideline for drinking water quality, 3rd edn. World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
This paper was selected from the 2nd Conference of the Arabian Journal of Geosciences (CAJG), Tunisia 2019
Rights and permissions
About this article
Cite this article
Omonona, O.V., Ekwe, A.C., Azuoko, GB. et al. Assessing the groundwater quality of a small-scale mining drainage basin using modified water quality indices. Arab J Geosci 14, 1566 (2021). https://doi.org/10.1007/s12517-021-07912-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-07912-8