Abstract
At Ras Kadhma embayment (north Kuwait), fabrics such as micrite envelopes, calcified microbial filaments, and micritic grain-to-grain bridges are observed in this modern sabkha environment in Kuwait Bay, associated with a tripartite facies classification. Three tidal zones were delineated by 8 vertical core samples, each marked by laterally diachronous units with different lithofacies and biofacies. Microbial populations (Lyngbya and Schizothrix), living within a distinct differentiated intertidal flat zone, were identified and their effects on sediments recorded. The extreme salinity, temperature, and chemical gradients in the shoreline environment have contributed to a microbial ecosystem that is trapping, binding, and biologically inducing CaCO3 precipitation, producing a variety of sedimentary structures now conventionally regarded as MISS (microbially induced sedimentary structures). The microbial fabrics are preserved in aggregates within the intertidal to the continental vadose zone. Core samples and outcrops were collected and analyzed mineralogically, chemically, and microbiologically. Field mapping on a meter scale reveals a concentric zonation, subtidal, intertidal, and supratidal zones, distinguished by textural and biological differences. Distinct lithofacies reveal varying stages of biomineralization with optimum conditions developed in the intertidal and lagoon sediments enhanced by microbial populations. The results of the study contribute to the understanding of the complex interaction between microorganism forming mats, the tidal flat sediments, and the physical parameters that control this setting in Kadhma Bay. Recurring colonization of these siliciclastic sediments in the intertidal zone will be permanently terminated in the coming decades by advancing intertidal sand bars resulting from the destruction of the Tigris-Euphrates delta.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
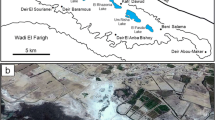
Ras Kadhma embayment is a small 2-km2 area in the northwest Arabian Gulf within Kuwait Bay (Fig. 1). The region is recognized for its prolific microbial sedimentary structures in both carbonate-rich and siliciclastic-dominant environments (Picha and Saleh 1977; Al-Yamani et al. 2004). Kuwait Bay is a shallow basin (approximately 8 m deep) with high salinity (36.5 to 43.5‰) (Jacob 1980; Al-Bader et al. 2011), fed by sediments from the Tigris-Euphrates delta (Al-Yamani et al. 2007). The area is influenced by anticlockwise currents in the gulf and the dominant shamal NW winds, together with anthropogenic influences (Al-Hurban et al. 2008). Tidal flats are in decline worldwide due to climate change, dams, storms, and habitat modification (Murray et al. 2015). The Tigris-Euphrates delta suffered irreparable damage during the First Gulf War by actions that led to water diversion and subsequent erosion of the delta. The tidal flat environments rimming Kuwait Bay are destined for cumulative destruction due to the sediment shifts inherent in the building of the coastal Silk City (2-m inhabitants), oil pollution, and siliciclastic bar encroachment (Ahram 2015). The purpose of this article is to account for the progression of sediment stabilization and cementation events in the advent of isolation by prograding tidal sand bars generated by sediment flux from the erosion of the Tigris-Euphrates delta. We propose (i) to record a new intertidal lagoon sub-environment in a modern tidal flat setting (Ras Kadhma coastline, Northern Arabian Gulf) in which a microbial influence is paramount to the construction and stabilization of sediments and meniscus cements induced by biomineralization, (ii) to focus only on surficial sediments as most of the sediments from the cores are loose sediments and there is no evidence to show the formal presence of microbial mat within the deeper layer of cored sediments and (iii) to describe a siliciclastic environment within the Arabian Gulf that exhibits a range of microbial textures in the intertidal zone.
Although a fraction of the size of other well-documented areas like Hamelin Pool (Jahnert and Collins 2013), Ras Kadhma embayment shares sediment cohesion and rheology aspects that are comparable with other renowned examples (Cuadrado et al. 2012). Physiographically, the embayment has a horseshoe shape open to the gulf but is nested between encroaching tidal sand bars to the east and manmade spits to the west (Fig. 1b). The tidally influenced areas can be divided into the supratidal zone that is inundated only under extreme events (storms, spring tides), the intertidal zone affected by daily normal tides, and the subtidal zone as the permanently submerged areas (Fig. 1a). The source of sand bars and organic carbon is supplied by the sediments of the nearby Tigris-Euphrates delta which delivers fluviatile sediments to the southern gulf by anticlockwise rotation of sediment-laden currents (AlShuaibi et al. 2012).
Microbes occupy a very broad range of niches in the natural world. These microorganisms, including cyanobacteria, are the key organisms in the formation of microbialites by organomineralization processes (Dupraz et al. 2009; Riding 2011). Microbialites are organo-sedimentary structures including stromatolites, thrombolites, and similar structures that occur as domes and columns in shallow waters. Recently, the Arabian Gulf is one of the most cited of microbial mat systems in the world because it produces microbially induced sedimentary structures (MISS), on a variety of sediment substrates (Paul and Lokier 2017). Noffke and Awramik (2013) added that the interaction between microbial communities and sedimentary dynamics can produce sedimentary structures called microbialites. They also showed two main types of the microbialites: (1) MISS (e.g., erosional remnant and pockets) as a result of biostabilization, leveling, baffling, trapping, and binding and (2) multilayered forms (e.g., stromatolites and thrombolites) that form in carbonate systems when mineral particles (carbonates) precipitated on EPS (microbial extracellular polymeric substance).
Stromatolitic growths have recently been discovered on the Quaternary sediments in the southwest corner of the study area (AlShuaibi et al. 2015) suggesting that the microbial-induced stabilization began at least in the Holocene. The study area is part of the greater Arabian Gulf basin (Alsharhan and Kendall 2003) and conforms to the subdivisions of gulf shorelines (i.e., subtidal, intertidal, and supratidal facies). The most cited microbialites are carbonate (e.g., aragonite, calcite, and dolomite) in composition, and studies on siliceous, phosphatic, manganese, iron, and sulfate examples are less known. Microbial communities living in marine, freshwater, and terrestrial environments induce trapping and binding of detrital particles and precipitate minerals. They are widely regarded as the Earth’s earliest ecosystem and the oldest fossilized bacteria in Earth Planet history are included in MISS, from the 3.48-Ga-old Dresser Formation, Pilbara Block, Australia (Noffke et al. 2013).
A key requirement for sandy sediments of intertidal environments to be colonized by microbial populations, leading to the formation of microbial mats (Krumbein 1983; Noffke 2010), is a low rate of sedimentation (Cuadrado et al. 2012). Baffling grains trap and bind grains in the growing mats (Noffke et al. 1997; Gerdes et al. 2000). The mats baffle and trap grains; they bind the grains into the mat matrix while the mats protect the sedimentary surface against attrition referred to as biostabilization. Microbial extracellular polymeric substances (EPS), which are produced by microbes, are important in providing nucleation sites and facilitating sediment trapping even when the environment is stressed (Decho 1990), as in our present desert site. Noffke et al. (2001) defined biofilms to be aggregates of microbial cells and their extracellular polymeric substances (EPS) which develop on all kinds of interfaces by adsorption (chemical absorption). Growth takes place during favorable ecological conditions (Noffke 2010). The mats grow on different types of substrate, mostly on submerged or moist surfaces, and occur in many environments including offshore transitions (Pazos et al. 2015). They colonize environments ranging in temperature from − 40 to 120 °C (Schieber et al. 2007). These mats and their microbes bind and trap minerals that lead at the end to lithification of sediments (Noffke et al. 1997). Noffke and Awramik (2013) showed that microbial communities in these mats interact with the surrounding sediments and even affect erosion, deposition, and deformation caused by wave action and currents.
Geological setting
The study area is located on a narrow headland in Ras Kadhma, Kuwait. The area is confined by the Jal Az-Zor escarpment to the west and by the Arabian Gulf to the east. Previous limited studies identified this tidal flat sedimentary cover overlying Late Quaternary alluvial fans (Al-Zamel and Al-Sarawi 1998). The area has an extremely low annual rainfall (less than 14 mm) with summer daily temperatures ranging between 45 and 47 °C (Al-Zamel and Al-Sarawi 1998). The area is located in northwest Kuwait Bay between 29° 23′ 46′′ N and 47° 44′ 38′′ E and is influenced by the nearest major fluvial source, Shatt Al-Arab River, the distal zone of the Tigris-Euphrates delta. Ras Kadhma embayment has a shallow shelf with an average depth of < 10-m deep (AlShuaibi et al. 2015). The embayment is subjected to semi-diurnal tides that range between 3.5 and 4.3 m (Hayes 1977).
The Ras Kadhma area contains coastal deposits of tidal flats, sabkhas, and sand dunes on a shallow ramp. The area is associated with low-energy waveforms and is composed of laminated or rippled clay, silt, and fine sand. The sedimentary record mainly consists of peritidal carbonates and intercalated mixed carbonate-siliciclastic deposits, with elevated contents of organic matter (AlShuaibi et al. 2012). Discontinuity surfaces are expressed as abrupt facies changes revealed by field mapping.
Sampling and methodology
A detailed geological map was constructed prior to sampling of the facies in the embayment. This map consists of 3 facies identified as subtidal, intertidal, and supratidal zones based on mineralogy, chemical components, geomorphological features, and biological and microbiological association (Figs. 1 and 2). The field units identified in the geological map are as follows:
Subtidal facies
This zone is submerged by the sea most of the time and briefly exposed at the low tides. Muddy tidal flats on the bay side are exposed to waves and tides. It can be distinguished from other tidal zones by muds and very fine-grained sands with bicarbonate (Fig. 1a). It is characterized by high biological diversity (Table 1). The proliferation of crabs and crustaceans suggests that the subtidal zone is well aerated with oxygen which encourages bioturbation of the organic-rich sediments and, hence, poor preservation of preexisting microbial mats.
Intertidal facies
This zone is a coastal zone that is under the influence of tides occurring between open sea and land, which is submerged by seawater twice a day. Non-vegetated expanses of mud and sand within this zone are cut by NW-SE oriented narrow tidal channels. Also, these sediments are partially covered by tidal arcuate sandbars with a NE-SW orientation which are recognized as dissected sand lenses on the east side of the mapped area (Fig. 1a and 3b). The embayment is currently being wind-deflated on the landward side and concurrently being isolated by large sandbars on the shoreline. These encroaching sandbars are generated by the currents which deposit the sands gradually on the intertidal zone through high/low tides. Semicircular lagoons located in this zone are characterized by stable water with a thick brown micritic sediment. Microbial mats in the lower seaward side of this area are clearly observed on the fractures of the cemented beachrock crust (Fig. 3d). The upper intertidal zone which lies on the north side of the study area, just below the boundaries of the supratidal, is characterized by polygons of mud cracks (Fig. 3f). Additionally, the organo-sedimentary textures documented in the intertidal zone (Fig. 4) have a specific distribution regarding topography, erosion, water depth, temperature, and rate of evaporation. Each zone of the intertidal zone (i.e., lower, middle, and upper) has a characteristic microbial fabric because of the interplay between sediment supply and diurnal changes in tides. Smooth mats occur in the lower intertidal zone, where rapid deposition of organic matter onto sandy layers occurs by rapidly advancing tidal waters. Organo-sedimentary laminae are deposited during tidal cycles, as shown by pioneering microbial mats in Fig. 4. Polygonal shrinkage cracks with tufted textures are found in the upper intertidal zone, where evaporation is at the maximum.
Distinct lithologies present in the intertidal zone of Ras Kadhma Bay. (a) Intertidal lagoon. The arrow shows the hinterland of the thinly bedded beachrocks that are thinly coated with recently developed microbial mats. (b) Example of one of the arcuate sandbars that occur on the east side of the study area. (c) New fresh microbial mats (yellow arrow) covering intertidal lithified sediments. The scale (ruler) is 20 cm. (d) Close-up image of the stromatolitic beachrock. Notice how microbial mats fill the fractures between breccia clasts and provide a semi-cohesive jigsaw of clasts. Scale (pen) is 12 cm. (e) Beachrock clasts (blue arrows) are held in a brittle cohesive hardground (red arrows; exposed sediments) by microbial mats (yellow arrows). The scale (ruler) is 20 cm. (f) Mud cracks in salt marsh of the upper intertidal zone with the absence of mat growth due to high evaporation
Organo-sedimentary textures of the intertidal zone of Ras Kadhma tidal flats. Figures a, b, c, d and e are from the lower intertidal zone, f is from the middle, and g and h are from the upper zone of the intertidal. (a) Thin layer of light gray smooth microbial mats overlying interbedded mats and sandy layers (pink). Kuwaiti coin diameter = 26 mm. (b) Curled and rolled-up gray smooth mats exposing underlying unlithified sand. Feature produced during erosion by tidal scour. Note the semi-desiccated rim forming a collar. (c) Microbial mats filling fractures and draping the lithified stromatolitic beachrock. (d) Pitted microbial mats covering current ripples (baffling) and penetrated by bioturbation features. Linear features produced by Cerithidea gastropods. Scale (shovel) is 57 cm. (e) Microbial encrusted pellets on the rim of a large crater caused by bioturbation of mudflats by a colony of mudskippers. (f) Salt-encrusted black microbial mats (biostabilization) restricted to the stoss slope of small current ripples. This can be considered an example of a pioneering biostabilization feature of the stoss slope in comparison with the exposed lee slope of the sand (beige). (g) Polygonal shrinkage cracks in tufted microbial mats of the upper intertidal zone. (h) Wrinkled microbial mats with salt encrustations in the upper intertidal zone
Supratidal facies
This zone is above high tide and flooded only during high spring tides and storm surge. It is characterized by prolonged exposure, and the sedimentary deposits are typical of a coastal arid environment. It consists also of nabkhas and salt marshes. The nabkha dunes are fine to medium sandy-grained eolian deposits with distinctive flora, Salicornia europaea being the major species. The nabkhas are sporadically scattered along the supratidal zone and mostly have an elongated short domal shape with an average height of 23 cm and an average length of 2 m.
Profiles of the sedimentary history are recorded by vertical coring into soft sediment using plastic acrylic tubes. In early May, eight cores were collected (duplicated samples of four cores each) to recover sediments representative of the area (Fig. 1a). On the west side of the beach lies a lagoon within the intertidal zone with a maximum depth of 1 m of water that is rimmed by lithified mat-encrusted breccia. Samples were stored in an icebox, kept in aluminum foil, and transported to the laboratory for analysis. Furthermore, local seawater was sampled for comparison with the pore water chemistry of the cores. Also, surface consolidated sediment samples were collected that represent fresh mats and lithified mat. These siliciclastic sediments exhibit the full range of MISS structures (Noffke 2010), both vertically and in the literal sense.
All cores and surficial samples were subjected to petrographic, mineralogical, and microbial studies. A typical sample was dried in the oven for XRD and SEM preparation and subsequently homogenized. For geochemical investigations, both lithified and core samples were subjected to three laboratory analyses: pigment analysis, direct microscopy investigation, and growth chamber records. The laminae were homogenized by mixing 2 grams from each lamina. For XRD analysis, the representative lamina from each zone was extracted to obtain the mineralogical composition. Powdered samples were subjected to XRD analysis using the Siemens D5000 X-ray Diffractometer equipped with a position-sensitive detector. X-ray patterns were collected in an angular range between 10°and 80° 2Φ. Our diffractometers typically use Bragg-Brentano geometry. The computer program DIFFRACplus with the International Center for Diffraction Data (ICDD) library was used for mineral identification and semi-quantification. Samples were examined on a Supra 50 LEO variable pressure scanning electron microscopy (VPSEM) system equipped with a Rontec EDX analyzer.
In order to determine the chemistry of the sediments, both on-site analysis and lab analysis were performed. On-site wet chemistry for pore water proceeded as follows. After core sampling, parameters measured are pH, temperature, salinity, conductivity, and percentage of dissolved oxygen for the remaining water in each hole using multiparameter water probe. This was to ascertain the optimum conditions for microbial growth (Wetzel 2001). Twenty grams of sediment was added to 20 ml of water and stirred for 5 min. After settling, the pH of the clear solution was taken. Also, the specific conductivity of collected soil was taken using a conductivity meter. In addition, moisture percentage (versus solid percentage) of the three different facies have been measured.
Organic-rich samples were subjected to CHNS analysis to determine total C, H, N, and S content (%). CHNS analysis is based on the classical Dumas method where samples are combusted in a pure oxygen-rich environment by using the Elementar Vario Micro Cube machine before the introduction to the elemental analyzer. The field emission scanning electron microscope (FESEM) was used for the observation and characterization of materials on a nanometer to micrometer scale. The FESEM is a Zeiss field emission type with the LEO SUPRA 50VP (variable pressure). The electron column type is the Gemini Zeiss column with the Schottky field emitter. The settings used were acceleration voltage of 0.2–30 kV and a resolution of 1 nm at 15 kV and 1.9 nm at 1 kV. Samples were coated with a thin film of gold or platinum.
Microbial populations that inhabit the surface, interior of mats, and rimming grains were subjected to three experiments: pigment analysis was used to determine the amount of chlorophyll content that supported photosynthetic populations. Other techniques such as direct microscopy investigation and growth chamber experiments were applied to stimulate the growth of the microbial populations. The samples were kept in flasks under conditions that encouraged the perpetuation of the microbial populations over several weeks. Duplicates were performed. One gram of master mix was added to 100 ml of MN media (minimal media for algae), in a 250-ml flask and incubated in the growth chamber (Conviron machine type) for 2 weeks at 25–30 °C. Also, lithified sediments and mat samples were subjected to the same experiments.
Results
The hand specimen of cemented beachrock crust is grayish-brown in color, has medium grain size, and appears moderately sorted with crystalline carbonates and micritic peloids (calcite). An evidence of dissolution also appears through the identification of microkarstification features in the interior of lithified sediment. Petrographic details are shown in (Fig. 5). There are also lime clasts, mostly peloids, floating in a matrix with sub-rounded to rounded quartz grains that are cemented and coated with micrite. Some microfossils are present in this beachrock. Rounded to oval-shaped ooids with quartz nucleus were recognized. Brecciated lithoclasts with micritic cements also occur. The matrix is mostly micrite with minor microcrystalline calcite (sparite). Quartz grains themselves are coated by carbonates (Fig. 5 b, c, d, e, and f). XRD results of the cemented beachrock and the attached lithified microbial mats show that the breccia contains 42% quartz, 56% aragonite, and 2% calcite. In the case of grain-attached microbial mats, quartz is considerably reduced to 27%, calcite increases to 24%, and dolomite increases to 7%. Other minerals also present are montmorillonite, halite, microcline, and albite. Table 3 shows the average mineral content of the three tidal zones and lagoon, including calcite, aragonite, quartz, halite, albite, gypsum, feldspar, and minor fractions of clay minerals.
Petrographic investigation of beachrock. (a) Brecciated rounded carbonate grapestone (black arrow) cemented by mats (white arrow). From b to f photomicrographs of the sample. (b, c, and d) General view of brecciated beachrock showing its components: QTZ, for quartz grains; LM, lime clasts; LTH, lithoclasts; FSL, fossil; and arrows represent the micritic envelope which surrounds quartz grains and occasional lithoclasts. (e) Different stages of growth of micrite envelope around quartz grains (arrows). The presence of several generations of re-cemented quartz is evidence of the reworking of the grains. Micrite envelopes on coated quartz grains are enveloped by microbial colonies. (f) High magnification images of coated grains with biofilms; grains are bound together by micrite. Micrite trapped by microbes envelopes with two quartz grains in contact
Chemical parameters are shown in Table 2 indicating that the intertidal zone has the highest pH, temperature, salinity, dissolved oxygen, and conductivity. Total C, H, N, and S (CHNS) content shows that the subtidal zone has the maximum CHNS contents in comparison with the other facies (Table 2). The pH, specific conductivity, and moisture content are compared with a seawater sample to compare the physical/chemical differences between seawater and tidal zone pore waters. The intertidal zone pore fluids have the highest pH values. The salinity of the intertidal zone (44.7‰) is also higher than the subtidal zone (37.8 ‰). The biomass of the photosynthetic microorganisms in the samples from different tidal zones was measured, and the amount of chlorophyll content (Table 2) indicates that the intertidal zone has higher organic matter content, which reflects the large community of photoautotrophic microorganisms living there (Table 3).
The XRD results of the core samples indicate there are clear differences in mineralogical contents between the facies (Fig. 6). The percentage of calcite increases toward the marine basin, unlike quartz. The subtidal zone contains calcite (49%), quartz (19%), feldspars (2–8.5%), dolomite (5.5%), clay minerals (5.5%), and halite (3%). The percentages are very different in the supratidal. It does not contain the mineralogical diversity as compared with the other facies. It consists mainly of quartz (70.4 to 100%) with calcite (4.3 to 10.2%) with minor fractions of gypsum, salts, and clay minerals. Diffraction results of the beachrock show that it contains about 56% aragonite, 42% quartz, and 2% calcite, while the fresh microbial mats that cover the surface layers of the most intertidal sediments contain 27% quartz, 26% calcite, 23% montmorillonite (clay), 7% dolomite, 6% microcline (alkaline feldspar), 6% halite, and 5% albite. Together with minerals are microbes that inhabit the pores between mineral grains of a rock.
SEM investigations of microbial mats and cements reveal complex nanometer-scale interactions between microbial populations and the sedimentary grains. SEM micrographs reveal that various morphological forms of sediment grains and particles contain a variety of attached microbial reproductive bodies and profuse filamentous bridges between the grains, which are developed to a maximum in the intertidal zone and associated lagoon (Fig. 7). Carbonate micrite envelopes are laminated with the meniscus cement that was generated from microbial colonies preserved within the pore space. The supratidal zone examination is apparently barren of the microbial population. A representative collage of the relationships between microbes and minerals generated in situ is presented in Fig. 8. These microbial populations vary in shapes, types, and occurrences from one zone to another. After several weeks in the growth chamber, the microbial populations bloomed, and the mats clearly proliferated in the flasks. Recorded changes were noted in the dominant species. From the microbiological and SEM investigations above, filamentous cyanobacteria appear to be the most dominant microorganisms in the tidal sediment samples, Oscillatoriales (e.g., Lyngbya, Oscillatoria, and Microcoleus) and Synechococales (e.g., Schizothrix) orders in particular (Rippka et al. 1979).
SEM micrographs of partially lithified beachrock crust by mats and meniscus cement. (a) General view of the intertidal sediments that are cemented together. (b) Image that zooms in on the edges of grains showing the mucus-like organic EPSs (arrow). (c) Long filament (white arrow) with precipitated carbonate (black arrows) on its biofilms. (d and e) Filamentous microorganisms bridge the gap between grains (meniscus cementing), occluding the phreatic space. (f) High power image of a filament showing the function of EPS in binding fragments. (g) Filament with carbonate precipitation (white circle). (h) Quantitative and qualitative analysis for the marked point in Fig. 7g showing the presence of calcite (or proto calcite)
Photomicrographs of microbial genera living in the sediments and within the fresh microbial mats. (a) Colony of Oscillatoriales (Cyanobacteria). (b) Schizothrix (filamentous cyanobacteria) with particles on biofilms (arrows). (c) Oscillatoriales (probably Lyngbya—a genus of cyanobacteria). (d) Extended biofilms of Oscillatoriales (arrows). (e and f) Proto-carbonate grain attached to Lyngbya (arrows)
Discussion
Siliciclastic environments under arid conditions are considered to represent extreme environments for microbial colonies due to current energies, temperature, grazers, and ultraviolet light. In modern siliciclastic environments, microbes form organic layers that carpet the shorelines which can be preserved or eroded depending on current intensity leading to erosive ripples (Noffke et al. 2001). Dense mats that shelter sediments contribute to biostabilization or cohesion of the shoreline (Noffke et al. 2003) and are recognized as an important process in both the intertidal and subtidal settings (Cuadrado et al. 2012). Many studies elsewhere discuss the role of microbial mats in their ability to lithify the sediments and to build structures such as stromatolites. Such activities are (1) biostabilization, (2) microbial leveling, and (3) baffling, trapping, and binding (Noffke et al. 2001). The first one refers to the stabilization of sediments against erosion due to the filamentous bacteria network which resembles carpets that cover the sediment surface with its adhesive mucous secretions and envelope the mineral particles. The next term, microbial leveling, is the smoothening of a rippled surface until a planar surface forms. Planar mats are being buried and water migrates intra-sedimentary which forms the crinkles in the sand. This happens during periods of low erosion and low sediment deposition rates (Noffke et al. 2001). Mineral particles that are transported by currents or winds are transported across the mat surface in a way reminiscent of baffling where sediments are trapped by the filaments. After that, the grains will be incorporated into the growing biomass and bound to each other. Over time, these processes produce laminated patterns within the sediments and, after lithification, stromatolites form especially in carbonate environments (Noffke et al. 2003).
Many authors have demonstrated the wide occurrence of microbial mats and microbialites along the shore of Kuwait including, Duane and Al-Zamel (1999), Al-Zaidan et al. (2006), and Al-Mohanna et al. (2007). AlShuaibi et al. (2012) recorded oncolites forming in siliciclastic sediments occurring within the intertidal zone sediments on the northern coast of Kuwait. The southern coastal zones of Kuwait by contrast are dominated by carbonate precipitation in predominantly carbonate facies according to Duane and Al-Zamel (1999), in contrast to the northern shores of Kuwait where microbial populations are dominant in the siliciclastic sediments.
Extracellular polymeric substances (EPS) have trapped and preserved laminated textures in the intertidal zone at Ras Kadhma (Figs. 5, 7, and 8), in precisely this fashion and bind calcium cations that precipitate later as calcium carbonate (Wei et al. 2015). The formation of polymorphs of calcium carbonate is influenced by parameters such as pH and temperature (Santomauro et al. 2012). pH differences within the study area (Table 2) clearly support alkaline pressuring. Some microkarstification features appear in the sample and these features are aspects of dissolution and re-binding of clasts. Meniscus-type cements, which are observed in hand specimens and confirmed by microscopic and SEM images (Figs. 5, 7, and 8), are clearly formed in tidal flat environments and are related to filament calcification, trapping of percolating micrite, and microbially induced carbonate formation (Hillgartner et al. 2001). Meniscus cements have been interpreted as indicators for marine vadose diagenesis (Harris et al. 1985). The internal porosity of mineral coating supports microbial activity (Gülay et al. 2014). Percolating groundwater in the north of Kuwait is a result of the seepage of continental flux, mostly from within the Kuwait Group of sediments. The mixing of groundwater and seawater apparently created dissolution features and micritic envelopes in the stromatolitic beachrock in the study area during Holocene events (AlShuaibi et al. 2015).
The formation of microcrystalline micrite envelopes (˂ 10 and ˃ 60 μm) are considered to be constructive in nature. Grains’ boundaries are bridged often in a double convex shape resembling meniscus cements. Reworked clasts up to several centimeters in diameter display the same microfabrics (Fig. 3). Pores are occluded by micrite and suggest that the fabric acted as a stabilizing influence on the sediment at the surface which eventually led to lithification of the beach rock in the early Holocene. The microfabrics are developed to the maximum in the lagoonal zone within the western microbial mats of the lowermost intertidal zone. Calcified filaments are in great abundance in the lithified microbial mats of the intertidal zone and bind the grains between the brecciated fragments (Fig. 3d and 5). These micritic filamentous structures attain thicknesses ≈ 60 μ or more and can form hundreds of micron-thick meniscus bridges themselves between grains. They have other uses as well acting as baffles for the percolation of fluids bearing calcium ions. Comparable fabrics are known where grains aggregate in environments with low sedimentation rates and low-energy beachheads.
Many researchers focus on physical (e.g., water temperature) and chemical (e.g., pH) parameters that influence the growth of microbes, in particular, cyanobacteria (Paerl and Tucker 1995; Wetzel 2001; Al-Bader et al. 2011; Gao et al. 2012; Paul and Lokier 2017). Cyanobacteria occur over a wide temperature range and colonize in warm water temperatures (Paerl and Tucker 1995). Their populations in the gulf respond to natural environmental changes as they increase during summer due to maximum light intensity and water temperature (Al-Bader et al. 2011). Paul and Lokier et al. (2017) found that the coastal sabkhas of Abu Dhabi which are marked by high salinity and temperature are microbially mediated. They prefer alkaline conditions and can survive when pH is elevated even up to 9 (Gao et al. 2012). Moreover, photosynthetic activities increase the pH (Wetzel 2001), so high pH can be used as an indicator for their occurrence and dominance in the sediment. From field measurements (Table 2), all parameters (pH, temperature, salinity, dissolved oxygen, and conductivity) attain the highest values in the intertidal zone. That indicates the intertidal zone has the optimum conditions for photosynthetic microorganisms to flourish. It also has the highest chlorophyll content among the tidal zones (Table 2).
Overall, as temperature increases, salinity increases, and both are important for microbial population growth (Awata et al. 2012). From Table 2, it is noteworthy that salinity increases with the increase in temperature and evaporation of waters from the sediments. Other factors are important in the binding of sediment particles. The cations in seawater help in bridging negatively charged polymers between microbes and sediment surfaces (Gerbersdore and Wieprecht 2015). The dissolved oxygen concentrations are high in the more mixed water areas such as the intertidal and its lagoon, and this is interpreted as a consequence of the photosynthetic activity. Through photosynthesis, the microbes absorb CO2 and/or HCO3- and release OH- and that leads to an increase in pH and concentrations of carbonate anions within the solution (Santomauro et al. 2012). pH is highest in the intertidal zone followed by the supratidal and then the subtidal. Lagoon water has the lowest pH, while that in the open seawater is higher than lagoon water, and that probably reflects the absence of refreshing tides reaching the lagoon by advancing sandbars with time. Specific conductivity results show that the intertidal zone has the highest conductance. Table 2 also shows that the subtidal zone is elevated in C, S, and H, while N contents are low in all zones. High C and low N media are considered an optimum chemical medium for bacterial growth (Decho 1990). In addition, many species are capable of fixing N2, especially cyanobacteria (Kremer et al. 2008). For this reason, the low N2 contents may be an evidence of the existence of these species.
Microscopic investigations (Fig. 8) reveal the numbers and types of microorganisms that exist within the samples, while SEM (Fig. 7) shows the texture of grains and the morphology of various populations of microorganisms. Subtidal samples reveal a small number of microorganisms, especially the coccoidal and filamentous types. The lagoon has a large variety of microorganisms including filamentous bacteria and foraminifera. Crabs are grazers for microbial mats which are the primary source of nutrition for both benthic and pelagic macrofaunal species within Kuwait Bay (Al-Zaidan et al. 2006). A large number of crabs in the subtidal zone compared with the intertidal reflect the number of mats that are grazed and consumed (Table 1 and Fig. 2). By contrast, the supratidal sediments are barren of microorganisms due to its extreme summer temperature (up to 60 °C) and lack of moisture. SEM investigations of the cemented beachrock show that the grains are coated by microbial mat biofilms in thick bridging filaments.
The biomass of the photosynthetic microorganisms in the samples from different tidal zones was measured and it shows that the subtidal zone has the highest pigment content (Table 2). The meniscus cement of the intertidal beachrock clasts shows that the sediments support a large community of photosynthetic microorganisms. A comparison between chlorophyll content of the three zones indicates that the lithified mats from the lagoon have the highest pigment contents probably as a result of the protective nature of this environment and its high organic productivity.
The intertidal zone and its lagoon sediments are dominated by filamentous cyanobacteria (Lyngbya and Schizothrix), with sparse diatoms. There is no evidence of bacteria and algae in the supratidal sediments. It is apparent from the culture experiments that the filamentous microorganisms “Oscillatoriales” are the prime agents in coalescing mats (Cuadrado et al. 2012). These populations dominate in the subtidal, intertidal, lagoon, and the lithified mats with meniscus cement. Cyanobacteria biomass is at a maximum in the intertidal zone and its affiliate lagoon, where the pH ranges between 8 and 8.3, high temperature (30–32 °C), and high salinity (45–51‰) in comparison with the other zones. Filamentous bacteria occur in the mat network and they have glue-like surfaces (EPS) where grains are trapped. Families of cyanobacteria which are preferentially found in the area are Oscillatoriaceae (Lyngbya or Microcoleus) and Schizothrichaceae (Schizothrix) and are known binders and trappers of sediment (Noffke 2010; Riding 2007; Al-Bader et al. 2011; Frantz et al. 2015).
In Kuwait, Al-Bader et al. (2011) compared the seasonal diversity of planktonic cyanobacteria between the clear south shallow coastal waters (Khiran) of Kuwait with turbid north waters (Subiya). Chlorophyll measurements were higher in summer than in spring in both Khiran and Subiya. Their observations imply that filamentous cyanobacteria Lyngbya sp. dominated Khiran area during spring, while the opposite was observed during summer where most of Subiya clones were filamentous (Oscillatoriales, Lyngbya.). No filamentous species were found in Khiran. Besides, the highest Synechococcus diversity was apparent during the summer in both locations. It can be concluded that Synechococcus thrive under high summer temperature in a pure carbonate environment (Al-Bader et al. 2011) and the Oscillatoriaceae group (especially Lyngbya) dominates the waters of Subiya, where siliciclastic sedimentation is at work.
The Ras Kadhma embayment is one of the many semi-restricted active basins in the northern Arabian Gulf that has received continuous evaporite and siliciclastic deposition since the Pleistocene. The Holocene sedimentary record of the area shows sedimentary structures and textures similar to those exposed elsewhere in the gulf and most probably formed by similar processes. Shallow-water carbonates associated with marginal marine sediments on the southern shores of the Arabian Gulf (Butler et al. 1982) are recognized but with local differences. Rhythmites of laminated gypsum and nodular anhydrite are common mineral transformations in the supratidal environment of Abu Dhabi (Butler et al. 1982), while Kadhma embayment (this study) holds only minor fractions of gypsum and salts (halite) as shown in Fig. 6. The continental fluid flux of freshwater results in different modes of mineral alteration, being restricted in northern Kuwait due to dissimilar mixing of marine and continental fluids to the Trucial Coast. The supratidal zone differs in other ways to the conventional architecture due to the presence of nabkhas identified in Kuwait (Khalaf et al. 1995). The presence of microbialites in the embayment was observed as an earlier Holocene event (AlShuaibi et al. 2015). However, apart from this narrow strip of thrombolites in the supratidal-intertidal transition, there is no reason to assume that continuous microbial mat stabilization was interrupted since then. Encroachment by the intertidal dunes is an ongoing hazard for tidal flat preservation in the future. The Tigris-Euphrates delta destruction during the Gulf War released once stable sediment that has isolated various environments along the coast and continues to do so (Al-Yamani 2008).
Microbial mats flourish not only in Kuwait’s coastal areas but also in other Arabian Gulf countries such as the United Arab Emirates and Qatar sabkhas. The siliciclastic-carbonate sabkha in Qatar is invaded with microbial mats that are composed of cyanobacteria, purple sulfur and purple non-sulfur bacteria, halophilic bacteria, and sulfate-reducing bacteria (Slowakiewicz et al. 2016). The coastal sabkhas of Abu Dhabi, in the United Arab Emirates, are also highly microbially mediated sites (Bontognali et al. 2010). Paul and Lokier (2017) show that Abu Dhabi shoreline stabilization occurred during recent transgressions incorporating pore-filling aragonite cements, high Mg calcite, and dolomite cements, marked by relatively high salinity and temperature (Lokier and Steuber 2009). Distinct carbonate textures result from the interaction between microbial growth patterns; the number of trapped carbonate particles, produced by organomineralization (Burne and Moore 1987; Dupraz et al. 2009); and aragonitic cement. This interaction should be recorded in the sediment facies and may be responsible for the variability in the morphology of the internal fabrics.
Jahnert and Collins (2013) show microbial deposits developed at Shark Bay tidal flats (Table 4). They occur in a highly evaporative saline environment with three tidal flats. The stressed conditions of the intertidal/subtidal environment produced the microbial mats by trapping, binding, and inducing CaCO3 precipitation, producing stromatolites and thrombolytic and cryptomicrobial forms. Table 4 shows the main differences between this data (Kadhma Bay) and other contemporary sites.
Conclusions
Sedimentary environments were mapped on a basis of lithological classification and textural differences. The following processes and findings are fundamental in explaining microbial processes involved in sediment stabilization and biomineralization in the Ras Kadhma coastal sector:
-
1
Thin microbial laminae are described and interpreted as typical exposures of the tidal flat environments expected in the arid climate of the Arabian Gulf.
-
2
New divisions of a low sedimentation shoreline at Kadhma embayment are subdivided into subtidal, intertidal (and lagoon), and supratidal categories which were delineated by field mapping and laboratory studies. Tidal sandbars are continuously encroaching and choking the intertidal zone and consequently will lead to extensive supratidal development. The encroachment of the intertidal sandbars from the NE (Tigris-Euphrates delta) will overwhelm the intertidal zone within decades, if not before.
-
3
Diagenesis of the sediments was enhanced where conditions (defined by chemical parameters) were amenable to microbial growth and responsible for microbial blooming, trapping, binding, biologically inducing CaCO3 precipitation, and producing sedimentary MISS structures (Noffke et al. 1997).
-
4
Tidal zones in the area have smooth bottom gradient and low relief bathymetry which indicates low-energy tidal variations, allowing for microbial preservation.
-
5
Microbial communities were found to favor the intertidal zone forming profuse carpet-like mats with different fabrics, but by comparison, only a limited microbial population occurs in the subtidal sediments due to excessive grazing and diurnal tidal encroachment. The supratidal zone is devoid of microbial populations that may induce biomineralization.
-
6
Microbial mats thriving in the intertidal and intertidal lagoon areas trap and bind carbonate, quartz grains, and bioclastic fragments through the mucus-like EPS. The Oscillatoriaceae group (Lyngbya in particular) dominates the waters of Kuwait Bay, where siliciclastic sedimentation is at work which contrasts with south Kuwait (shoreline constructed by carbonate sedimentation), where the Synechococcus is dominant.
-
7
Members of the filamentous cyanobacteria form a bloom that contributes to lithification and stabilization of the surface sediments of the area. The intertidal zone is the most suitable environment for microbial mat development because it has the optimum conditions for growth, and the grazing by macrofauna is limited due to the prolonged exposure. Textures of the microbial mats reflect the relationship between sediment texture and the ability of microbes to physically access organic substances and significantly determine sediment water content, nutrient availability, and grade of erosion.
In conclusion, microbes are playing an important role in the lithification of recent tidal sediments; the mats stabilize the sediments and trap it. The early lithification leads to the formation of microbially induced sedimentary structures (MISS).
References
Ahram A (2015) Development, counterinsurgency, and the destruction of the Iraqi Marshes. Int J Middle East Stud 47(3):447–466
Al-Bader D, Rayan R, Mahmood H, Al-Hasan R, Bobby L, Eliyas M (2011) Comparison of the seasonal diversity of planktonic cyanobacteria in clear and turbid shallow coastal waters of Kuwait using culture-independent techniques. Kuwait J Sci Eng 38:163–183
Al-Hurban A, El-Gamily H, El-Sammak A (2008) Geomorphic changes in Ras Al-Subiyah area. Kuwait. Environ Geol 54:1377–1390
Al-Mohanna SY, George P, Subrahmanyam MNV (2007) Benthic microalgae on a sheltered intertidal mudflat in Kuwait Bay of the Northern Arabian Gulf. J Mar Biol Assoc India 49:27–34
Alsharhan AS, Kendall CGSC (2003) Holocene coastal carbonates and evaporites of the southern Arabian Gulf and their ancient analogues. Earth-Sci Rev 61:191–243
AlShuaibi A, Duane MJ, Mahmoud H (2012) Microbial-activated sediment traps associated with oncolite formation along a Peritidal Beach, Northern Arabian (Persian) Gulf, Kuwait. Geomicrobiol J 29:679–696
AlShuaibi AA, Khalaf FI, Al-Zamel A (2015) Calcareous thrombolitic crust on Late Quaternary beachrocks in Kuwait, Arabian Gulf. Arab J Geosci 8:9721–9732
Al-Yamani F (2008) Importance of the freshwater influx from the Shatt-Al-Arab River on the Gulf marine environment. In: Abuzinada AH, Barth HJ, Krupp F, Böer B, Al Abdessalaam TZ (eds) Protecting the Gulf’s Marine Ecosystems from Pollution. Birkhäuser Basel
Al-Yamani FY, Bishop J, Ramadhan E, Al-Husaini M, Al-Ghadhban AN (2004) Oceanographic Atlas of Kuwait’s Water. KISR, Kuwait, p 203
Al-Yamani FY, Bishop JM, Al-Rifaie K, Ismail W (2007) The effects of the river diversion, Mesopotamian Marsh drainage and restoration, and river damming on the marine environment of the northwestern Arabian Gulf. Aquat Ecosyst Health Manag 10:277–289
Al-Zaidan ASY, Kennedy H, Jones DA, Al-Mohanna SY (2006) Role of microbial mats in Sulaibikhat Bay (Kuwait) mudflat food webs: evidence from δ13C analysis. Mar Ecol Prog Ser 308:27–36
Al-Zamel A, Al-Sarawi M (1998) Late Quaternary Sabkha sedimentation along Kadhmah Bay Coast, Kuwait, Arabian Gulf. Arab Gulf J Sci Res 16:471–495
Awata T, Tanabe K, Kindaichi T, Ozaki N, Ohashi A (2012) Influence of temperature and salinity on microbial structure of marine anammox bacteria. Water Sci Technol 66:958–964
Bontognali TRR, Vasconcelos C, Warthmann RJ, Bernasconi SM, Dupraz C, Strohmenger CJ, McKenzie JA (2010) Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 57:824–844
Burne RV, Moore LS (1987) Microbialites: organo-sedimentary deposits of benthic microbial communities. PALAIOS 2:241–254
Butler GP, Harris PM, Kendall CGSC (1982) Recent evaporites from Abu Dhabi coastal flats. In: Depositional and Diagenetic spectra of Evaporites, 3rd edn. SEPM Core Workshop, Calgary, pp 33–64
Cuadrado DG, Carmona NB, Bournod CN (2012) Mineral precipitation on modern siliciclastic tidal flats colonized by microbial mats. Sediment Geol 271–272:58–66
Decho AW (1990) Microbial exopolymer secretions in ocean environments – their role(s) in food webs and marine processes. Oceanogr Mar Biol 28:73–153
Duane M, Al-Zamel A (1999) Syngenetic textural evolution of modern sabkha stromatolites (Kuwait). Sediment Geol 127:237–245
Dupraz C, Reid RP, Braissant O, Decho AW, Norman RS, Visscher PT (2009) Processes of carbonate precipitation in modern microbial mats. Earth Sci Rev 96:141–162
Frantz CM, Petryshyn VA, Corsetti FA (2015) Grain trapping by filamentous cyanobacteria and algal mats: implications for stromatolite microfabrics through time. Geobiology 13:409–423
Gao Y, Cornwell JC, Stoecker DK, Owens MS (2012) Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences 9:2697–2710
Gerbersdore SU, Wieprecht S (2015) Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 13:68–97
Gerdes G, Klenke T, Noffke N (2000) Microbial signatures in peritidal siliciclastic sediments: a catalogue. Sedimentology 47:279–308
Gülay A, Tatari K, Musovic S, Mateiu RV, Albrechtsen HJ, Smets BF (2014) Internal porosity of mineral coating supports microbial activity in rapid sand filters for groundwater treatment. Am Soc Microbiol 80:7010–7020
Harris PM, Kendall CG, Lerche I (1985) Carbonate cementation – a brief review. In: Carbonate Cements (eds. Schneidermann N, Harris PM). SEPM Spec Publ 36:79–95
Hayes MO (1977) Preliminary investigation of coastal processes and geology of Kuwait City waterfront. Technical paper, Kuwait Municipality, Kuwait, 37p
Hillgartner H, Dupraz C, Hug W (2001) Microbially induced cementation of carbonate sands: are micritic meniscus cements good indicators of vadose diagenesis. Sedimentology 48:117–131
Jacob PG (1980) Oceanographic data for Kuwait marine environment. Kuwait Institute for Scientific research. Technical Report, Kuwait, p 128
Jahnert RJ, Collins LB (2013) Controls on microbial activity and tidal flat evolution in Shark Bay, Western Australia. Sedimentology 60:1071–1099
Khalaf FI, Misak R, AI-Dousari A (1995) Sedimentological and morphological characteristics of some nabkha deposits in the northern coastal plain of Kuwait, Arabia. J Arid Environ 29:267–292
Kremer B, Kazmierczak J, Stal LJ (2008) Calcium carbonate precipitation in cyanobacterial mats from sandy tidal of the North Sea. Geobiology 6:46–56
Krumbein WE (1983) Stromatolites - the challenge of a term in time and space. Precambrian Res 20:493–531
Lokier S, Steuber T (2009) Large-scale intertidal polygonal features of the Abu Dhabi coastline. Sedimentology 56:609–621
Lokier SW, Andrade LL, Court WM, Dutton KE, Head IM, van der Land C, Paul A, Sherry A (2017) A new model for the formation of microbial polygons in a coastal sabkha setting. Deposit Record 3(2):201–208
Murray NJ, Ma Z, Fuller R (2015) Tidal flats of the Yellow Sea: a review of ecosystem status and anthropogenic threats. Austral Ecology 40:472–481
Noffke N (2010) Microbial mats in sandy deposits from the Archean era to today. Springer (publisher), Heidelberg, p 196
Noffke N, Awramik SM (2013) Stromatolites and MISS—differences between relatives. GSA Today 23:4–9
Noffke N, Gerdes G, Klenke T, Krumbein WE (1997) A microscopic sedimentary succession indicating the presence of microbial mats in siliciclastic tidal flats. Sediment Geol 110:1–6
Noffke N, Gerdes G, Klenke T, Krumbein WE (2001) Microbially induced sedimentary structures - a new category within the classification of primary sedimentary structures. J Sediment Res 71:649–656
Noffke N, Gerdes G, Klenke T (2003) Benthic cyanobacteria and their influence on the sedimentary dynamics of peritidal depositional systems (siliciclastic, evaporitic salty, and evaporitic carbonatic). Earth Sci Rev 62:163–176
Noffke N, Christian D, Wacey D, Hazen RM (2013) Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia. Astrobiology 13:1–22
Paerl HW, Tucker CS (1995) Ecology of blue-green algae in aquaculture ponds. J World Aquacult Soc 26:109–131
Paul A, Lokier SW (2017) Holocene marine hardground formation in the Arabian Gulf: shoreline stabilization, sea level and early diagenesis in the coastal sabkha of Abu Dhabi. Sediment Geol 325:1–3
Pazos PJ, Gutiérrez C, Fernández DE, Heredia AM, Comerio M (2015) The unusual record of Nereites, wrinkle marks and undermat mining trace fossils from the late Silurian–earliest Devonian of central-western margin of Gondwana (Argentina). Palaeogeogr Palaeoclimatol Palaeoecol 439:4–16
Picha F, Saleh AM (1977) Quaternary sediments in Kuwait. Kuwait J Sci 4:169–184
Riding R (2007) The term stromatolite: towards an essential definition. Lethaia 32:321–330
Riding R (2011) Microbialites, stromatolites, and thrombolites. In: Reitner J, Thiel V (eds) Encyclopedia of Geobiology: Encyclopedia of Earth Science Series. Springer, Heidelberg, pp 635–654
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Santomauro G, Baier J, Haung W, Pezold S, Bill J (2012) Formation of calcium carbonate polymorphs induced by living microalgae. J Biomater Nanobiotechnol 3:413–420
Schieber J, Bose PK, Eriksson PG, Banerjee S, Sarkar S, Altermann W, Catuneau O (eds) (2007) Atlas of microbial mat features preserved within the siliclastic rock record. Atlases in Geoscience 2. Elsevier, Amsterdam
Slowakiewicz M, Whitaker FF, Thomas L, Tucker M, Zheng Y, Gedl P, Pancost RD (2016) Biogeochemistry of intertidal microbial mats of Qatar: new insights from organic matter characterization. Org Geochem 102:14–29
Wei S, Cui H, Jiang Z, Liu H, He H, Fang N (2015) Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz J Microbiol 46:455–464
Wetzel RG (2001) Limnology: lake and river ecosystems. Academic Press, San Diego, p 1006
Acknowledgments
The authors would like to thank Kuwait University for funding the MSc thesis which led to this manuscript. We would also like to extend our thanks to the people that assisted the research in the laboratory. Special thanks are given to Dr. Huda Mahmoud and her research assistants (Department of Biological Sciences, Kuwait University) for their continuous support and assistance in the microbiological section. Our sincere appreciation also extends to all lab technicians and staff members in the Department of Earth and Environmental Science, Nanoscopy Center, the National Unit for Environmental Research and Services (NUERS), and in the Research Sector Project Unit (RSP Unit) in College of Science, Kuwait University. The authors are particularly grateful to the reviewers who helped focus the thrust of the article and greatly improved the final draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsibile Editor: Haroun Chenchouni
Rights and permissions
About this article
Cite this article
Ahmad, E.A., Duane, M.J. Selective microbial-induced biomineralization of a siliciclastic zoned coastline in a constricted modern tidal flat, Kuwait (Northern Arabian Gulf). Arab J Geosci 13, 1017 (2020). https://doi.org/10.1007/s12517-020-05941-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05941-3