Abstract
In the current research, solidification/stabilization (S/S) treatment of the contaminated soil using hydraulic binders and additives was used to (1) reduce the mobility of organic and inorganic contaminants and (2) compare the ability of various binders in fixing contaminants. The samples were collected from Franco-Tunisian Petroleum Company, located in Sidi Litayem, Sfax (Southern Tunisia). Leaching tests were performed on contaminated soil, containing metallic elements, and hydrocarbons. Calcium aluminate cement (CAC), ordinary Portland cement (OPC), and ground-granulated blast-furnace slag (GGBFS), additives especially the bentonite and water, were used for S/S treatment. The obtained standard specimens were subjected for treating after treatment the leachability of pollutants, compressive strength (CS), and XRD analysis. The results of analysis conducted on contaminated soils showed that concentrations of metallic elements were in the range of 9.08–427 mg/kg and 15,520 mg/kg of organic compound. Next, 10% of the used binder improved the immobilization of pollutants and gave a satisfactory CS exceeding 1 MPa. Thus, the CAC is more effective in reducing the leachability of metal contaminants than OPC + GGBFS and produces much higher strength, which was of the order of 2.41 MPa. The mechanical characterization was confirmed by XRD analysis. The lowest values of organic compounds are presented in mixtures treated by 10% of used binder, indicating the effectiveness of those with the presence of 10% of bentonite. This work shows that 10% (OPC + GGBFS) + 10% bentonite improved the immobilization of metallic elements and hydrocarbons, thus proving its efficiency due to its low cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, with the inevitable rise in population and industrialization, the continuous production and consumption of energy has led to large quantities of waste. The latter is growing in volume and in toxicity, and the problem will be further aggravated. Increasingly, our everyday waste production consists mainly of organic constituents, such as hydrocarbons and toxic chemical elements, namely alkali and metallic elements and these toxic products are combined with a plethora of other chemicals, which eventually impact public human health and environment quality (Qian et al. 2006). It has been stated that a perfect sustainable society should not generate waste exceeding its own capacity of waste treatment (Stren et al. 1992). So, the question is: how can we change the way we consume so as to produce less waste, while using all waste as a resource? The sustainable management of waste is, therefore, and above all, the responsibility of states and territory governments, which manage waste in accordance with their respective legislation, policies, and programs.
Different waste treatment technologies such as remediation technologies, containment, thermal treatments, physicochemical treatments, solidification/stabilization (S/S) treatment, and recycling and composting (Ji et al. 2004) were developed. Not only is S/S treatment using hydraulic binders the most promising (Al-Ansary and Al -Tabbaa 2007), but also a remedial technology that has attracted widespread use around the world. Besides, S/S processes have emerged as an efficient method for the treatment of wastes polluted with potentially toxic metals due to its versatility, efficiency (Falciglia et al. 2012).
This treatment involves mixing hydraulic binders into the waste to transform it into a solid material with stable pH, low mobility, and leachability of pollutants (Zhou et al., 2006). According to Van der Sloot et al. (2007), this will create the minimum threat to the environment by transforming hazardous wastes into non-hazardous or less-hazardous wastes.
Previous research works have found that the S/S treatment is a reliable and an appropriate technology for the treatment of contaminated soils (Scanferla et al. 2009; Voglar and Lestan 2011; Jin et al. 2016). It is a commonly used land remediation method that aims to improve the mechanical properties and the leaching resistance of the contaminated soil (Jin et al. 2016). The range of operating conditions that result in acceptable treatment of some commonly used binders has been investigated. It was shown that about 20% binder dosage can satisfy most leaching criteria (Kogbara et al., 2014).
The US Environment Protection Agency (EPA) has recognized that the use of ordinary Portland cement (OPC) as a binder is the best demonstrated available technology. It has also been reported that the OPC is the most widely used binder in the S/S technique (Vandeperre et al. 2008). According to Gu et al. 2015, the treatment of contaminated soil using the OPC is widely used in road, rail, and airport construction. Navarro et al. (2011) have examined the effectiveness of this cement in contaminated soil by using leaching experiments and geochemical modeling. The viability of the cement-based S/S technology was evaluated on 40 site representative soil samples from Cinkarma brownfield using OPC (Volgar and Lestan 2010). The introduction of OPC and cement kiln dust as hydraulic binders has improved the properties of arsenic-contaminated soils especially strength, durability, permeability, and volume stability (Tariq and Yanful, 2013). Furthermore, Barth et al. (1989) have revealed that using OPC in the S/S of metallic elements containing waste is effective in reducing the leaching of these elements. Laforest and Duchesne (2005) have used the ground-granulated blast-furnace slag (GGBFS) and OPC to evaluate the retention of hexavalent Cr.
These authors proved that GGBFS was more efficient than OPC in fixing Cr at lower concentrations. However, with concentrations above 2000 mg/l, OPC was more efficient. Jin et al. (2016) demonstrated that although the treatment of the in situ solidified/stabilized soil using novel MgO-bearing binders alone provided negligible strength to the soil, it improved the immobilization capacities on both organic and inorganic contaminants. Furthermore, the substitution of MgO by 90% of GGBFS increased the strength and decreased the permeability remarkably (Wang et al. 2015). Special cements such as calcium aluminate cement (CAC) (Conner 1990; LaGrega et al. 1994) have also been studied. The CAC pastes containing Pb, Cu, and Zn and the leaching tests presenting 99.9% retention were studied by Navarro-Blasco et al. (2013). Regarding the immobilization of Cr, the lower pH and the higher content of Fe present in CAC could improve its ability to immobilize Cr6+ to Cr3+ since Cr6+ is more soluble than Cr3+ (Navarro-Blasco et al. 2013).
In the current research work, OPC was selected as the most used cement to investigate the leaching properties and the unconfined compressive strength of the solidified/stabilized soil. GGBFS was used both to improve the mechanical behavior of the soil, including the unconfined compressive strength in combination with the cement and reduce the environmental impact of OPC (Wang et al. 2015). Al Ansary and Al Tabbaa (2007) showed that the combination of 20% of GGBFS-OPC and 30% of lime-OPC with low oil content led to the immobilization of the pollutants and the reduction in oil concentration. CAC was applied in view of its rapid hardening. Bentonite was used as the soil binder, additive desiccant due to its absorption properties and a degreasing agent (Carmody et al. 2007). Economically, it was used to optimize the amount of hydraulic binders and water in a cementing matrix.
The main objectives are (1) comparing the ability of various binders in the fixation of contaminants and (2) assessing their effects on the properties of S/S materials and curing conditions.
Material and methods
Contaminated soil sampling
A sampling campaign of contaminated soil was conducted directly from the oil site of the Franco-Tunisian oil company located in Sidi Litayem (SIT 11) of the region of Sfax (Southern Tunisia) (34° 53′ 13.82″ N 10° 31′ 52.41″ E) (Fig. 1). Outdoor contaminated soil samples were taken from the surface (0–30 cm), during the period extending from December 2015 to January 2016. These samples, containing metallic elements and salts, and rich in organic constituents such as petroleum hydrocarbons, have a dark brown color. They were uniformly mixed before further experimental work. The studied soil before treatment is sand-colored clayey (S0). Sampling was carried out using a manual coring of cylindrical specimens (diameter = 4 cm; height = 8 cm) according to the AFNOR NF X31-210 French standard relative to the characterization of ultimate industrial stabilized water (Recueil 1994). In fact, sampling was conducted in control soil named (SIT11-2) (34° 53′ 15.61″ N 10° 31′ 49.16″ E) and polluted soils (SIT11-1: P1, P2, P3, and P4) (34° 53′ 15.35″ N 10° 31′ 51.34″ E) (Fig. 1). Then, contaminated soil samples were air-dried at ambient temperature and sieved at 2 mm, for the analysis of chemical and physical properties in the laboratory of Research unit in Geotechnical Environmental and Civil Materials.
Contaminated soil characterization
For the analyses of the total copper (Cu), cadmium (Cd), chromium (Cr), zinc (Zn), nickel (Ni), and lead (Pb) concentrations in contaminated soil samples, about 2 g of the dried soil were digested in a mixture of concentrated acids (nitric acid, fluorhydric acid, and perchloric acid) according to the EPA 3052 guideline (EPA 1996). The digested samples were then diluted to 50 ml with double distilled water, and filtered through Whatman filter paper into acid-washed polyethylene sample bottles. After filtration, the samples were determined for Cu, Cd, Cr, Zn, Ni, and Pb using an atomic absorption spectroscopy AAS with the air-acetylene flame (Analytik Jena model ZEEnit 700PC). The detection limits (mg/l) were 0.015 for Cd, 0.01 for Pb, 0.002 for Zn, 0.001 for Ni, 0.004 for Cu, and 0.01 for Cr.
Leaching tests were performed on contaminated soil, containing metallic elements, and hydrocarbons, according to the AFNOR NF X31-210 French standards. The leaching test was determined by putting in contact about 50 g of the contaminated soil sample and 500 ml of distilled water, during 24 h of continuous agitation. Non-ionic detergent polyoxyethylene 20 sorbitan monooleate Tween 80 was chosen as an additive (Voglar and Lestan 2011) to make the organic matter containing in the soil more hydrophilic (Table 1). The solution was separated from the solid residual fraction by filtration through 0.45-μm filter. The filtrated leachates were then subjected to chemical analysis of different parameters in the laboratories of the Faculty of Sciences of Sfax (Tunisia) and the Olive Tree Institute of Sfax (Tunisia). Metallic elements Cu, Cd, Cr, Zn, Ni, and Pb were measured in triplicate using an atomic absorption spectrometry. The contaminated soil pH was measured in a soil/distilled water ratio of 1/2.5 by the electrometric method using a pH meter equipped with a glass electrode (Louati et al. 2017). Soxhlet extraction apparatus was used for the extraction of organic compounds from the contaminated soil (Kumar and Kothiyal 2011).
The mineralogical analysis of prepared mixtures is determined by X-ray diffraction. The estimate of the percentage of each mineral is affected by the integration of the area of the most intense peak (Mosbahi et al. 2014; Mosbahi et al. 2017).
All XRD data were collected under the same experimental conditions using an X’pert HighScore plus PANalytical diffractometer. Philips PW type diffractometer PANalytical X Pert Pro MPD (θ–2θ) system, equipped with a copper anticathode, was used CuKα radiation source under 40 kV/40 mA and an angular range of 3–70° 2θ for bulk rocks and 3–30° 2θ for fine fraction, a step size of 0.017° 2θ, and a counting time of 10 s/step to determine mineral composition.
Tested formulations
The treated soil specimens were prepared for various formulations of hydraulic binders and additives, according to the AFNOR NF X31-211 French standard (diameter = 4 cm; height = 8 cm) (Table 1). Indeed, hydraulic binders, additives, contaminated soil sample, and water were put into the container and mixed for 4 mn, using magnetic mixer in order to achieve a uniform admixture (Wang et al. 2014). The obtained smooth paste was subjected to compaction tests using standards cylindrical tube models with an inside diameter of 4 cm and a height of 8 cm for the production of standard specimens in which leaching tests, chemical analyses, compressive strength (CS) measurements, and mineralogical analysis by X-ray diffraction were carried out after 28, 60, and 90 curing days. In fact, CS were determined in triplicate using MATEST CYBER-TRONIC. Leaching tests were performed in accordance with the AFNOR NF X31-211 French standards by putting in contact a specimen and distilled water using liquid to solid ratio (L/S) of 10:1, during 24 h of continuous agitation. The solution was filtered through 0.45-μn filter (GF/C) and tested for pH using a pH meter of HACH. Metallic element concentrations were analyzed using an atomic absorption spectrometry (Tromp et al. 2012). The rest of liquid from the batch-leaching test was transferred into a 1000-ml separatory or conical bottomed funnel for organic extraction, which consists in adding 30 ml of dichloromethane (DCM) and shaking it for 2 min. The complete extracted sample was then poured into a container, whose weight was recorded before pouring, for DCM evaporation at 48 h. Finally, the mass of the residual was recorded (Ouellet-Plamondon 2011). Table 1 presents the list of the tested cementations formulations for soil S/S.
Results and discussion
Contaminated soil chemical characterization
The results of chemical characterization carried out on contaminated soil are shown in Table 2.
The results of the analysis carried out on the acid attack solutions of soil indicated that the zinc (Zn) is the dominant element in comparison with other metallic elements. Its concentration is 427.59 mg/kg followed by chromium (Cr) 210.79 mg/kg, nickel (Ni) 206.8 mg/kg, Copper (Cu) 128 mg/kg, lead (Pb) 96.7 mg/kg, and cadmium (Cd) 9.08 mg/kg.
The results of the analysis conducted on soil leachates indicated an alkaline soil with a pH average value equal to 8.26. Furthermore, except for Zn, the concentration of metallic elements is higher than the maximum levels allowed by the Tunisian standard of wastewater discharge (N.T106-002). Thus, the concentrations of these elements exceeded largely the recommended threshold, thus requiring a pretreatment of the contaminated soil before discharge into the environment. Table 2 also shows that the mean concentration of organic compound in the contaminated soil is in the order of 15,520 mg/kg, which prevents the intake and the hydration of the mixture and presents large difficulties for the treatment.
Treated soil chemical characterization
The results of analysis conducted on treated soil leachates are shown in Tables 3 and 4 respectively. The pH values for the 12 mixtures varied from 8 to 11 with an average of about 9, indicating an alkaline soil. Besides, these values increased in comparison with those observed in soil before treatment, which could be due to the cements’ hydration reaction (CAC and OPC + GGBFS). It could also be explained by the fact that the leaching phenomenon of the chemical species of the solid phase is less intense (Table 3).
In general, an increase in the retention of most metallic elements according to the proportioning of different binders and according to time was observed. Table 4 shows that the mean concentrations of Cr, Cu, Pb, Zn, Cd, and Ni for 3 months were less important than those observed in contaminated soil leachates. In addition, the observed leachate concentrations of different elements, except those of Pb, Cd, and Ni, were less important than those presented by the Tunisian standard. Table 4 also reveals a total retention of Cu for the mixtures M2 and M6 after 28, 60, and 90 curing days and of Zn, Cd, and Ni for the mixtures M2, M6, and M12 during 3 months.
Moreover, the retention of metallic elements was more important in mixtures M6 and M12, indicating that the use of 10% of CAC and 10% of GGBFS + OPC as shown in Table 2 plays an important role in stabilizing these elements, especially after 28 and 90 curing days where most elements were undetected. Furthermore, it should be noted that the leachate concentrations of metallic elements for the mixture M6 (10% CAC with 10% bentonite) were less than those detected in the mixture M12 (10% OPC + GGBFS with 10% bentonite). This could be explained by the fact that the CAC reduced the leachability and the diffusion of metal contaminants more efficiently than OPC even in the presence of the GGBFS. On the other hand, the addition of 10% bentonite plays an important role in the absorption of metallic elements in the cementing matrix (M’leyeh et al. 2002). Voglar and Lestan (2011), who tested the efficiency of these two binders (CAC and OPC) in stabilizing the metallic elements in a cementing matrix, confirmed our results. Indeed, these authors found that the metallic element concentrations in the soil treated by the OPC were more important than those treated by the CAC, especially for the case of Cu. It was of the order of 3.41 ppm (> 0.5 ppm) in the case of soil treated by the OPC, while it was undetected in the case of the soil treated by the CAC (Voglar and Lestan 2011).
Compressive strength evaluation
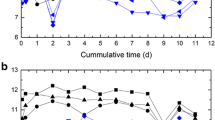
Compressive strength (CS) measurements were conducted on obtained specimens in order to examine the changes of strength characteristics (Louati et al. 2017). The evolution of the CS with time curing for different binder proportioning is presented in Fig. 2. This figure shows, except for the mixtures M1, M5, and M9, an increase in the compressive strength with time. An increase in the CS with cement content (CAC or OPC + GGBFS) was also observed. In fact, calcium silicate hydrate (C-S-H) is the main product of the hydration of OPC and is primarily responsible for the strength in cement-based materials, and when GGBSF was added, Ca(OH)2 was able to react with SiO2, Al2O3. Therefore, this produced more C-S-H gel (Wang et al. 2014). The decrease in the CS according to the bentonite proportioning (CAC = 5%) for the mixtures M3 and M5 could be explained by the fact that the bentonite can produce a negative impact on strength development. Actually, the bentonites which are foliated clay inflate with water that is why the CS of cement decreases. However, heating bentonite loses water and resists to compression which may be used as a result in future research work. Thus, the use of bentonite is not investigated on strength development but it has an important role in metallic element retention as mentioned previously. The CS for the mixtures M1, M3, M5, M7, M9, and M11 did not exceed 1 MPa standard for landfill disposal of class 3 industrial wastes treated in comparison with the other mixtures. This could be due to the low amount of used cement (5%), even in the presence of 5% of bentonite. Except for mixtures M5 and M9, all CS values exceeded 0.4 MPa. According to Malviaya and Chaudhary (2006), the minimum desired compressive strength for stabilized material should be estimated based on the design loads. For example, the US EPA considered the CS value at 0.35 MPa as a satisfactory compressive strength for materials placed on it in a landfill. Moreover, in the UK, the desired 28-day compressive strength is 0.7 MPa, but the value that is as low as 0.35 MPa is also considered acceptable, depending on the test specimen (Hills and Pollard 1997). The CS values observed in mixtures M8 and M12 are lower than those observed in mixtures M2 and M6 (Fig. 2). Furthermore, the highest values of the CS are measured in the mixture M2 (10% CAC), especially after 90 curing days where the mean value is about 2.41 MPa. Voglar and Lestan (2011) have shown that the CAC is more effective in reducing the leachability and diffusion of metal contaminants than OPC, thus producing much higher strength.
Organic compound leachability
To assess the effectiveness of S/S in immobilizing organic compounds, the leachability of these elements is also a key factor (Wang et al. 2015). Figure 3 and 4 reveal that the organic compounds detected in different treated soil mixtures are very weak in comparison with those observed in contaminated soil (15,520 mg/kg). Indeed, the values varied between 6.12 and 312 mg/kg, where mixtures M1 and M7presented the highest values in the order of 200 and 312 mg/kg, respectively, mainly explained by the less percentage of the used binders (CAC and OPC + GGBFS) in the order of 5%. The lowest values are presented in mixtures M6, M12, M8, and M2, respectively, indicating the effectiveness of the used cements (CAC and OPC + GGBFS) with the presence of 10% of bentonite. This is because of the high absorption ability of organic compounds by cements and, especially, by the bentonite (Gillman 2005). Furthermore, bentonite was considered, in the current research, as a degreasing agent.
Treated soil mineralogy
This current research was evaluated by the treatment of the soil using the XRD analysis. X-ray diffraction was important in order to know the mineralogical composition of the treated soil in comparison with contaminated soil. In fact, the mixtures M2, M6, M8, and M12 were selected. These mixtures gave satisfactory results regarding compressive strength and metallic element immobilization. The mineralogical analysis detected in M2 revealed the presence of clay minerals, such as smectite and kaolinite, as well as accessory minerals, such as quartz and calcite (Fig. 5). For M6, the mineralogical analysis has shown that this mixture is very rich in quartz, calcite, clay mineral, such as smectite and kaolinite (Fig. 6). M8 showed the presence of ettringite and thaumasite (Fig. 7). M12 revealed the abundance of quartz and gypsum and the presence of calcite (Fig. 8).
The presence of the quartz and the detection of phyllosilicates in mixture M2 proved the natural origin of these elements and the addition of bentonite in M6. The polluted soil (S0) is clayey sand (Fig. 4). Furthermore, new mineral phases compared to contaminated soil S0 like magnetite, portlandite, manganpyrosmalite, and fayolite appeared (Figs. 5 and 6). These minerals ensure the durability of mixtures, which is confirmed with compressive strength results. The presence of ettringite and thaumasite in M8 reduces the mixture durability in comparison with the mixture M2, which shows the higher strength (Fig. 7). The high proportions of gypsum in M12 could be explained by the high proportion of cement (OPC + GGBFS) of the order of 10%. Besides, the abundance of ettringite compared to fayolite, magnetite, portlandite, and cementite was detected in this mixture. These minerals contained iron oxides emanating from the slags (metallurgy) used in the mixture which is rich in irons (Fig. 8).
Conclusions
In this work, the physical and chemical performance of the ex situ S/S-treated soil using novel binders (CAC and GGBFS) and additives have been investigated. Even at low proportioning of binders, this remediation retained the organic compound (like hydrocarbon) very well with the retention efficiencies of 97–99%. However, although the concentrations of most metal were lower than the standards and showed a decrease between 28 and 90 curing days, those of Pb, Cd, and Ni were higher in some mixtures. In this case, it is recommended to increase the proportioning of binders. We conclude that the retention of the total organic compound is better than that of metals. In fact, the use of 10% of CAC and 10% of OPC + GGBFS gave satisfactory results when the compressive strength exceeded 1 MPa (desired resistance). However, the result showed that CAC reduced the leachability of metallic elements and produced a higher compressive strength that is more efficient than OPC + GGBFS. Similarly, although adding 10% of bentonite to 10% of CAC or to 10% OPC + GGBFS decreased the strength, it improved the immobilization capacities on both inorganic and organic contaminants more than the use of binder alone. Therefore, bentonite has a great power of retention of the total organic and inorganic compounds. Although the use of CAC as a binder in the S/S gives better results for the strength and retention of the metal elements, it is very expensive. Hence, the use of OPC + GGBFS is satisfactory, thanks to its availability and its reduced price compared to the CAC, giving results that are not more aloof than those of CAC whether for the resistance or retention of metals. Finally, the XRD analysis in this research work confirmed the mechanical characterization by the formation of new minerals compared to contaminated soil.
References
Al-Ansary MS, Al-Tabbaa A (2007) Stabilisation/solidification of synthetic petroleum drill cuttings. J Hazard Mater 141:410–421. https://doi.org/10.1016/j.jhazmat.2006.05.079
Barth E, Percin P, Arozarena M, Zieleinswski J, Dosani M, Maxey H, Hokanson S, Pryately C, Whipple T, Kravitz R, Cullinane M, Jones L, Malone P (1989) Stabilization and solidification of hazardous wastes. Noyes Data Corporation, New Jersey
Carmody O, Frost R, Xi Y, Kokot S (2007) Absorption of hydrocarbons on organo-clays—implications for oil spill remediation. J Colloid Interface Sci 305:17–24. https://doi.org/10.1016/j.jcis.2006.09.032
Conner JR (1990) Chemical fixation and solidification of hazardous waste. Van Nostrand Reinhold, New York http://www.acee.gc.ca/050/documents_staticpost/cearref_8989/STP-0203.pdf
EPA (1996) Method 3052. Microwave assisted digestion of siliceous and organically based matrices. In: test methods for evaluating solid wastes: physical/chemical methods, EPA SW-846, 3rd edn. Chapt. 3, Section A, vol. I (Inorganic Analytes). US Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, DC, pp 3052–1–3052-20
Falciglia PP, Cannata S, Romano S, Vagliasindi FGA (2012) Assessment of mechanical resistance, γ-radiation shielding and leachate γ-radiation of stabilised/solidified radionuclides polluted soils: preliminary results. Chem Eng Trans 28:127–132. https://doi.org/10.3303/CET1228022.
Gillman GP (2005) Hydrotalcite: leaching-retarded fertilizers for sandy soils, in: Management of Tropical Sandy Soils for Sustainable Agriculture. FAO Document Repository, Khon Kaen, pp 107–111
Gu K, Jin F, Al-Tabbaa A, Shi B, Liu C, Gao L (2015) Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilization. Eng Geol 195:53–62. https://doi.org/10.1016/j.enggeo.2015.05.025
Hills CD, Pollard SJT (1997) Influence of interferences effect on the mechanical, microstructural and fixation characteristics of cement-solidified hazardous waste forms. J Hazard Mater 52:171–191. https://doi.org/10.1016/S0304-3894(96)01806-7
Ji GD, Yang YS, Zhou Q, Sun T, Ni JR (2004) Phytodegradation of extra heavy oil-based drill cuttings using mature reed wetland: an in situ pilot study. Environ Int 30:509–517. https://doi.org/10.1016/j.envint.2003.10.003
Jin F, Wang F, Al-Tabbaa A (2016) Three-year performance of in-situ solidified/stabilised soil using novel MgO-bearing binders. Chemosphere 144:681–688. https://doi.org/10.1016/j.chemosphere.2015.09.046
Kogbara RB, Al-Tabbaa A, Stegemann JA (2014) Comparisons of operating envelopes for contaminated soil stabilised/solidified with different cementitious binders. Environ Sci Pollut Res 21(5):3395–3414
Kumar V, Kothiyal NC (2011) Distribution behavior of polycyclic aromatic hydrocarbons in road side soil at traffic intercepts within developing cities. Int J Environ Sci Technol 8(1):63–72 https://springerlink.bibliotecabuap.elogim.com/article/10.1007/BF03326196
Laforest G, Duchesne J (2005) Immobilization of chromium (VI) evaluated by binding isotherms for ground granulated blast furnace slag and ordinary Portland cement. Cem Concrete Res 35:2322–2332. https://doi.org/10.1016/j.cemconres.2004.12.011
LaGrega MD, Buckingham PL, Evans JC (1994) The Environmental Resources Management Group: Hazardous Waste Management. McGraw-Hill Inc, New York, pp 641–704
Louati D, Selmani S, Choura M (2017) Evaluation and optimization of solidification/stabilization treatment of the exhausted oil-based drilling sludge (Southern Tunisia). Appl J Envir Eng Sci 3(2):144–152
M’leyeh A, Srasra E, Cheref A (2002) Adsorption of heavy metals by natural clays of Borj Chekir, SW of Tunis, Proc. Inter Symp Envir Poll Cont and W Manag: 7–10, T (EPCOWM’2002), p 533–546
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: a review. J Hazard Mater 137:267–276. https://doi.org/10.1016/j.jhazmat.2006.01.065
Mosbahi M, Khlifi M, Jamoussi F, Tlili A (2014) Influence of the halokinesis on the clay mineral repartition of upper Maastrichtian–Ypresian in the Meknassy-Mezzouna basin, centerwestern Tunisia. Arab J Geosci 7:3881–3899. https://doi.org/10.1007/s12517-013-1050-y
Mosbahi M, Khlifi M, Jamoussi F, Tlili A (2017) Valorization of Coniacian-middle Campanian clay minerals of the Meknassy-Mezzouna region (centerwestern Tunisia) in the clinker manufacturing. Arab J Geosci 10:349. https://doi.org/10.1007/s12517-017-3124-8
Navarro A, Cardellach E, Corbella M (2011) Immobilization of Cu, Pb and Zn in mine contaminated soils using reactive materials. J Hazard Mater 186:1576–1585. https://doi.org/10.1016/j.jhazmat.2010.12.039
Navarro-Blasco I, Duran A, Sirera R, Fernández JM, Alvarez JI (2013) Solidification/stabilization of toxic metals in calcium aluminate cement matrices. J Hazard Mater 260(89):103. https://doi.org/10.1016/j.jhazmat.2013.04.048
Ouellet-Plamondon C M (2011) Characterisation and performance of innovative alumino silicates for soil mix technology permeable reactive barriers. Ph D Thesis, Cambridge University, UK.
Qian G, Cao Y, Chui P, Tay J (2006) Utilization of MSWI fly ash for stabilization/solidification of industrial waste sludge. J Hazard Mater 129:274–281. https://doi.org/10.1016/j.jhazmat.2005.09.003
Recueil (1994) Soils quality, Environment. AFNOR French norms, Paris
Scanferla P, Ferrari G, Pellay R, Ghirardini AV, Zanetto G, Libralato G (2009) An innovative stabilization/solidification treatment for contaminated soil remediation: demonstration project results. J Soils Sediments 9:229–236. https://doi.org/10.1007/s11368-009-0067-z
Stern R, White R, Whitney J (1992) Sustainable cities: urbanization and the environment in international perspective. Westview Press inc, Boulder CO Oxford, 5 May
Tariq A, Yanful EK (2013) A review of binders used in cemented paste tailings for underground and surface disposal practices. J Environ Manag 131:138–149. https://doi.org/10.1016/j.jenvman.2013.09.039
Tromp K, Lima AT, Barendregt A, Verhoeven JTA (2012) Retention of heavy metals and poly-aromatic hydrocarbons from road water in a constructed wetland and the effect of de-icing. J Hazard Mater 203(204):290–298. https://doi.org/10.1016/j.jhazmat.2011.12.024
Van der Sloot HA, Van Zomeren A, Meeussen JCL, Seignette P, Bleijerveld R (2007) Test method selection, validation against field data, and predictive modelling for impact evaluation of stabilised waste disposal. J Hazard Mater 141:354–369. https://doi.org/10.1016/j.jhazmat.2006.05.106
Vandeperre LJ, Liska M, Al-Tabbaa A (2008) Hydration and mechanical properties of magnesia, pulverized fuel ash, and Portland cement blends. J Mater Civ Eng 30:375–383. https://doi.org/10.1061/(ASCE)0899-1561(2008)20:5(375)
Voglar GE, Lestan D (2011) Efficiency modeling of solidification/stabilization of multi-metal contaminated industrial soil using cement and additives. J Hazard Mater 192:753–762. https://doi.org/10.1016/j.jhazmat.2011.05.089
Voglar GE, Lestan D (2010) Soldification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. J Hazard Mater 178:926–933. https://doi.org/10.1016/j.jhazmat.2010.02.026
Wang F, Wang H, Al-Tabbaa A (2014) Leachability and heavy metal speciation of 17-year old stabilised/solidified contaminated site soils. J Hazard Mater 278:144–151. https://doi.org/10.1016/j.jhazmat.2014.05.102
Wang F, Wang H, Jin F, Al-Tabbaa A (2015) The performance of blended conventional and novel binders in the in-situ stabilisation/solidification of a contaminated site soil. J Hazard Mater 285:46–52. https://doi.org/10.1016/j.jhazmat.2014.11.002
Zhou Q, Milestone NB, Hayes M (2006) An alternative to Portland cement for waste encapsulation, the calcium sulfo aluminate cement system. J Hazard Mater 136:120–129. https://doi.org/10.1016/j.jhazmat.2005.11.038
Acknowledgments
The authors would like to thank all the personnel in the Cement Laboratory of Gabes for help. They would also extend their thanks to Mrs. Leila Mahfoudhi, Emeritus Teacher of English in the Sfax Faculty of Sciences, for having proofread and polished the language of the manuscript.
Funding
This work was supported by the Research Unit of Environmental Geotechnics and Civil Materials, University of Sfax, National School of Engineering of Sfax.
Author information
Authors and Affiliations
Corresponding author
Additional information
Professor Mohamed Jamel Rouis died on 23 November 2017.
Rights and permissions
About this article
Cite this article
Bougharraf, N., Louati, D., Mosbahi, M. et al. Comparison of the effectiveness of different binders in solidification/stabilization of a contaminated soil. Arab J Geosci 11, 348 (2018). https://doi.org/10.1007/s12517-018-3668-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3668-2