Abstract
This study describes the determination of lead and pH in Setif soils. Soil samples from the town of Setif were taken from a total of 100 subsurface soils, systematically sampled (regular 1 × 1 km grid). The lead concentration was determined by atomic absorption and the average lead concentrations ranged from 24 to 384 mg kg−1. The distribution of the different concentrations of lead and iso-concentration was distributed on the map of the exchange site with Arc GIS software. Compared with their local soil background values, higher concentrations of Pb were observed to different extents. The distribution of Pb concentrations has been explained by urban traffic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial, agricultural, and urban development has been accompanied by real health problems related to environmental pollution. Industrial companies release into the environment a significant number of pollutants such as lead, elements likely to contaminate the environment (water, air, soil, plants) and to have a real impact on human health (Garnier 2005). The environmental risks associated with soil pollution in Pb are increasingly worrying.
Anthropogenic lead in soil pollution is mainly due to automobile traffic, industrial activities (Delmas et al. 2002), sewage treatment, waste incineration, agricultural use of fertilizers and pesticides, the production of paints, and pigments (Oulhote et al. 2011; Triantafyllidou and Edwards 2012), which may have adverse effects on ecosystems (Facchinelli et al. 2001).
Bioavailability or lead in soil is dependent on soil properties (Luo et al. 2014) and an assessment of lead toxicity based on total metal concentrations may therefore overestimate or underestimate the actual availability and risk of lead in soil. It can also threaten the health of animals and humans along the food chain. However, only a fraction of the total concentration of metals in the soil is available to be absorbed and have toxic effects on soil organisms, defined as the bioavailability of metals (Peijnenburg et al. 2007).
In Algeria, lead tetraethyl is still used to improve the performance of gasoline. It is used for its anti-knocking role. It also allows lubricating the valves of the engines (Guibet and Montagne 2011).
The global consumption of fuels containing lead in Algeria reached 2.71 million tons in 2017 (1.18 million tons in normal gasoline, 1.53 million tons in super gasoline: Journée d’études sur la médiatisation de l’essence sans plomb SONATRACH Division Raffinage Production des Essences sans Plomb Post-Réhabilitation des Raffineries 23 Mai 2012).
The harmful effect of lead on the environment and on human health has been widely studied (Delon 1986; Cunningham and Berti 1993; Pichard 2002; Dumat et al. 2006; Al-Dabbas et al. 2015; Etchevers et al. 2014; Hojati 2017). Lead containing petrol seems to be the main source of contamination in the cities. This has led governments in developed countries to ban the addition of lead to fuels (Dela Guardia et al. 1983).
The purpose of this study is to determinate the concentration of lead in the soil of the city of Setif, one of the most populated cities in Algeria. Samples were taken from an area of approximately 100 km2 including the city and surrounding areas.
Materials and methods
All chemicals and reagents were obtained from Sigma Aldrich and were of analytical grade or equivalent.
Site description
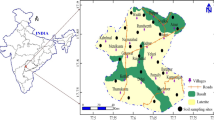
The city of Setif is located in the east of Algeria, in a highland region; it is 300 km east of the capital Algeria and rises to 1100 m altitude. It covers an area of 127 km2, with a large population, estimated at more than 410,000 inhabitants (2015). It occupies a predominant position among the cities of the highlands. It is also a major crossing point because it is crossed by the East-West Main road, as well as the N° 09 national road and the N° 5 national road (Fig. 1). Setif soils are generally sandy to clayey in texture and mostly classified as arid soils and are calcareous. Minerlogically, most of the soils are dominated by kaolinite, illite, smectite, and chlorite-typical for most arid and semi-arid soils (Djenba 2013).
Sampling
The sampling points were systematically distributed in the town of Setif and its surroundings, based on a regular 1 × 1 km grid. Thus, 100 grid cells were sampled (Fig. 2). In the city center, where most soils were very anthropogenic, samples were collected from gardens, cemeteries, and parks. Geographic coordinates were taken at each sampling point using a GPS. Samples were taken in February, March, and April 2015 at a depth of 0 to 2 cm. The soil samples were dried in the open air and sieved to 2 mm, according to the French standard NF X31-101 (French Agency for Standardization (AFNOR) 1994) and kept in closed plastic bags until analysis.
Measurement of the concentration of lead in the soil
To determine the concentration of lead in the soil, we followed two steps:
Mineralization
The digestion step is an essential step because it limits the interferences related to the organic matter. In our study, wet mineralization was favored and carried out as follows: A 5-g sample of the soil sample was digested in a solution containing 5 mL of nitric acid (65%), 10 mL of hydrochloric acid (37%), and 10 mL of the water. The whole is kept in a sand bath set at a temperature of about 300 °C until complete drying of the sample. Fifty milliliters of water is then added to the sample. After stirring and filtration, the concentration of lead is determined by flame atomic absorption spectroscopy (FAAS).
Calibration and quantification
The lead concentration was determined by flame atomic absorption spectroscopy (FAAS). This method was the most feasible and could be suitably adapted to a higher measurement concentration of more than 30 elements; the residual concentration of Pb was determined by atomic absorption spectrometry (Analytik Jena NovAA 400). A calibration curve (1–5 mg L−1) lead was prepared by appropriate dilution of a stock standard solution of lead nitrate (equivalent to 1 g L−1 lead) (Fig. 3). The lead wavelength (λ max) is equal to 283.3 nm. The software used to determine the absorbance is Win AAS (Version 3.15.0). The detection limit of this device is 0.25 ppm (Amiard et al. 1987). A Cyber Scan pH 510 digital pH meter equipped with a combined glass electrode-calomel was used for the pH measurement.
The standard was given by the following: Abs = 0.004 [Pb2+], where [Pb2+] is in milligram per liter. Furthermore, the coefficient of determination (R2) was found to be 0.997.
Estimation of lead content in gasoline
The analysis of lead in gasoline marketed in the town of Setif was carried out by X-ray fluorescence using a 9-W Panalytical Epsilon 3 spectrophotometers. This energy-dispersive X-ray spectrophotometer is designed for elemental analysis. The system is controlled by a computer on which the OMNIAN analysis software is installed. The sample is placed under helium flow during the analysis. The X-ray fluorescence spectrum was recorded with a silver filter with a thickness of 100 μm with a potential difference of 30.00 kV and a current of 300 μA.
pH measurement
Accurately weighed 10 g of the ground and sieved soil sample was weighed in a clean glass beaker of 50 mL and 25 mL deionized water was added to form a 1:2.5 soil/water slurry solution. Then, the beaker, containing the mixture, was placed on an automatic stirrer and stirred for 30 min. For the measurement of pH, the meter was calibrated with the standard buffer solutions of pH 4 and 7 before use. The pH measurements of the soil/water mixtures were carried out immediately after the soil samples were brought to the laboratory. The measurements were done by immersing the calibrated pH meter probes into the upper part of the slurry solution of the mixtures until the readings were stable (Odiyo et al. 2005).
Results and discussions
Results
The X-ray fluorescence analysis of gasoline marketed in the town of Setif shows that it contains 0.982 g L−1 of lead. This value corresponds to 1.53 g of tetraethyl lead (PTE) per liter of gasoline. It also contains much lower contents the following elements as Br (Fig. 4).
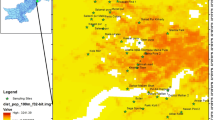
The results of the atomic absorption analysis of lead in our samples collected in the town of Setif are summarized in Table 1. Based on the results shown in Table 1, the distribution of the different concentration of lead and iso-concentration was distributed on the exchange site map with Arc GIS software (Fig. 5). The average lead concentration in the town of Setif is 67.705 mg kg−1. A minimum of 24.097 mg kg−1 was measured for sample No 53 and a maximum of 383.621 mg kg−1 was measured for sample N° 26. Generalized low-level contamination raised the overall concentrations to 30–100 mg kg−1(Davies 1995).
Discussion
Algeria is still using petrol containing lead tetraethyl (PET) as an antiknock additive at a concentration of 1.53 g L−1. This leads to soil pollution by lead. The city of Setif is suffering from this pollution. It is much more pronounced inside the city is especially in the city center where the road traffic is high. It is less important outside the city, especially in areas with no roads. Total Pb concentrations have a median of 67.705 mg kg−1 with a considerable range (24.097 and 383.621 mg kg−1). Thirteen percent of total Pb concentrations are less than 30 mg kg−1, but elsewhere, widespread low-level contamination has raised overall concentrations to 30–100 mg kg−1 (67%). In our study, the standard AFNOR, French Standard X 31-151 of 100 mg kg−1 is exceeded in 20% of the points of the city with a maximum of 383.621 mg kg−1 at position N° 26 (AFNOR 1994). This point is located next to a NAFTAL fuel distribution station. Most of the contaminated soil is located in the city center; the other points are located in the industrial zone ready for a road heavily used by vehicles, where the traffic is heavy.
Several studies have shown that lead deposition at ground level is positively correlated with road traffic density (Nawazish et al. 2012). High levels of lead in urban areas are mainly attributable to automobile exhaust and traffic emissions, in particular, leaded petrol and lubricating oils (Sharma and Dubey 2005).
Such an increase in lead concentrations in areas with heavy traffic has been observed in other cities. Indeed, despite the strong increase in the use of unleaded fuels in European countries, the level of lead in urban soils remains high due to the non-biodegradability of metals (Sánchez-Martin et al. 2000; Imperato et al. 2003). According to the study department French roads and motorways, most of the lead particle deposition takes place in the first 20 m of the road. Ground-level lead concentrations are inversely proportional to the distance from the road (Sánchez-Martin et al. 2000; Imperato et al. 2003; Setra 2004; Genc and Ulupinar 2010; Teju et al. 2014) indicating their linear dependence to the traffic density to the vehicular emissions. In developing countries, however, lead tetraethyl-containing gasoline is still widely used. For example, in Algeria, 89% of gasoline consumption is leading (Maas et al. 2010).
These data point to the existence of a causal link between the density of road traffic and the contamination of the environment by heavy metals, especially lead.
The chemical properties of lead in the soil are related to pH values; it is the factor that most influences the mobility and bioavailability of lead (Rieuwerts et al. 1998). For this type of study, pH is not only important in itself but also because it depends on the greater or lesser availability of the waterborne elements. At the pH values obtained (soils of 7.7–8.4, considered moderately alkaline), most of the metals in this range are in the form of low-solubility salts, being soluble at acidic pH values, as in the case of manganese, which means that it is not very likely to encounter toxicity problems in plants caused by trace elements (McBride 1994; Itanna 1998). This study showed us that pH did not influence the concentration of Pb measured in the 100 samples.
Conclusion
Pollution status, relationships with soil properties, and the main sources of Pb in topsoils from different land uses in the city of Setif, one of the most populated cities in Algeria, were studied. Compared with their local soil background values, higher concentrations of Pb were observed to different extents. Accordingly, industrial and urban soils showed higher concentrations and enrichments of Pb, rather than other land uses.
The spatial distribution of Pb concentrations has been explained by urban traffic. Lead concentrations showed spatial autocorrelation and high values were distributed over a large area. The sampling strategy of this study (regular grid with a mesh size of 1 × 1 km) is adequate to describe such models. The sampling strategy can be a useful tool for providing land managers with spatial data over large areas from a land management perspective. In addition, this work has allowed us to see the real impact of land use. Factories and road traffic have a significant effect on environmental pollution and in particular soils. The petrol containing lead tetraethyl is also a major source of environmental contamination by lead. Soil pH did not influence the concentration of Pb.
References
Al-Dabbas MA, Ali LA, Afaj AH (2015) Determination of heavy metals and polycyclic aromatic hydrocarbon concentrations in soil and in the leaves of plant (Eucalyptus) of selected locations at Kirkuk-Iraq. Arab J Geosci 8(6):3743–3753
Amiard JC, Pineau A, Boiteau HL, Metayer C, Amiard-Triquet C (1987) Application of atomic absorption spectrophotometry using Zeeman effect to the determination of eight trace elements (Ag, Cd, Cr, Cu, Mn, Ni, Pb and Se) in biological materials. Water Res 21(6):693–697
Association Française de NORmalisation (AFNOR) (1994) Qualité des sols. Recueil de normes Françaises. AFNOR Edition, Paris
Cunningham SD, Berti WR (1993) Remediation of contaminated soils with green plants: an overview. Vitro Cell Dev Biol - Plant 29(4):207–212
Davies BE (1995) Lead. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie Academic and Professional, London, pp 206–223
Dela Guardia M, Durrieu F, Voinovitch IA, Louvrier J (1983) Amélioration par emploi de tensio-actifs de la sensibilité et de la répétabilité du dosage du plomb en spectrométrie d’absorption atomique avec atomisation électrothermique. Spectrochim Acta B 38(4):617–624
Delmas C, Larpin L, Legret M, Astruc M (2002) Mobility and adsorption capacity of Pb and Zn in a polluted soil from a road environment: laboratory batch experiments. Environ Technol 23(4):381–390
Delon J (1986) Approche de la toxicologie des garages. Comité Hygiène Industrielle.
Djenba S (2013) Geological and geotechnical characteristics of the soils in the region of Sétif. 1st Annual International Interdisciplinary Conference, AIIC 2013, 24-26 April, Azores, Portugal– Proceedings, pp. 484–490
Dumat C, Quenea K, Bermond A, Toinen S, Benedetti MF (2006) Study of the trace metal ion influence on the turn-over of soil organic matter in various cultivated contaminated soils. Environ Pollut 142(3):521–529
Etchevers A, Bretin P, Lecoffre C, Bidondo M-L, Strat YL, Glorennec P, Tertre AL (2014) Blood lead levels and risk factors in young children in France 2008-2009. Int J Hyg Envir Health 217(4–5):528–537
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114(3):313–324
Garnier R (2005) Toxicité du plomb et ses dérivés. Pathologie professionnelle et de l'environnement, pp. 1–15
Genc A, Ulupinar E (2010) Transport of lead (Pb2+) ions through silty-clayey soils under acidic conditions. Transport Porous Med 84(3):699–709
Guibet C, Montagne X (2011) Carburants liquides, caractéristiques et principes généraux, technique d’ingénieur Combustibles fossiles, Editions T I
Hojati S (2017) Pollution assessment and source apportionment of arsenic, lead and copper in selected soils of Khuzestan Province, southwestern Iran. Arab J Geosci 10(23):528
Imperato M, Adamo P, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ Pollut 124(2):247–256
Itanna F (1998) Comparative study on soil pollution with toxic substances on farmlands close to old and new industrial sites in Ethiopia. Bull Chem Soc Ethiopia 12(2):105–112
Journée d’études sur la médiatisation de l’essence sans plomb (2012) Production des Essences sans Plomb Post-Réhabilitation des Raffineries SONATRACH Division Raffinage
Luo W, Verweij RA, Van Gestel CAM (2014) Assessment of the bioavailability and toxicity of lead polluted soils using a combination of chemical approaches and bioassays with the collembolan Folsomia candida. J Hazard Mater 280:524–530
Maas S, Scheifler R, Benslama M, Crini N, Lucot E, Brahmia Z, Benyacoub S, Giraudoux P (2010) Spatial distribution of heavy metal concentrations in urban, suburban and agricultural soils in a Mediterranean city of Algeria. Environ Pollut 158(6):2294–2301
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, Inc., New York
Nawazish S, Hussain M, Ashraf M, Ashraf MY, Jamil A (2012) Effect of automobile related metal pollution (Pb2+ et Cd2+) on some physiological attributes of wild plants. Int J Agric Biol 14(6):953–958
Odiyo JO, Bapela HM, Mugwedi R, Chimuka L (2005) Metals in environmental media: a study of trace and platinum group metals in Thohoyandou, South Africa. Water SA 31(4):581–588
Oulhote Y, Le Bot B, Poupon J, Lucas JP, Mandin C, Etchevers A, Zmirou-Navier D, Glorennec P (2011) Identification of sources of lead exposure in French children by lead isotope analysis: a cross-sectional study. Environ Health 10:75
Peijnenburg WJGM, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67(2):163–179
Pichard A (2002) Plomb et ses dérivés. Fiche INERIS-DRC-01-25590-ETSC-Api/SD-N°00df257_version2.doc, p.1-83
Rieuwerts JS, Thornton I, Farago ME, Ashmore MR (1998) Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chem Spec Bioavailab 10(2):61–75
Sánchez-Martin MJ, Sánchez-Camazano M, Lorenzo LF (2000) Cadmium and lead contents in suburban and urban soils from two medium-sized cities of Spain influence of traffic intensity. Bull Environ Contam Toxicol 64(2):250–257
Setra, service d’études techniques des routes et autoroutes (2004) la pollution des sols et des végétaux à proximité des routes : les éléments traces métalliques. Note d’information, p. 73
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17(1):35–52
Teju E, Megersa N, Chandravanshi BS, Zewge F (2014) Lead accumulation in the roadside soils from heavy density motor way towns of eastern Ethiopia. Bull Chem Soc Ethiop 28(2):161–176
Triantafyllidou S, Edwards M (2012) Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Crit Rev Environ Sci Technol 42(13):1297–1352
Acknowledgments
The authors thank the Material, Environment, and Energy Laboratory team (UR14ES26) of the University of Gafsa, Tunisia, for their technical help.
Funding
The authors would like to thank MESRS and DGRSDT (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et Direction Générale de la Recherche Scientifique et du développement technologique, Algeria) for financial support via the PRFU program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Abdullah M. Al-Amri
Rights and permissions
About this article
Cite this article
Sellami, S., Zeghouan, O., Lassaad, M. et al. Determination of lead concentrations in the soils of Setif City, Eastern Algeria. Arab J Geosci 13, 929 (2020). https://doi.org/10.1007/s12517-020-05977-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05977-5