Abstract
Bio-mediated soil improvement has been recently introduced as a novel link between biotechnology and geotechnical engineering. Microbial induced carbonate precipitation (MICP) is one of the most promising bio-mediated methods which have been developed based on biologically driven urea hydrolysis. In the present study, an indigenous bacterium that can be used in MICP is identified in the native soil of Sirjan, Southern Iran. This bacterium, called Acinetobacter calcoaceticus Nima (ACN), was compared with the standard Sporosarcina pasteurii (SP), with respect to their performance in the improvement of the mechanical properties of biologically treated soil samples. During a 40-day period of the experiment, direct shear and shear wave velocity tests were conducted to investigate the influence of the treatments on soil properties. Also, stereo microscope images were used to investigate the precipitation on the inter-particle contacts. It was observed that the bio-mediated techniques can significantly improve the mechanical properties of the soil samples. For instance, while the shear wave velocity of the bacteria-free soil was measured as 175 m/s, SP-treated soil has the shear wave velocity of 526 m/s. This value was even higher for the ACN-treated samples (588 m/s) indicating that the local bacteria performed considerably better than the standard SP when used for the native soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil materials usually do not meet engineering requirements, mainly in terms of shear strength and deformability. Consequently, soil improvement techniques, also known as ground treatment, have been extensively used in many geotechnical engineering projects. Traditionally, there exist two main categories of ground improvement techniques namely mechanical compaction and chemical stabilization (Ibragimov 2015). The selection of the appropriate improvement method is accomplished based on various criteria such as soil type, percentage of fines, area and depth of the improvement, and availability of the material. A common approach is the use of Portland cement, lime, and chemical stabilizers, such as epoxies, silicates, and polyurethanes for filling the soil voids and gluing its solid particles. However, almost all of these chemicals, except for sodium silicate, are toxic and hazardous material, and hence have raised serious environmental concerns. For instance, in 1974, acrylamide grout caused water poisoning in Japan, resulting in the ban of nearly all chemical grouts (Karol 2003).
Bio-mediated soil improvement techniques have been recently introduced into the geotechnical engineering practice as environmentally friendly solutions for sustainable construction. For instance, lignin-based biofuel co-products (BCPs) have been used to stabilize pavement subgrade soil materials with low quality (Uzer 2016). The bacterial potential in the improvement of the mechanical properties of different soils has been also studied by a number of researchers (e.g., Rajasekar et al. 2017; Kano et al. 2017). Biodegradation of the soil’s bacteria results in the increase of its shear strength (Grabiec et al. 2017) and both compressive and shear wave velocities, Vp and Vs (Akyol et al. 2017). The bacterial effects have also been influential in improving the compressive and tensile strength of cement mortars (Al-Salloum et al. 2017). In all cases, the potential impacts of the bio-treatment on the engineering properties of soils can be reasonably estimated through the examination of previously researched non-bio-mediated treatment methods. For instance, evaluation of the potential increase in shear strength of sands from bio-mediated calcite precipitation will be similar to the previous studies examining the use of gypsum, cement, and epoxy for soil improvement (Neelands and James 1963; Acar and El-Tahir 1986; Ismail and David 2001; Avseth et al. 2000; Sharma and Fahey 2003).
Microbial induced carbonate precipitation (MICP) in the soil is a new method for increasing soil strength based on the activity of bacteria. This interdisciplinary approach (biotechnology and civil engineering) has been used to solve geotechnical problems, such as slope instability, soil erosion and scour, seepage of levees and cutoff walls, settlement and bearing capacity of shallow foundations, and earthquake-induced liquefaction (Hamdan et al. 2017). As stated in Jiang and Soga (2016), MICP treatment can reduce the cumulative erosion weight, erosion rate, and axial strain relative to non-MICP soil. The magnitudes of hydraulic conductivity for all tested samples before the erosion process fall into a range from 5.5 × 10−5 to 8.0 × 10−3 m/s.
Owing to its climatic diversity, Iran has various temperature and humidity regimens, and a wide variety of soil types can be found in the country. Because of the arid climate of the southern parts of the country, resistant bacteria (e.g., spore-forming) are the dominant population in the native soils of these regions. In this study, for the first time, Acinetobacter calcoaceticus strain Nima is isolated from the native soil of Sirjan, Southern Iran, and used for MICP. Acinetobacter calcoaceticus is a soil bacterial species of the genus Acinetobacter. It is a no motile, gram-negative coccobacillus and grows under aerobic conditions. This bacterium produces the urease and the biological calcium carbonate cement forms. Several soil samples are made and examined through direct shear and shear wave velocity tests. The results are compared with those obtained from the application of the standard Sporosarcina pasteurii (SP) bacteria.
Theoretical background
Due to the existence of water and nutrients, soil media are considered to be ideal habitats for different species of microorganisms, including microbes and bacteria. Microorganisms are highly adaptable and have been survived for over 3.5 billion years (Stotzky 1997). Identification, characterization, and classification of microorganisms are usually accomplished according to their shape, type of cell wall, biochemical transformation, nutrients, and DNA/RNA sequences (Chapelle 2001). The bacteria which produce the urease enzyme, e.g., Bacillus, Sporosarcina, Spoloactobacilus, Clostridium, and Desulfotomaculum, have been recently used in soil improvement practice (Kucharski et al. 2008). This technique, also known as bio-treatment, influences the formation of fine-grained soils and changes the engineering properties of coarse-grained soils.
Living organisms can produce minerals, such as silicates, carbonates, and calcium, in a process that is referred to as biomineralization (Lowenstam and Weiner 1989). These minerals may be synthesized under two distinct mechanisms, namely biologically controlled and biologically induced mineralization. In the former, the process is controlled irrespective of the environmental conditions, while in the latter, the formation of minerals is significantly influenced by extracellular metabolic activities of the microorganisms as well as the environmental conditions (Dhami et al. 2013). The produced minerals can function as a clogging factor to seal the pores of soil skeleton and thus reduce its permeability, and/or as a cementations factor to increase the strength and stiffness of the soil.
Among the existing bio-treatment techniques, MICP which is a biologically induced process has been widely used to improve the mechanical and hydraulic properties of soils. The extent of soil improvement using this technique depends on the environmental factors, including the physical and chemical properties of the soil such as water content, mineralogy, particle size distribution, and texture. It has been reported in the literature that the improvement due to biomineralization will slow down and eventually stop as the population of microorganisms increases (e.g., De Muynck et al. 2010). This can be attributed to the increasing salinity of the medium, resulting from the microbial activities, which in turn controls the population growth.
Materials and methods
In this study, Sirjan’s native soil was used. The soil was classified in accordance with the Unified Soil Classification System (ASTM 2012 Standard D 2487-11). The tested soil is uniform sand with medium-size grains and is classified as poorly graded sand with silt (SPFootnote 1-SMFootnote 2). The physical and mechanical properties of the soil are summarized in Table 1, and its grain size distribution curve is given in Fig. 1.
The methodology adopted for this study is based on urea hydrolysis and precipitation of calcium carbonate in the presence of Ca2+ ions (Whiffin et al. 2007; Van Paassen et al. 2009; Dejong et al. 2010; Chou et al. 2011; Burbank et al. 2012; He et al. 2013):
After depositing the sediment, a coating and bonding between soil particles is created, which in turn results in the increase of compressive strength of the specimens. In addition to the bacteria, reactive substances, i.e., urea and calcium ions, should be present in the environment (these are supplied by adding the required chemical solutions). Direct shear and shear wave velocity measurements were carried out to evaluate the soil’s mechanical properties. The effect of time on the soil’s strength was also studied. Furthermore, the bacteria were cultivated as isolated from the soil. Stereo microscopy was utilized to visually assess the performance of the locally cultivated bacteria.
Selection of microorganisms
Preliminary studies were performed on the identification of different types of urease-producing bacteria and their capability to produce enzymes. Subsequently, the SP (PTCC = 1645) was selected as the witness. This bacterium was supplied from the collection of microorganisms of Iran’s Scientific and Industrial Research Center.
The topsoil was discarded and samples required for isolation of microbes were obtained from 10-cm layers below the soil surface. Ten grams of each soil sample was mixed with 90 cm3 of 0.9% salt solution and incubated at 30 °C in a shaker incubator at 100 rpm for 2 h. A total of 1 cm3 from the soil-borne bacterial suspensions was serially diluted (in the range of 10−1 to 10−6) and each dilution was plated in triplicate on Nutrient Agar (NA) medium. The plates were incubated aerobically at 30 °C for 24 h. After the incubation period, the plates were observed for any kind of growth, and the distinct colonies were sub-cultured. The screening for urease-producing bacteria was conducted using urea broth medium in test tubes. The color changes from pale yellow to pink-red indicated positive urease activity, as shown in Fig. 2.
Identification of urease-producing bacteria
The urease-producing bacteria were identified based on morphological, biochemical, and 16S rDNA gene sequencing and phylogeny analysis. Phylogenetic affiliation of the screened strain was ascertained by 16S rDNA gene sequence analysis. In order to find the most similar available sequences, a BLAST search was done in the NCBI database. 16S rDNA sequence data of most closely related species was retrieved and used in a tree construction to demonstrate the taxonomy of this isolate. Figure 3 shows the inferred phylogenetic relationships derived from neighbor-joining analysis of the 16S rDNA gene sequence of the Acinetobacter calcoaceticus Nima (ACN) with the most validly described species of the genus Acinetobacter. The numbers located at the crotch mean the reliability of the cluster descending from that node; the higher the number, the more reliable is the estimate of the taxa that descend from that node.
Genomic DNA was extracted from a pure culture of isolated bacteria using a DNA extraction kit (Cinnagen Cat. No.: DN8115C), according to the manufacturer’s instructions. PCR amplification was performed in a total volume of 50 μl mixture, containing 4 μl templates DNA (50 ng), 5 μl of F primer (10 pmol), 5 μl of R primer (10 pmol), 1 μl of 10 mM 4 dNTP mix, 1.5 μl of 50 mM MgCl2, 5 μl of 10× PCR buffer, 0.5 μl of Taq DNA Polymerase (5 U μl−1, Fermentas, Germany), and 28 μl of sterile distilled water and micro-centrifuged briefly. An initial denaturing step of 94 °C for 3 min was followed by 25 cycles of amplification (1 min at 94 °C, 1 min at 56 °C, 1.5 min at 72 °C) and a final extension step at 72 °C for 5 min. PCR products were separated by electrophoresis of 5 μl of PCR product in a 1% agarose gel for 2 h and by staining with ethidium bromide. Amplification products were stored at − 20 °C. The clean PCR product was subjected to cycle sequencing in both directions. Phylogenetic analysis was carried out as follows: the sequences were edited using Bioedit V.5.0.9 (Hall 1999). A BLAST search at the NCBI genome database server was conducted to identify the nearest neighbors (Altschul et al. 1990). Alignment, phylogenetic, and molecular evolutionary analyses were conducted using MEGA version 5 (Tamura et al. 2011). Bootstrap tests and reconstruction were performed 1000 times to confirm the reliability of the phylogenetic tree (Felsenstein 1985). The nucleotide sequence of the 16S rRNA gene of bacterial strain (Sporosarcina pasteurii strain Nima) reported in this study has been deposited in GenBank under Accession No of “MF662810.”
Preparation of the samples
Both types of bacteria were separately cultured in liquid form, 1000 cm3 capacity, in a nutrient broth environment. Bacterial culture was performed in a liquid medium containing 15 g/1000 cm3 casein-peptone, 5 g/1000 cm3 soymeal-peptone, 5 g/1000 cm3 NaCl, and 20 g/1000 cm3 urea and pH was adjusted to 7.3. For the culturing and heating period, a shaker encoder and temperature of 30 °C was used, and the bacteria containers were put in the incubator. The growth velocity of the ACN in the initial stages was rather slower than the standard SP as depicted in Fig. 4.
SP bacteria had the maximum live weight on the fifth day as 1.5 × 109 bacteria per cm3, and the population reached its minimum on the 15th day. On the other hand, the maximum population of live ACN bacteria was observed on the sixth day as 1.4 × 109 bacteria per cm3 (see Fig. 5).
Bacteria-free soil samples for direct shear test were prepared at the optimum moisture content, i.e., 12% as shown in Table 1. On the other hand, for the samples containing bacteria, the required amount of bacteria solution (in place of water), 12%, was added to the dry soil, for each Sporosarcina pasteurii and Acinetobacter bacteria type. Eighteen samples were prepared for direct shear testing on different days. Inside the direct shear molds were coated by grease. We used a thin plastic layer which rests on top of the greased layer. All test samples were made in plastic layer. After that, samples were removed from the mold and kept in appropriate condition until the test days.
The samples for shear wave velocity measurement (microseismic test) were made in polyethylene tubes; depending on the desired density, the required amount of water for bacteria-free samples and the required amount of bacteria solution for the samples containing bacteria have been used for densities 60%, 80%, and 95% respectively 4.7%, 8.4%, and 11.0%. It will be noted that the soil samples are not sheared during this test (shear wave velocity measurement). The soil properties for these samples are given in Table 1.
All the samples (including bacteria-free condition) were sheared in all direct shear tests. The prepared samples from the previous step, with the same size as required by the direct shear test, were put into the shear mold and placed in the machine accordingly. The rate of shearing was set as 1 mm/min and gauge readings were made every 30 s. Once the test is completed, the lateral load cell number remains constant. After completion of the test, calculations were made, and the maximum shear stress diagram was plotted according to the two fixed vertical stresses (0.2 kg/cm2, and 1 kg/cm2). It will be noted that in microseismic, for measuring shear wave velocity, the samples are not sheared.
Because the soil inside of the cylinder should be compact, initially, metallic tubes with 80-mm diameter and 300-mm height were used for the specimens of the microseismic test. However, the disturbance of shear waves impacted the accuracy of measurements, and thus, polyethylene tubes were used instead (Law et al. 1989). The polyethylene tube is less capable of absorbing the shear wave and has the role of retaining the soil sample only (Szabo and Wu 2000). Due to the need for pores to form the sediment, different densities have been considered in this experiment (relative densities of 60%, 80%, and 95%). At all stages of the test, the sensor directly contacts the soil. On the other hand, specimens with a diameter of 90, 75, 63, and 50 mm were made from a soil sample in identical conditions, and it was observed that the shear wave velocity of soil was different for each of the diameters. For these samples, as the diameter (cross-section) decreased, shear wave velocity increased. However, the samples with diameters of 105, 120, and 150 mm had similar shear wave velocity. Therefore, it was concluded that the use of a pipe with a diameter greater than 105 mm had no effect on the outcomes of the test (and this size was selected).
If there is a big gap between soil particles, the possibility of the formation of sediment is low and vice versa. In total, nine samples were made at three relative densities and for the three types of soil, i.e., bacteria-free, SP-treated, and ACN-treated. The microseismic tests were scheduled for a period of 40 days. For each test, the specimen was put on a timber, as the test medium. As illustrated in Fig. 6, two sensors were installed at the two ends of the timber: one accelerometer right on the timber, and one shear wave sensor on the specimen.
Direct shear tests were conducted to evaluate the influence of bio-treatments on the shear strength of the samples. Eight samples of each soil were made (with dimensions of 10 cm × 10 cm × 5 cm) and tested every 5 days (over a period of 40 days). The last set of samples prepared for the test was those used for stereo microscopy. Stereo microscopy samples of SP- and ACN-treated soils with relative densities of 60%, 80%, and 95% were made at diameters of 40 mm and 30 mm.
Results and discussion
Microseismic tests
Each test was repeated ten times and the results were recorded after further data processing, e.g., omitting outliers and inaccurate data and applying a smoothing filter. Shear wave velocities of the three different soils during the test period are given in Table 2. It is evident that the shear wave velocity in bacteria-treated samples has significantly increased (nearly 100%) after 40 days. This indicates the improvement of stiffness properties of the treated specimens. Moreover, the native bacteria (ACN) showed even a better contribution to the improvement of the soil’s stiffness compared to the standard SP bacteria. On the other hand, in absence of the bio-treatment, the soil’s stiffness shows a relatively constant trend (the small changes in shear wave velocity can be attributed to the gradual drying of the samples).
Figure 7 depicts the ratio of compressional and shears wave velocities (Vp/Vs) for the three soils over time. It can be observed that the ratio decreased in the case of bio-treated soil specimens. According to Pride (2005), this reduction of Vp/Vs is synonymous with the reduction of the soil’s porosity. In Fig. 7, the highest porosity reduction is observed for the ACN-treated samples with a relative density of 80%. Porosity reduction can be regarded as a positive functionality of this treatment which enhances both mechanical and hydraulic properties of the soil.
Direct shear tests
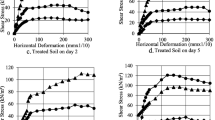
The results of the direct shear test during the 40-day period indicated that despite the untreated (bacteria-free) samples, which had a relatively constant strength, the shear strength of the bio-treated soils has considerably increased over time. Figure 8 shows the direct shear test results for SP- and ACN-treated samples. It can be observed that the ACN-treated samples have better overall performance, especially at higher normal stress where the 40-day strength is almost 10% higher than the SP-treated ones. Figure 9 depicts the variations of the angle of internal friction for the two types of soils over the 40-day period. According to this figure, both bio-treatment techniques caused the friction angle to drastically increase (from 28° to around 60°). Likewise, this figure shows that the indigenous ACN bacteria give better results regarding the improvement of shear strength of the granular soil of Sirjan area.
Stereo microscopy
The stereo microscopy produces two-dimensional images that allow for visual identification of depositions created on the soil particles’ surfaces. During the precipitation process, the soil particles’ surfaces are initially coated with a thin layer of minerals. Subsequently, a solid inter-particle bond is formed from the sediments, which is the main cause of porosity reduction and soil improvement. In essence, the mechanism of bacteria movement is in such a way that they tend to be concentrated around the contact points where more food is present.
Figure 10 shows stereo microscopy images of the samples of bio-treated soil at different relative densities. In this figure, the chains of bacteria which result in higher strength and stiffness are highlighted with white circles. This figure suggests that ACN-treated soil with Dr = 80% has the largest number of bacteria chains and thus is the case which bacteria-treatment has performed best. Also, according to Fig. 10, the local ACN bacteria form a longer chain within the soil medium compared to the standard SP bacteria.
Concluding remarks
This paper presented the results of an experimental study on the bio-treatment of granular soils using MICP. Two types of bacteria, namely the standard SP and the indigenous ACN, were used to improve the mechanical properties of the sandy soil of Sirjan (Southern Iran). Laboratory experiments were conducted to identify the biological behavior of the bacteria (particularly their growth rate). After initial investigations on the biological characteristics of the microorganisms, soil specimens were made and treated over a period of 40 days.
Microseismic test results revealed that both bacteria-treated soils become stiffer over time as the measured shear wave velocity of the samples showed a meaningful increase. While the bacteria-free soil had a shear wave velocity around 175 m/s and ACN-treated soil samples had a shear wave velocity increase up to 588 m/s after 40 days. The shear wave velocity of SP-treated samples, in similar conditions, was 526 m/s.
According to the results of microseismic tests, the highest increase rate of the samples treated with the two types of bacteria at different densities belonged to ACN 60%. It can be concluded that treatment for relatively loose and low-density soils has been more effective. On the other hand, the shear wave velocity of ACN 95% specimen reached the value of 588 m/s over time. It is noteworthy that, the best performance regarding the increase of shear wave velocity for bacteria-free soil (due to soil drying only) was 1.33 times, which was observed at a relative density of 60%.
In the direct shear tests, the highest shear strength was related to the ACN-treated soil. The shear strength in this case (at vertical stress 1 kg/cm2) was 2.25 times higher than the shear strength in the case of SP. At the lower vertical stress (0.2 kg/cm2), this ratio was around 3. When the soil was treated with ACN bacteria, the angle of internal friction, which is one of the most important soil geotechnical factors, was more than 2 times greater compared to the standard treatment with SP for treated soil with ACN.
According to stereo microscopy results, the amount of sediment formation and bacterial activity in the density of 80% was highest for both bacterial species. It seems that the higher degrees of compaction do not leave sufficient space for bacterial activities. This study showed superior performance of indigenous ACN bacteria compared to the standard SP. The findings of this research suggest that the use of native bacteria) ACN in this case) should be considered as a viable option for the purpose of bio-mediated soil improvement.
Notes
Sand-Poor
Sand-Mo (Swedish word for silt)
References
Acar YB, El-Tahir ET (1986) Low strain dynamic properties of artificially cemented sand. J Geotech Eng 112(11):1001–1015. https://doi.org/10.1061/(ASCE)0733-9410(1986)112:11(1001)
Akyol E, Bozkaya Ö, Dogan NM (2017) Strengthening sandy soils by microbial methods. Arab J Geosci 10(15):327. https://doi.org/10.1007/s12517-017-3123-9
Al-Salloum Y, Abbas H, Sheikh QI, Hadi S, Alsayed S, Almusallam T (2017) Effect of some biotic factors on microbial-induced calcite precipitation in cement mortar. Saudi J Biol Sci 24(2):286–294. https://doi.org/10.1016/j.sjbs.2016.01.016
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
ASTM (2012) Standard Practice for Classification of Soils for Engineering Purposes (Unified Classification System). ASTM Standard D 2487-11; ASTM International, West Conshohocken. https://doi.org/10.1520/D2487-11
Avseth P, Dvorkin J, Mavko G, Rykkje J (2000) Rock physics diagnostic of North Sea sands: link between microstructure and seismic properties. Geophys Res Lett 27(17):2761–2764. https://doi.org/10.1029/1999GL008468
Burbank M, Weaver T, Lewis R, Williams T, Williams B, Crawford R (2012) Geotechnical tests of sands following bio induced calcite precipitation catalyzed by indigenous bacteria. J Geotech Geoenviron 139(6):928–936. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000781
Chapelle FH (2001) Ground-water Microbiology and Geochemistry. Wiley, New York
Chou CW, Seagren EA, Aydilek AH, Lai M (2011) Biocalcification of sand through ureolysis. J Geotech Geoenviron 137(12):1179–1189. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000532
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36(2):118–136. https://doi.org/10.1016/j.ecoleng.2009.02.006
Dejong JT, Mortensen BM, Martinez BC, Nelson DC (2010) Bio-mediated soil improvement. Ecol Eng 36(2):197–210. https://doi.org/10.1016/j.ecoleng.2008.12.029
Dhami NK, Sudhakara Reddy S, Mukherjee A (2013) Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol 4:314. https://doi.org/10.3389/fmicb.2013.00314
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Grabiec AM, Starzyk J, Stefaniak K, Wierzbicki J, Zawal D (2017) On possibility of improvement of compacted silty soils using bio deposition method. Constr Build Mater 138:134–140. https://doi.org/10.1016/j.conbuildmat.2017.01.071
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series (Vol. 41 No. 41 p. 95–98). Information Retrieval Ltd., London, pp c1979–c2000
Hamdan N, Kavazanjian E Jr, Rittmann BE, Karatas I (2017) Carbonate mineral precipitation for soil improvement through microbial denitrification. Geomicrobiol J 34(2):139–146. https://doi.org/10.1080/01490451.2016.1154117
He H, Zhang Z, Wang J, Li K (2013) Compressive properties of nano-calcium carbonate/epoxy and its fiber composites. Compos Part B 45(1):919–924. https://doi.org/10.1016/j.compositesb.2012.09.050
Ibragimov MN (2015) Design and implementation of soil stabilization by grout injection using hydrofracking technology. Soil Mechanics and Foundation Engineering 52(2):100–108. https://doi.org/10.1007/s11204-015-9313-0
Ismail AF, David LIB (2001) A review on the latest development of carbon membranes for gas separation. J Membr Sci 193(1):1–18. https://doi.org/10.1016/S0376-7388(01)00510-5
Jiang N, Soga K (2016) The applicability of microbially induced calcite precipitation (MICP) for internal erosion control in gravel–sand mixtures
Kano S, Moriwaki T, Ochi K (2017) A study on the bio-treatment technique of ground improvement with urease microorganisms which live in Japan. In: Geotechnical Hazards from Large Earthquakes and Heavy Rainfalls. Springer Tokyo, pp 441–448. https://doi.org/10.1007/978-4-431-56205-4_41
Karol RH (2003) Chemical grouting and soil stabilization, revised and expanded, vol 12. CRC Press
Kucharski ES, Cord-ruwisch R, Whiffin V, Al-thawadi SM (2008) United States patent
Law YF, Johnston KW, Routh HF, Cobbold RSC (1989) On the design and evaluation of a steady flow model for Doppler ultrasound studies. Ultrasound Med Biol 15(5):505–516. https://doi.org/10.1016/0301-5629(89)90103-8
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press
Neelands RJ, James AN (1963) Formulation and selection of chemical grouts, with typical examples of their field use. Grouts and Drilling Muds in Engineering Practice, 150–155
Pride SR (2005) Relationships between seismic and hydrological properties. In: Hydrogeophysics. Springer, Dordrecht, pp 253–290. https://doi.org/10.1007/1-4020-3102-5_9
Rajasekar A, Moy CK, Wilkinson S (2017) Stimulation of indigenous carbonate precipitating bacteria for ground improvement. In: IOP Conference Series: Earth and Environmental Science, Vol 68, No. 1. IOP Publishing, p 012010. https://doi.org/10.1088/1755-1315/68/1/012010
Sharma SS, Fahey M (2003) Degradation of stiffness of cemented calcareous soil in cyclic triaxial tests. J Geotech Geoenviron 129(7):619–629. https://doi.org/10.1061/(ASCE)1090-0241(2003)129:7(619)
Stotzky G (1997) Soil as an environment for microbial life. In: van Elsas JD, Trevors JT, Wellington EMH (eds) . Taylor & Francis, Modern soil microbiology, pp 1–20
Szabo TL, Wu J (2000) A model for longitudinal and shear wave propagation in viscoelastic media. J Acoust Soc Am 107(5):2437–2446
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. https://doi.org/10.1093/molbev/msr121
Uzer AU (2016) Evaluation of freezing-thawing cycles for foundation soil stabilization. Soil Mech Found Eng 53(3):202–209. https://doi.org/10.1007/s11204-016-9386-4
Van Paassen LA, Harkes MP, Van Zwieten GA, Van der Zon WH, Van der Star WR, Van Loosdrecht MC (2009) Scale up of BioGrout: a biological ground reinforcement method. In: Proceedings of the 17th international conference on soil mechanics and geotechnical engineering. Lansdale, IOS Press, pp 2328–2333. https://doi.org/10.3233/978-1-60750-031-5-2328
Whiffin VS, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24(5):417–423. https://doi.org/10.1080/01490450701436505
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sanjay Kumar Shukla
Rights and permissions
About this article
Cite this article
Mohammadizadeh, M., Ajalloeian, R., Nadi, B. et al. Experimental study on soil improvement using local microorganisms. Arab J Geosci 13, 469 (2020). https://doi.org/10.1007/s12517-020-05450-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05450-3