Abstract
In this study, a comprehensive survey was performed on the fire-induced landforms, belowground and near surface gas emissions, and soil properties. In addition, the effect of the soil cover on the gas emissions and soil properties was examined. The survey zone was divided into three areas based on the surface features. Sponges, vents and cracks, and the fissures dominated in area A, and area B exhibited no obvious features but was covered with a layer of sand soil. Area C was characterized by degraded vegetation. A comparison of the composition and concentration of belowground and near surface gases was performed among the three areas. Topsoil samples were collected at a 0–5-cm depth in the three areas, and soil properties were measured and analyzed. The average CO, CO2, CH4, and H2 concentrations below the ground surface in area B were lower than those in area A. The detected near surface CO and CH4 mainly originated from obvious openings. Damage to the soil ecology was primarily caused by the replacement of O2 by CO2. Soil thermal and humidity anomalies were obvious in area A, but scarcely occurred in areas B and C because of solar heating. The soil pH was the lowest in area A, which was closely related to the total soil contents. The organic matter contents in area B were higher than those in area A because of the increase in the particles released from underground coal fires, and area C attained the highest organic matter contents due to vegetation withering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Underground coal fires are natural hazards which the coal experiences oxidation, thermal accumulation, and eventually spontaneous ignition (Liang et al. 2016), generally occurring in exposed coal seams, coal storage piles, or coal waste piles (Jiang et al. 2017). Coal fires consume non-renewable coal resources and release tremendous amount of toxic gases (e.g., CO and SO2), greenhouse gases (e.g., CO2 and CH4), and trace elements (e.g., As, F, and Hg). In addition, underground coal fires contribute to land subsidence due to volume loss of coal seams (Kuenzer and Stracher 2012), and threaten the lives and properties of local residents. To data, research on underground coal fires in the environment has focused on the assessment of soil gas emissions, underground water, and soil pollution (Song and Kuenzer 2014).
Pone et al. collected and analyzed gas combustion by-products from gas vents in the Witbank and Sasolburg coalfields, and assessed the health risk to local communities (Pone et al. 2007). Carras et al. measured greenhouse gas emissions originating from spoil piles and waste coal dumps, and studied the difference in gas emissions among three areas characterized by the extent of spontaneous combustion (Carras et al. 2009). Temporal and spatial gas (e.g., CO2, CO, Hg, and hazardous pollutants) trends of vent dynamics were extensively investigated (Dindarloo et al. 2015; Engle et al. 2012; Garrison et al. 2017; Hower et al. 2009, 2011, 2013; O’Keefe et al. 2010, 2011). Gaseous emissions may condense in the mouth of gas vents due to the sharp decrease in temperature, and the characteristics of the secondary minerals of the condensates have been investigated, including the components and the formation mechanisms (Dias et al. 2014; O’keefe et al. 2018; Oliveira et al. 2019). Some gaseous emissions are highly soluble, and liquor leaching may lead to severe acid drainage, resulting in pollution of underground water (Querol et al. 2011). In addition, the toxic trace elements (e.g., As, F, Hg, and Se) emitted by the underground coal fires may become enrich in gas vents and soils (Hong et al. 2018; Liang et al. 2018; Li et al. 2018), thus causing soil contamination. Changes in soil properties could also influence the distribution of soil heavy metals and polycyclic aromatic hydrocarbons (PAHs) in coalfields, which in turn could aggravate the destruction of terrestrial ecosystems. Querol et al. found that mobility of certain trace pollutants was enhanced under acidic conditions, such as Li, Ti, Sc, V, Cr, Mn, Co, and Ni. In addition, Al-K-Fe-NH4+-bearing sulfates easily dissolved under acidic conditions (Querol et al. 2008). Garrison et al. observed that the concentrations of monoaromatic hydrocarbons (BTEX) and PAHs in soils were higher in areas with high soil matter contents due to their enhanced absorption capacities (Garrison et al. 2016). Wang et al. observed that soil Hg and Cd exhibited notable correlations with total carbon contents, and suggested that soil Hg and Cd were related to organic matter (Wang et al. 2019). Therefore, the study of soil properties contributes to explaining the formation and distribution of certain soil pollutants.
A small, but growing, body of research has been conducted to investigate the impact of underground coal fires on the soil properties in coal fire areas. Tobin-Janzen et al. found changes in resident microbial populations in boreholes impacted by Centralia, and Pennsylvania anthracite coal fires, and the soil temperature, pH, and chemical composition were analyzed (Tobin-Janzen et al. 2005). Zhang et al. performed physicochemical characterization of soil samples in the mouths of vents, and microbial communities at high temperatures (Zhang et al. 2013). Zeng et al. investigated surface vegetation coverage, heavy metal distribution, and organic matter, and determined that coal fires can affect soil properties via organic matter digestion (Zeng et al. 2018). However, most of the research on soil properties has focused on vents or surface openings, in addition to the soil gas emissions.
The aims of this investigation are to (1) analyze the concentrations of hazardous pollutants in various soils layers in Wuhushan coal fires not confined to vents or surface openings and (2) collect and analyze surface soil samples to conduct the soil physicochemical characterization in the investigated area. In addition, the effect of backfill consisting of sandy soils on the gas emissions and soil properties is examined.
Materials and methods
Site description
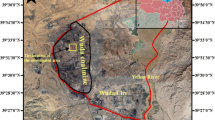
The Wuda coalfield is located in the northwest of Wuda District, and including three coal mines: Wuhushan, Suhaitu, and Huangbaici (Fig. 1), covering an area of 35 km2. The study area is a backfilled region in the northern Wuhushan mine, which belongs to the no. 10 fire zone. The climate in the area is characterized as a strongly continental, semiarid climate, with an average precipitation of approximately 168 mm/a (Song and Kuenzer 2017). The land surface of the Wuhushan coalfield is dominated by bare rocks covering sandy soil, and the vegetation density is very low (Song et al. 2015). The Wuhushan coal mine started construction in 1965. The no. 9, no. 10, no. 12, no. 13, no. 15, no. 16, and no. 17 coal seams are the main coal seams in the Wuhushan mine, among which the no. 9, no. 10, and no. 12 exhibit a high propensity of spontaneous combustion with sulfur contents up to 3.42%. Notably, in-ground coal fires occur when shallow coal seams are exposed at the Earth’s surface (Shan et al. 2019). The destructive and disorderly operation of small-scale private mine results in the spread of coal fires. Since 2006, a coal fire suppression project has been implemented in the area. Coal mines are typically contracted to individuals, and they are permitted to excavate surface rocks, remove spontaneous combustion coal, and sold any mineable coal, but they are responsible for backfilling. However, exaction has destroyed the landscape, and new fissures and cracks have thus been created, which has led to the expansion of the coal fire range. The above scheme has been terminated by the local government, and a fire suppression project was gradually implemented towards standardization in 2011. Follow-up work was organized and implemented by a professional fire-fighting engineering office (Liang 2018). The burning coal was excavated, the ground surface was covered with sandy soil, and sowing grass were sown for ecological restoration purposes. This method obtained promising results, and the area of shallow and visible coal fires was estimated to have decreased by ~60%, while the risk of spreading remains (Jiang et al. 2017). In the research zone, the intensity of shallow coal fires notably decreased, and recovery of vegetation was observed along the edge of the survey zone. However, the overlying soil did not prevent hidden combustion of coal seams. Nearly all the surfaces were devoid of vegetation in the high-temperature zone. Visible surface flames were extinguished, but fumes persisted scattered throughout the central area.
Gas concentrations were measured on a variety of surfaces grouped into three categories based on the degree of combustion of the underground coal fire:
-
1)
Surfaces with spontaneous combustion and obvious features, including cracks (Fig. 3b), sponges (Fig. 3c), fissures (Fig. 3d), and vents (Fig. 3e). This region was defined as area A.
-
2)
Surface with spontaneous combustion but without any obvious features covered by sandy soils. This region is defined as area B.
-
3)
Surfaces characterized by withered plants (Fig. 3e). The vegetation degradation area occurred along the periphery of the investigated area (Fig. 2). It represented the reclamation area, in which coal fires had been extinguished, and grass seeds were sown for ecological restoration. This region was defined as area C.
The coal fire-induced landforms can be roughly divided into three types among the backfilled regions in area A, including cracks and vents, sponges, and fissures. Cracks and vents are distributed in the southeastern sampling areas, and obvious black smoke is observed. The maximum width of the cracks is approximately 70cm, and they are possible pathways for the ventilation of the underground coal fires (Kuenzer and Stracher 2012). Compared to the original landform (Engle et al. 2012), the observed vents and cracks contained no visible minerals or tars (Fig. 3b). The soil cover may contribute to the lack of tar and minerals. The fissures are mainly distributed in the northwestern part of the investigated area. The fissures are linear in the surface soil, and they are common outbound pathways of hot gas emissions (Kuenzer and Stracher 2012). The in situ fissures range from a few centimeters, and are extremely narrow (<0.5 cm), and white and yellow salt minerals could be observed besides the fissures. When hot particulate emissions cool, the substances absorb water and change into white salts which mainly consist of alunogen (Querol et al. 2011). The yellow salts mainly comprise native sulfur, which is generated the reaction: SO2+2H2S→3S0+2H2O (Querol et al. 2008). The presence of the sulfur also suggests that the coal fire is located deep below the ground surface, because sulfur only remains stable below 100°C (Gürdal et al. 2015), which is also confirmed by measurement of the temperature in situ (Fig. 4). Sponges are a type of sponge-like contaminated soil (Liang et al. 2018), commonly found on the surface of the backfilled regions, and are mainly distributed in the southeast, as shown in Fig. 3e.
Soil sampling
A soil and gas sampling campaign was carried out in August, 2019. A total of 88 soil samples at depths ranging from 0 to 5 cm was collected with a Teflon tool at closely spaced intervals (10m), of which 30 samples were collected in area A, 46 samples were collected in area B, and the remaining samples were collected in area C.
Surface gas concentration measurements
H2S, SO2, and H2 were measured in situ, and monitoring was performed with a portable gas analyzer containing a self-priming pump. The range and accuracy of the emission analyzer are 0–2000 ppm and ± 0.1%, respectively, for H2 and 0–100 ppm and ± 1%, respectively, for SO2 and H2S. The probe of the sampling device was hammed to a depth of approximately 5 cm at each grid point, and connected to the gas inlet of the analyzer. In area A, a rubber tube was directly inserted into the vents and cracks. Measurement was recorded until the date remained stable during the process of measurement. CO, CO2, CH4, C2H6, C2H4, C2H2, N2, and O2 were analyzed via capillary gas chromatography (GC-4000A, Beijing), and the accuracy assessed via standard gas mixture was less than 5%, while the reproducibility was higher than 10%. Belowground gases were drawn with a gas-tight syringe, and injected into cleaned aluminum foil sampling bags. Moreover, near-surface gases were directly drawn with a gas-tight syringe at a vertical distance of 70 cm from the position corresponding to the belowground gases. The airbags were transferred to the laboratory within 24 h.
Soil chemical analyses
The soil pH was determined with various pH meters, and the organic matter content was measured via potassium dichromate oxidation. The soil humidity was measured with a potable digital hygrometer. The total salt contents were determined via measurement of the electrical conductivity of the soil leaching solution. The distribution of the soil pH and total salt contents in the investigated area were interpolated via the inverse distance weighting method in ArcGIS 10.4, as shown in Fig. 6.
Surface temperature measurement
The surface temperature was measured with an infrared thermal imager (Fortric220s, USA), and the measurement range and accuracy of the imager are −20 to 150 °C and ±0.1 °C, respectively. The highest temperature in the images (e.g., the vent in Fig. 3) was selected as the representative temperature. The surface temperature and soil moisture were also interpolated via the inverse distance weighting method in ArcGIS 10.4, as shown in Fig. 3.
Statistical analysis
A statistical t test was performed to statistically determine whether the soil properties in the different areas, including the soil organic matter content, soil pH, and total salt contents, were significantly different (p≤0.01).
Results and discussion
Analysis of the soil physical properties
The ground temperature in area A is significantly higher than that in area B (Fig. 3b), which indicates that the temperature is mainly transmitted to the surface via convection through the cracks and fractures. The variation in the ground temperature is high among the various fire-induced landforms. The maximum temperature in the cracks reaches 354°C (Table 4), significantly higher than that at the sampling points, which indicates that the fire font may lie in the southeastern part of the investigated area.
The cracks are linearly oriented from SW to NE, and it is inferred that the underground coal fires move from SE to NW based on the cracks that are orthogonal to the fire spreading direction. The average surface temperature of the sponges is the highest, and the sandy soil is very dark gray because of combustion and particle emission. The surface temperature of the fissures ranges from the 36.4 to 261 °C (Table 4), and the average temperature is the lowest among all the points with obvious features (Table 4).
The soil humidity is correlated with the combustion degree of the underground coal fire. The contours of the soil humidity are dense in areas where the combustion degree is high (Fig. 3). The soil near the sponges obtained the highest water contents, followed by the fissures (Table 4). Humid soil is almost undetectable in the degraded area due to the influence of solar radiation. In area B, at 25% of the points, humid soil is detected, mostly ranging from 2.1 to 10.1% (Table 4), and humid soil mainly occurs at the center of the high-temperature zone (Fig. 3). Research has shown the coal may contain more than 20% moisture by weight (Gürdal et al. 2015), and the vapor may seep to the surface along fissures within the bedrock by the buoyancy generated via heating, and it may accumulate in soil near the fissures and vents, thus increasing the soil humidity. The average soil humidity near fissures and sponges is higher than that near the vents and cracks (Fig. 3), because the former are more likely to act as outbound ventilation pathways (Kuenzer and Stracher 2012).
Analysis of the belowground gas concentration
Coal fire gases are transported to the surface through two primary mechanisms: advection through vents and fractures (vent emissions) and diffusion through the overburden (O’keefe et al. 2018). Gases are released in area A via advection, while diffusion dominates in area B. CO2, CO, and CH4 are the dominant gases in the research zone with additional components such as H2, SO2, H2S, C2H4, C2H2, and C2H6. These gaseous products may be largely generated via the thermal degradation of the biopolymers occurring in coal seams. The CO2 concentrations are relatively high in the vicinity of the fire front (Fig. 6), with the highest CO2 concentration reaching 11 vol%. The concentrations of CO2 seem to exhibit no correlation with the surface temperature, which is contrary to the conclusions of Hower et al. (2009). This probably occurs because gas emissions are more likely dominated by the underground fire itself, not the surface temperature. In addition, the distance to the fire or the presence of obstacles also influences the ground temperature and gas emissions (Garrison et al. 2017). The variation in the belowground CO2 concentration in area A is wide (Table 1), and is influenced by the fire depth, ventilation area, and relative position of the fire front to the vent (O’keefe et al. 2011; Song et al. 2019, 2020). The average CO2 concentration in area A is 5.4 vol% (Table 1), significantly exceeding the OSHA 8-h limit of 5 vol%. In the area B, the average CO2 concentration is of the same order of magnitude as that in area A (Table 2), which indicates a relatively high permeability, of the thin calcite-cemented sandstone occurring below surface soil layer. The variation differs within a range of 3 orders of magnitude in area B (Table 2), and there are two main reasons for the high variation. On the one hand, cracks, vents, and fissures are established before landfilling, but may remain still active despite the sandy soil cover. On the other hand, dense fissures and tectonic discontinuities occur within various strata of the formations.
The vegetation cover is low in the investigated area (Fig. 4f), of which areas A and B are covered with little or no vegetation. Kuenzer et al. found that toxic gases and underground heat are responsible for the deterioration of vegetation (Kuenzer et al. 2007). A strongly negative relationship is found between the soil CO2 and O2 concentrations in areas A and B (Fig. 5), which indicates that the soil O2 is displaced by CO2 produced by the underground coal fire. Patil et al. suggested that a minimum soil O2 concentration ranging 12–14% could sustain a healthy root environment, or the plant would wither due to a decreased root respiration (Patil et al. 2010). However, the maximum O2 concentration in the degraded area the least affected by underground coal fires is lower than 10 vol% (Table 3). Therefore, anoxic conditions are also a major factor causing ecological environmental damage.
Compared to the vents and cracks in the Heshituoluogai coal fire area (Wang et al. 2017), H2 is detected at most points in area A, and the average H2 concentration is 401 ppm (Table 1), which is significantly higher than that in the Heshituoluogai coal fire area. The high concentration of H2 indicates that the underground coal fire occurs in the advanced heating phase. The CO and CH4 concentrations in area A are lower than the CO2 concentration, indicating that the system is approaching near-complete combustion conditions. CO, ranging from 9 to 6008 ppm (Table 1), far exceeds the 8-h OSHA limit of 50 ppm. The CH4 concentration ranges from 4 to 2274 ppm (Table 1). In comparison to Tiptop coal-mine (O’keefe et al. 2018), the high CH4 concentration also indicates that the coal fire occurs in the stage of intense combustion. The CO and CH4 concentrations in the area B are significantly lower than those in area A, while the range of the concentrations is large, ranging from 4 to 3530.3 ppm and 1 to 2276 ppm (Table 2), respectively. C2H4 is detected in almost 76.5% of the analyzed samples in area A, and its concentrations reach up to 74 ppm (Table 1). Compared to C2H4, C2H6 is detected in almost 23.3% of the analyzed samples, and C2H4 and C2H6 are detected only in trace amounts: in fewer than 39% and 7%, respectively, of the analyzed samples (Table 1). H2S and SO2 are highly concentrated in area A, ranging from 0.72 to 26.4, and 2 to 100, respectively (Table 1).
In the degraded vegetation area, H2, SO2, and H2S are not detected. The belowground CO and CH4 concentrations are low, averaging approximately 7.11 ppm and 10.9 ppm, respectively (Table 3). A moderately negative relationship is found between the soil CO2 and CH4 concentrations in area A (r=0.727, p<0.01, n=30), indicating that they have the same origin, and most of the gases may be generated from the combustion of coal. The CO concentration does not seem related to the CO2 concentration in areas A and B. This indicates that the system occurs in an unsteady state, and CO may be rapidly converted into CO2. Although landfills may prevent the emission of CO, SO2, H2S, C2H2, C2H4, and C2H6, the average CO2 and CH4 concentrations are the same order of magnitude in areas A and B (Table 3). This indicates that the degree of greenhouse gas emissions through soil diffusion in the backfilling area also contributes to the burden of the atmosphere. Therefore, the estimation of greenhouse gas emission through soil diffusion in backfilling areas deserves attention.
Analysis of the near-surface gas concentration
The near-surface CO2 concentration is relatively high in area A, and the maximum value is below the OSHA 8-h limit of 0.5 vol% (Table 1). The contours of the near-surface CO2 concentration are dense in the vicinity of the high-temperature zones, but sparse in the area far from the high-temperature zones (Fig. 6). The average concentration of CO2 is 839 ppm in the vicinity of the degraded vegetation area (Table 3), almost unaffected by underground coal fires. Near-surface CH4 and CO are also detected in area A, but their concentrations are lower than their corresponding belowground concentrations (Table 1). The maximum ground concentration of CO is 68 ppm in area A (Table 1), which is slightly higher than the eight-hour OSHA limit of 50 ppm. Obviously, gases are diluted by air as they are emitted, but temperature inversions can cause potential health risks in the surrounding regions of coal fire areas (Hower et al. 2009). The correlation of the belowground CO2 concentration with near-surface CO2 concentration in area A is positive but not significant (R2=0.38, p<0.01, n=30), and in area B, there seems to be no association (R2=0.17, P=0.01, n=58). This suggests that gases generated from the vents, cracks, and fissures mainly affect the near-surface CO2 concentration distribution. The near-surface CO and CH4 concentrations in area B are in line with these same rules. The near-surface CH4 concentrations at same locations are even higher than their corresponding belowground CH4 concentrations due to the influence of vent advection. H2, SO2, H2S, C2H4, C2H6, and C2H2 are below the detection limits in the near-surface zone (Table 2).
Analysis of the soil total salt contents and soil pH
Area A exhibits the lowest soil pH, with an average value of 5.35, and it mostly ranges from 4.03 to 6.67 (Table 4). Several studies of the soil pH distribution impacted by underground coal fires can be referenced for comparison. Tobin-Janzen et al. found that the soil pH in surface soil samples (at a depth from 0 to 20 cm) collected in boreholes indicated slightly acidic to acidic conditions throughout the Pennsylvania mine fire area (6.35–4.11) (Tobin-Janzen et al. 2005). Wang et al. measured the soil properties around the vents, and found that the soil pH and total salt contents near vents varied although they originated from same coal seam, and the data indicated that the soil pH was correlated with the total salt contents (Wang et al. 2020). The soil pH observed in this study is not consistent with the above reports because of the difference in the environment among the areas. However, these studies all indicate that the soil pH near the vents and cracks is generally acidic.
The average soil pH varied greatly among the landforms in area A, and the sponges in the area exhibited the lowest soil pH (0.95–1.99), followed by the vents and cracks (1.57–7.18), while the soil pH near the fissures was the highest (3.09–7.79) (Table 4). The negative correlation between the soil pH and the total salt contents (r=0.859, p<0.01) indicated that the soil pH is significantly influenced by the soil salt in area A (Fig. 7). A source for a high acidity may be hydrolysis of the sulfate, while Fe3+-Fe(OH)2+ and HSO4−-SO42− could maintain a pH of approximately 1, while the dissolution of alunogen and the hydrolysis of other Al sulfates may be responsible for a pH values of approximately 4 (Querol et al. 2011). Most samples retrieved from the fissures indicated near-neutral pH, and the significant leachable content of gypsum (high Ca and SO42−) may be unaffected by pH. A small number of samples collected from the fissures even indicated alkalinity, and NH4Cl and organic condensates could account for this alkalinity (Querol et al. 2011). Sulfate in area A could be generated by the reaction of SO2 with the soil moisture, which is confirmed by the high SO2 concentration in the area (Fig. 7). The average soil pH was 7.19 in area B, and significantly lower than that in area A (Fig. 8). This indicates that the soil cover weakens the acidity, which plays a positive role in reducing environmental hazards. Compared to area A, the total salt contents were significantly lower than those in area B (Fig. 8), and the contours of the SO2 concentration were relatively sparse (Fig. 7). SO2 was even not detected in the degraded vegetation area (Fig. 7). The soil pH in area B was relatively higher than that in area C (Fig. 8), but the t test showed that there was no significant difference between these two groups.
Analysis of the organic matter contents
The average organic carbon contents in the degraded vegetation area are the highest, with an average content of 17.62 mg/g, which is significantly higher than that in the other areas (Fig. 9). This probably occurs because the carbonated particles generated via vegetation withering increase the soil organic carton contents by mixing with the soils (Cui et al. 2012; Zhao et al. 2016). In comparison, the organic matter contents in the Huangbaici coal fire area decreased with increasing soil temperature, because the heat effect of the coal fire digested the organic matter (Wang et al. 2020). We draw a nearly opposite conclusion: the average organic carbon contents in the high-temperature zone (area A) seem be higher than those in the surrounding area, and the mean average organic carbon contents in area A are significantly higher than those in area B (Fig. 9). The particles released through the fissures within the strata from the underground coal fires may increase the organic carbon contents (Wang et al. 2019). In addition, the landfilling with yellow sandy soil in area B affects the organic carbon contents because the sandy soil contains relatively low organic contents.
Limitations and future research directions
-
1)
Breathing cycles are the common phenomena of certain coal fires. Engle et al. observed that the CO2 vent emissions in Wyoming coal fires significantly differ between two consecutive days, while the CO and H2S showed no significant differences (Engle et al. 2012). Dindarloo et al. found that the CO concentration increased in the middle of the day when outside air temperature increased, and declined in the afternoon when the outside air cooled (Dindarloo et al. 2015). The temporal variability in diffuse gas emissions remains a research gap (Engle et al. 2013). Therefore, the temporal variability of in vent emissions and diffuse gas emissions in coal fire areas should be considered.
-
2)
CO2 generally stems from three sources in coal fire areas: native CO2, CO2 produced from coal combustion, and CO2 produced from the oxidizing of native CH4 (Ide and Orr 2011). Ide and Orr used 13C isotope signatures to determine the fractions of CO2 emitted from coal combustion in coal seams (Ide and Orr 2011). Carras et al. found that the emission rate of greenhouse gases originating from low-temperature oxidation of coal waste is similar to the emission rates due to biological activity (Carras et al. 2009). In our study, CO2 and CH4 in areas A and B were qualitatively inferred to originate from coal combustion based on their relatively high concentrations and Pearson correlation analysis, as discussed in the “Analysis of the belowground gas concentration” section. A portion of CH4 and CO2 may originate from microbial activity in the degraded area. Quantitative analysis of the source is required in the research zones, which could determine contribution of the greenhouse gases emitted from coal combustion to the pollution of the local environment.
3) Soil chemical properties are sensitive to gas and heat intrusions as discussed in the “Limitations and future research directions” section, such as the soil pH and organic carbon. The mobility of trace metals and changes in microbial communities is reliably related to the soil pH and organic carbon (Rasheed et al. 2013). For example, the soil Hg and Cd contents were related to the organic matter (Wang et al. 2019), and Fe3+ concentration was relatively high near the vents (Querol et al. 2011). Tobin-Janzen found that the ribotype diversity of microbial populations decreased within individual boreholes with increasing temperature (Tobin-Janzen et al. 2005). Therefore, whether the change of soil biochemistry properties could be adopted a complementary index to monitor underground coal fires deserves further research.
Conclusion
Surveys were conducted in the Wuda coal fire area to investigate the impact of underground coal fires on the surface landform, soil gas emissions, and soil properties. Backfilling of sandy soil imposes certain effect on gas emissions and soil properties. These observations lead to the following conclusions:
-
1)
Little vegetation occurs in the area seriously affected by underground coal fires, such as in area A and area B. Fissures, cracks and vents, and the sponges are the main fire-induced landforms in the research zone.
-
2)
A comparison of the components and concentrations of gases is performed among area A, area B, and the degraded vegetation area. The CO2 and CO concentrations peak at 11 vol% and 6008 ppm, respectively, in area A, far exceeding OSHA safety limits. In area B, the CO2 and CO concentrations are relatively low, with average concentration of 9.84 vol% and 3530 ppm, respectively. SO2, H2S, C2H6, and C2H4 highly concentrate in area A, ranging from 0.72 to 26.4, 2 to 100, 6 to 128, and 2 to 74 ppm, respectively. The soil CO2 concentrations are correlated with CH4 but seem to be unrelated to CO. Low soil O2 concentrations damage the healthy root environment for soil O2 is displaced by CO2. CO and CH4 are detected near the ground surface, while the other gases are below the detection limits. Backfill of soil mitigates the emission of SO2, H2S, C2H2, and C2H6, but does not seen to reduce emissions of the greenhouse gases in the research zone. The near-surface gas concentration mainly originates from the obvious openings. Although coal-fire gas emissions are diluted by air, the maximum of near surface CO concentration exceeds the OSHA 8-h exposure limit, which is a hazard to geologists at the site.
-
3)
Thermal surface and soil moisture anomalies are mainly dominated by obvious openings. There seems no relationship between the surface temperature and gas concentration due to solar heating. The sponges exhibit the lowest soil pH (0.95–1.99), followed by the vents and cracks. A negative correlation is found between the soil pH and the total salt contents in area A (r=0.859, p<0.01). Hydrolysis of Al and Fe sulfates originating from soil salts accounts for the relatively low pH values. The particles released through the fissures may increase the organic carbon contents in area A, while the carbonated particles stemming from vegetation withering cause an increase in the organic carbon contents in the degraded vegetation area. Compared to the area A, the soil in area B attain relatively high soil pH values and relatively low organic carbon contents, which suggests that the soil cover helps improve the quality of the soil environment.
References
Carras JN, Day SJ, Saghafi A, Williams DJ (2009) Greenhouse gas emissions from low-temperature oxidation and spontaneous combustion at open-cut coal mines in Australia. Int J Coal Geol 78:161–168. https://doi.org/10.1016/j.coal.2008.12.001
Cui XY, Hao JM, Zhao SS, Sang Y, Wang HQ, Di XY (2012) Temporal and spacial changes of total soil organic carbon content as affected by an experimental forest fire in the Greater Xing’s a mountain. J Soil Water Conserv 26:195–200
Dias CL, Oliveira MLS, Hower JC, Taffarel SR, Kautzmann RM, Silva LFO (2014) Nanominerals and ultrafine particles from coal fires from Santa Catarina, South Brazil. Int J Coal Geol 122:50–60. https://doi.org/10.1016/j.coal.2013.12.011
Dindarloo SR, Hood MM, Bagherieh A, Hower JC (2015) A statistical assessment of carbon monoxide emissions from the Truman Shepherd coal fire, Floyd County, Kentucky. Int J Coal Geol 144-145:88–97. https://doi.org/10.1016/j.coal.2015.03.012
Engle MA, Radke LF, Heffern EL, O’keefe JMK, Hower JC, Smeltzer CD et al (2012) Gas emissions, minerals, and tars associated with three coal fires, Powder River Basin, USA. Sci Total Environ 420:146–159. https://doi.org/10.1016/j.scitotenv.2012.01.037
Engle MA, Olea RA, O’Keefe JMK, Hower JC, Geboy NJ (2013) Direct estimation of diffuse gaseous emissions from coal fires: current methods and future directions. Int J Coal Geol 112:164–172. https://doi.org/10.1016/j.coal.2012.10.005
Garrison T, Hower JC, Fryar AE, D’Angelo E (2016) Water and soil quality at two eastern-Kentucky (USA) coal fires. Environ Earth Sci 75:574. https://doi.org/10.1007/s12665-016-5380-4
Garrison T, O’Keefe JMK, Henke KR, Copley GC, Blake DR, Hower JC (2017) Gaseous emissions from the Lotts Creek coal mine fire: Perry County, Kentucky. Int J Coal Geol 180:57–66. https://doi.org/10.1016/j.coal.2017.06.009
Gürdal G, Hoşgörmez H, Özcan D, Li X, Liu H, Song W (2015) The properties of Çan Basin coals (Çanakkale—Turkey): spontaneous combustion and combustion by-products. Int J Coal Geol 138:1–15. https://doi.org/10.1016/j.coal.2014.12.004
Hong XP, Liang HD, Chen Y, Liu YH, Shi YY (2018) Distribution of fluorine in the surface dust of Wuda coal base, Inner Mongolia of Northern China. J Geochem Explor 188:390–397. https://doi.org/10.1016/j.gexplo.2018.02.012
Hower JC, Henke K, O’Keefe JMK, Engle MA, Blake DR, Stracher GB (2009) The Tiptop coal-mine fire, Kentucky: preliminary investigation of the measurement of mercury and other hazardous gases from coal-fire gas vents. Int J Coal Geol 80:63–67. https://doi.org/10.1016/j.coal.2009.08.005
Hower JC, O’Keefe JMK, Henke KR, Bagherieh A (2011) Time series analysis of CO concentrations from an Eastern Kentucky coal fire. Int J Coal Geol 88:227–231. https://doi.org/10.1016/j.coal.2011.10.001
Hower JC, O’keefe JMK, Henke KR, Wagner NJ, Copley G, Blake DR et al (2013) Gaseous emissions and sublimates from the Truman Shepherd coal fire, Floyd County, Kentucky: a re-investigation following attempted mitigation of the fire. Int J Coal Geol 116-117:63–74. https://doi.org/10.1016/j.coal.2013.06.007
Ide ST, Orr FM (2011) Comparison of methods to estimate the rate of CO2 emissions and coal consumption from a coal fire near Durango, CO. Int J Coal Geol 86:95–107. https://doi.org/10.1016/j.coal.2010.12.005
Jiang WG, Jia K, Chen Z, Deng Y, Rao PZ (2017) Using spatiotemporal remote sensing data to assess the status and effectiveness of the underground coal fire suppression efforts during 2000–2015 in Wuda, China. J Clean Prod 142:565–577. https://doi.org/10.1016/j.jclepro.2016.03.082
Kuenzer C, Stracher GB (2012) Geomorphology of coal seam fires. Geomorphology 138:209–222. https://doi.org/10.1016/j.geomorph.2011.09.004
Kuenzer C, Zhang JZ, Tetzlaff A, van Dijk P, Voigt S, Mehl H, Wagner W (2007) Uncontrolled coal fires and their environmental impacts: Investigating two arid mining regions in north-central China. Appl Geogr 27:42–62. https://doi.org/10.1016/j.apgeog.2006.09.007
Li CH, Liang HD, Chen Y, Bai JW, Cui YK (2018) Distribution of surface soil mercury of Wuda old mining area, Inner Mongolia, China. Hum Ecol Risk Assess Int J 24:1421–1439. https://doi.org/10.1080/10807039.2017.1413536
Liang YC (2018) Emission characteristics of typical pollutants of coal fire in Wuda, Inner Mongolia. Dissertation, China University of Mining and Technology (Beijing), Beijing
Liang YC, Liang HD, Zhu SQ (2016) Mercury emission from spontaneously ignited coal gangue hill in Wuda coalfield, Inner Mongolia, China. Fuel 182:525–530. https://doi.org/10.1016/j.fuel.2016.05.092
Liang Y, Zhu SQ, Liang HD (2018) Mercury enrichment in coal fire sponge in Wuda coalfield, Inner Mongolia of China. Int J Coal Geol 192:51–55. https://doi.org/10.1016/j.coal.2018.03.006
O’keefe JM, Henke KR, Hower JC, Engle MA, Stracher GB, Stucker JD et al (2010) CO(2), CO, and Hg emissions from the Truman Shepherd and Ruth Mullins coal fires, eastern Kentucky, USA. Sci Total Environ 408:1628–1633. https://doi.org/10.1016/j.scitotenv.2009.12.005
O’keefe JMK, Neace ER, Lemley EW, Hower JC, Henke KR, Copley G et al (2011) Old Smokey coal fire, Floyd County, Kentucky: estimates of gaseous emission rates. Int J Coal Geol 87:150–156. https://doi.org/10.1016/j.coal.2011.06.005
O’keefe JMK, Neace ER, Hammond ML, Hower JC, Engle MA, East J et al (2018) Gas emissions, tars, and secondary minerals at the Ruth Mullins and Tiptop coal mine fires. Int J Coal Geol 195:304–316. https://doi.org/10.1016/j.coal.2018.06.012
Oliveira MLS, Pinto D, Tutikian BF, da Boit K, Saikia BK, Silva LFO (2019) Pollution from uncontrolled coal fires: continuous gaseous emissions and nanoparticles from coal mines. J Clean Prod 215:1140–1148. https://doi.org/10.1016/j.jclepro.2019.01.169
Patil RH, Colls JJ, Steven MD (2010) Effects of CO2 gas as leaks from geological storage sites on agro-ecosystems. Energy 35:4587–4591. https://doi.org/10.1016/j.energy.2010.01.023
Pone JDN, Hein KA, Stracher GB, Annegarn HJ, Finkleman RB, Blake DR et al (2007) The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int J Coal Geol 72:124–140. https://doi.org/10.1016/j.coal.2007.01.001
Querol X, Izquierdo M, Monfort E, Alvarez E, Font O, Moreno T, Alastuey A, Zhuang X, Lu W, Wang Y (2008) Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int J Coal Geol 75:93–104. https://doi.org/10.1016/j.coal.2008.04.003
Querol X, Zhuang X, Font O, Izquierdo M, Alastuey A, Castro I et al (2011) Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: a review and new experimental data. Int J Coal Geol 85:2–22. https://doi.org/10.1016/j.coal.2010.09.002
Rasheed MA, Lakshmi M, Rao PLS, Kalpana MS, Dayal AM, Patil DJ (2013) Geochemical evidences of trace metal anomalies for finding hydrocarbon microseepage in the petroliferous regions of the Tatipaka and Pasarlapudi areas of Krishna Godavari Basin, India. Pet Sci 10:19–29. https://doi.org/10.1007/s12182-013-0245-x
Shan B, Wang G, Cao F, Wu D, Liang WX, Sun RY (2019) Mercury emission from underground coal fires in the mining goaf of the Wuda Coalfield, China. Ecotoxicol Environ Saf 182:109409. https://doi.org/10.1016/j.ecoenv.2019.109409
Song ZY, Kuenzer C (2014) Coal fires in China over the last decade: a comprehensive review. Int J Coal Geol 133:72–99. https://doi.org/10.1016/j.coal.2014.09.004
Song ZY, Kuenzer C (2017) Spectral reflectance (400–2500 nm) properties of coals, adjacent sediments, metamorphic and pyrometamorphic rocks in coal-fire areas: a case study of Wuda coalfield and its surrounding areas, northern China. Int J Coal Geol 171:142–152. https://doi.org/10.1016/j.coal.2017.01.008
Song ZY, Kuenzer C, Zhu HQ, Zhang Z, Jia YR, Sun YL, Zhang JZ (2015) Analysis of coal fire dynamics in the Wuda syncline impacted by fire-fighting activities based on in-situ observations and Landsat-8 remote sensing data. Int J Coal Geol 141-142:91–102. https://doi.org/10.1016/j.coal.2015.03.008
Song ZY, Wu DJ, Jiang JC, Pan XH (2019) Thermo-solutal buoyancy driven air flow through thermally decomposed thin porous media in a U-shaped channel: towards understanding persistent underground coal fires. Appl Therm Eng 159. https://doi.org/10.1016/j.applthermaleng.2019.113948
Song ZY, Huang XY, Jiang JC, Pan XH (2020) A laboratory approach to CO2 and CO emission factors from underground coal fires. Int J Coal Geol 219. https://doi.org/10.1016/j.coal.2019.103382
Tobin-Janzen T, Shade A, Marshall L, Torres K, Beblo C, Janzen C et al (2005) Nitrogen changes and domain bacteria ribotype diversity in soils overlying the Centralia, Pennsylvania underground coal mine fire. Soil Sci 170:191–201. https://doi.org/10.1097/00010694-200,503,000-00005
Wang YM, Li XQ, Wang WZ, Guo ZX (2017) Experimental and in-situ estimation on hydrogen and methane emission from spontaneous gasification in coal fire. Int J Hydrog Energy 42:18728–18733. https://doi.org/10.1016/j.ijhydene.2017.04.192
Wang G, Cao F, Shan B, Meng M, Wang W, Sun RY (2019) Sources and assessment of mercury and other heavy metal contamination in soils surrounding the Wuda Underground Coal Fire Area, Inner Mongolia, China. Bull Environ Contam Toxicol 103:828–833. https://doi.org/10.1007/s00128-019-02734-7
Wang HY, Zhang JP, Zhang L, Wang JL, Xu ZH (2020) Gas emission and soil chemical properties associated with underground coal fires, Wuda Coalfield, Inner Mongolia, China. Nat Resour Res 29:3973–3985. https://doi.org/10.1007/s11053-020-09696-2
Zeng Q, Dong J, Zhao L (2018) Investigation of the potential risk of coal fire to local environment: a case study of Daquanhu coal fire, Xinjiang region, China. Sci Total Environ 640-641:1478–1488. https://doi.org/10.1016/j.scitotenv.2018.05.135
Zhang T, Xu J, Zeng J, Lou K (2013) Diversity of prokaryotes associated with soils around coal-fire gas vents in MaNasi county of Xinjiang, China. Antonie Van Leeuwenhoek 103:23–36. https://doi.org/10.1007/s10482-012-9782-3
Zhao ZX, Li ZC, Zhou JG, Cheng CF, Zhao RY, Sun JJ (2016) Effect of fire on soil organic carbon of natural Pinus massoniana forest in north subtropical area of China. Chin J Ecol 35:135–140
Funding
This work was supported by National Natural Science Foundation of China (no. 51874313) and National Key R&D Program of China (no. 2018YFC0808101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Wang, H., Zhang, J., Zhang, L. et al. A survey on the gas emissions and soil properties near the surface in a coal fire area. Arab J Geosci 14, 384 (2021). https://doi.org/10.1007/s12517-021-06737-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-06737-9