Abstract
The Pliocene-Pleistocene Ghari Chandan Formation consists of lacustrine-fluvio-deltaic deposits exposed in the Attock-Cherat Range. Three beds of bentonite deposits occur in the upper part of the formation. An average thickness of each layer ranges from 0.4 to 1 m with a total strike length of ~5 Km. Montmorillonite and saponite clay minerals with admixtures of quartz, feldspar, muscovite, biotite, and hornblende were identified in these samples. Compositionally these beds are similar and comprised of dioctahedral smectite. The differential thermal analyses of bentonite reveals high thermal stability of the deposits. Detailed mineralogical and geochemical investigations suggest that the Garhi Chandan deposit is a mixture of calcic and sodic bentonite derived from andesitic and trachyandesitic source rock. Heavy metal concentration reduces the overall adsorbing capacity of the deposit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bentonite, which is the commercial name for montmorillonite was first used for clay in 1890 after its first discovery in the Upper Cretaceous tuff near Benton, Wyoming. Bentonite forms from the alteration of volcanic ash, predominantly consisting of smectite minerals, montmorillonite (Na0.2Ca0.1Al2Si4O10(OH)2(H2O)10), hectorite (Na0.4Mg2.7Li0.3Si4O10(OH)2), saponite(Ca0.1Na0.1Mg2.25Fe2+ 0.75Si3AlO10(OH)2•4(H2O)),beidellite(Na0.5Al2.5Si3.5O10(OH)2•(H2O)), and nontronite (Na0.3Fe3+ 2Si3AlO10(OH)2•4(H2O)) (Christidis and Dunham 1993; Cara et al. 2000). The most common accessory minerals present in bentonite include quartz, feldspar, mica, and hornblende, which can have a great impact on the industrial value of a deposit. The interlayer in clay minerals such as smectite are not only hydrated but are also expandable between individual smectite sheets that varies depending on the type of interlayer cations present (Odom 1986). The diagnostic physical properties such as favorable color result in high market value of bentonite and can be used in many industries particularly electrical, ceramics, pharmaceuticals, cosmetics, toiletries, and detergents. The main producing countries of bentonite are the USA, Greece, Italy, Spain, Turkey, and other Mediterranean regions (Christidis et al. 1997). Crystal shape, size, cation exchange capacity, hydration, swelling, thixotropy (the gel becoming firm on rest condition and reverting to fluidity when shaken), bonding capacity, impermeability, plasticity, dispersibility, and tendency to react with organic compounds make bentonite an important industrial mineral in Pakistan. This study shows in detail the geochemical, mineralogical, and industrial characteristics of the Pliocene-Pleistocene Ghari-Chandan bentonite deposit, which is situated between 33° 45′ N to 33° 55′ N latitude and 71° 40′ E to 71° 50′ E longitude about 40 to 45 km southwest of Peshawar in the Attock Cherat Range (Figs. 1 and 2).
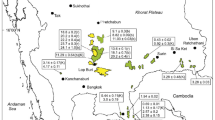
Regional geological gap showing exposed parts of the Higher Himalaya, Lesser Himalaya and Sub-Himalaya in Pakistan. The MMT separates the Higher Himalaya from the Kohistan Island Arc. The Khairabad/Panjal Thrust separates the Higher Himalaya from the Lesser Himalaya, and the MBT separates the Lesser Himalaya from the Sub-Himalaya, which is bounded to the south by the Main Frontal Thrust. The inset showing detail regional geology of the study area, which lies in the hanging wall of the Khairabad/Panjal Thrust Fault within the Higher Himalaya
Regional geological map showing major structures, basins, and location of the study area within the Attock-Cherat Range (Modified after Hussain et al. 1990)
Geological setting
The E-W trending Himalayan orogenic belt, which is comprised of the Tethyan Himalaya, Higher Himalaya, Lesser Himalaya, and Sub-Himalaya, separated by the Main Mantle Thrust from the Kohistan Island Arc in the north; to the south, it is marked by the Main Frontal Thrust; to the west, the left-lateral Chaman Fault separate it from the Afghan Block, and in the east, this immense orogenic belt is bounded by the right-lateral Sagain fault, which is exposed in Myanmar(LeFort 1975; Yin 2006). The Sagain Fault separates the Indian plate from the Eurasian plate. The Khairabad-Panjal Thrust Fault within the Indian Plate, which has been considered tectonically equivalent to the Main Central Thrust separates the Higher Himalaya from the Lesser Himalaya in the south. The Khairabad-Panjal Thrust Fault is marked by the thrusting of the Attock-Cherat Range over the Lesser Himalaya. Our study area Garhi Chandan, which is the part of Greater Himalaya, is located in the hanging wall of the Khairabad-Panjal Thrust Fault (Fig. 1).
The Precambrian to Cenozoic rocks are exposed in different parts of the area, and at places, these rocks are covered by the quaternary alluvium deposits. The Attock-Cherat Range has been divided into three blocks on the basis of structural and lithological characteristics (Fig. 2; Hussain et al. 1989; Pogue et al. 1999). The Northern Block consists of the Manki, Shahkot, Utch-Khattak, and Shekhai formations of the Precambrian age. Slate, stormatolitic limestone, and limestone with chert lenses are the major lithologies of these formations (Kazmi and Jan 1997; Shah 2009). The Khairabad Thrust Fault occurs to the south of the Northern Block and serves as the northern boundary of the Central Block. The Central Block consists of the Precambrian Dakhner Formation that consists of low-grade metamorphic argillites (claystone, siltstones with some sandstone). The Hissartang Thrust Fault serves as the southern boundary of the Central Block. The Southern Block lies to the south of the Hissartang Thrust Fault. It consists of the Palaeozoic Darwaza, Hissartang and Inzari formations. The regional Hissartang Thrust Fault in the Attock-Cherat Range is concealed by the Quaternary alluvium of the Nizampur Basin. The three blocks are collectively bounded by the Peshawar Basin to the north, Nizampur Basin to the south, and Campbellpore Basin to the east (Fig. 2; Yeats and Hussain 1987).
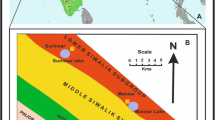
According to Hussain et al. (1989), the Paleocene stratigraphic sequence of the Attock-Cherat Range, which comprises of the Hangu, Lockhart, and Patala Formations unconformably overlie the Paleozoic sequence of the Southern Block (Fig. 3). The contact between the Paleozoic and Paleocene stratigraphic sequences is marked by a well-developed 1–2-m thick laterite marker bed. The Paleocene stratigraphic sequences in the region are unconformably overlain by the fluvial Miocene Murree Formation. The exposed Miocene Murree Formation in the study area is overlain by the Pale red and greenish yellow mudstone of the newly introduced Ghari Chandan Formation.
Detailed geological map of the Garhi Chandan area (Modified after Hussain 1992)
Methodology
Fieldwork
A total of 20 samples weighing each in the range of 350 to 400 g were collected from different exposed bentonite horizons distinguished on the basis of variation in color, for example, olive gray, brownish, and pinkish brown.
X-ray diffraction analyses
Sample preparation for XRD (oriented sample)
Samples collected in the field were crushed in the Geoscience laboratory, Islamabad. The powdered samples were sieved through 200 mesh; the American Society for Testing and Materials (ASTM) was used for the preparation of random powder slides. The analyses were carried out by Panalytical X’PertPRO Diffractometer (XRD) at 45 Kv and 40 mA with CuKα radiations scanning speed at 0.05°/s. In the first step, all samples were scanned by XRD and compared X-ray diffractograms to the International Centre for Diffration Data (ICDD) PDF 4 + 2009 database of over 600,000 known compounds to identify each mineral phase. A 3-g sample was placed in a 300-ml beaker and mixed with de-ionized water and left overnight. The suspension was centrifuged for 20 min and allowed to settle down. The suspension was taken from a depth and placed on a glass slide. The slides were then allowed to dry in air and referred to as normal slide. The samples were then analyzed in a diffractometer 8°/min from 2° to 40° 2θ degrees. The normal slide was then placed in a desiccator containing ethylene-glycol (glycol) for overnight. This glycolated slide was again analyzed at 8°/min from 2° to 40° 2θ degrees. This technique is useful to study mixed-layer clays. Organic molecules of glycol are able to penetrate the inter-layer space of clay minerals, which show expansion such as smectite, halloysite, and some mixed-layer clay minerals.
Mineral identification
The diffraction pattern of the samples was interpreted using software Xpert High Score Plus and manually. Minerals identified in pressed powder samples are quartz, montmorillonite, illite, muscovite, calcite, kaolinite, and albite. The oriented samples are montmorillonite and saponite.
Differential thermal analyses
Differential thermal analyses were carried out to analyze dehydration and dehydroxylation of seven samples using SHIMADZU Analysis Work Station TA-60WS at 20 °C/m from 35 to 1200 °C using sample weight of approximately 20 mg.
X-ray fluorescence spectrometry
Chemistry of 20 samples was determined using 3370 Rigaku WD-XRF for all major elements. Glass disk prepared by taking 0.4 g of volatile free sample with 4.0 g lithium tetra borate 1:10 ratio and after adding two drops of 5 % lithium iodide (LiI), heated in a bead sampler at 1050 °C for 6 min in a platinum crucible using fundamental parameter method, which has an excellent calibration procedure, management of volatile components and calculation method to measure XRF intensities. Trace element chemistry of the deposit was analyzed by Press pellet.
Scanning electron microscope analyses
Microscopic structures of bentonite were studied using scanning electron microscope (Model JEOL JSM 6610LV. Compositional analyses were carried out using EDS (OXFORD X-MAX 20 mm2. Powder sample is dispersed on special aluminum stubs; carbon paint is a better choice as particles are held firm in to the adhesive. Carbon coating is well applied all around the particles to avoid charging of the particles under the SEM.
Results and discussion
Interstratified 0.4- to 1-m-thick bentonite beds consist of montmorillonite (smectite), quartz, illite, muscovite, calcite, kaolinite, and albite. XRD results are shown in Table 1.
The XRD pattern of an oriented sample shows (001) peak on d = 14.5 Å, of montmorillonite (Fig. 4a). The expansion of the basal spacing from 14.5 to 17.05 Å after glycol addition (Fig. 4d), indicate the presence of sodium bentonite (Table 1; Fig. 4a) 0.060- peak (also called 06–33) of the d value allows the distinction between dioctahedral and trioctahedral clay minerals. In most of the studied samples, the 060-peak is constrained between 1.49 to 1.50A°, which indicate the composition of ideal dioctahedral Na-montmorillonite and Na-beidellite. G-5 at 2Ə 62.15, 61.98°, and 61.89°; the d values are 1.497, 1.495, and 1.497, respectively, and confirmed the presence of ideal dioctahedral Na-montmorillonite (M100 B0) which indicates single phase at temperature >375 °C (Yamada et al. 1991).
Understanding exchangeable cations (Na+, K+, Ca+ and Mg+) in fine-grained clay mineral interlayer sheets are of high importance to know the industrial applications (Evans 1993). Soils having high concentration of smectite (Na-montmorillonite) can undergo up to 30 % volume change due to wetting and drying, such as soil-termed swelling bentonites, is of great industrial demand (Yamada et al. 1991; Lim et al. 2013). Ca-montmorillonite does not have swelling property (Evans 1993). The weight loss from room temperature up to 1200 °C can be divided into three steps (Fig. 5). The first one, up to 300 °C, represents break out of water molecules from the surface and from the interlayer space, including water molecules coordinating exchangeable cations. The second and third regions are connected with de-hydroxylation of clay minerals (Andrejkovicova et al. 2006).
Significant endothermic peak between 105 and 115 °C (Fig. 5b) indicates the removal of adsorbed and interlayer water of the clay. An exothermic peak at 545.15, 546.84, and 547.58 °C is due to de-hydroxylation. Other significant peaks at 990.21, 990.66, and 991.97 °C were observed due to the breakdown of montmorillonite structure, which shows the thermal stability of montmorillonite related to the crystalline structure (Ralph et al. 1942). Garhi Chandan bentonite possesses high thermal stability, fine crystal size, and mono-mineralic composition in fraction <2 μm, which make it a capable material to be used as a catalyst to speed up reaction (Stankovic et al. 2011), cation exchanger, and can be used as oil well drilling mud and water sealer (Highley 1990; Evans 1993) .
The chemistry of selected samples shows high silica content and presence of alkali and alkaline oxides (Table 2). Forty-two to 66 % of SiO2 suggests that the Ghari Chandan deposit is derived from erosion in the hinterland/denudation zone. The percentage of Al2O3 varies between 4 and 18 % and Fe2O3 ranges from 0.62 to 8.57 %. Higher value of CaO in some samples indicates the presence of plagioclase and calcite, which is also identified through XRD pattern. The high percentage of Na+ does not exhibit high swelling capacity of bentonite because the presence of plagioclase also increases the quantity of sodium (Christidis et al. 1997). G-2, G-4, G-5, and G-6 have a high concentration of magnesium, which can reduce the bentonite swelling capacity (Lim et al. 2013). Results from selected samples indicate that bentonite from the Garhi Chandan area is the mixture of sodium and calcium bentonite. Samples G-2, G-4, G-5, and G-6 have large loss on ignition (LOI) values which also confirm the presence of calcite in our samples. The presence of calcite in bentonite related to the alteration process in these samples (Delgado et al. 1993). Ca-bentonite contains opal, alkali feldspar, biotite, and pyrite, while Na-bentonite contains less plagioclase feldspar, opal, kaolinite, and illite. Y, Zr, Th, Co, and Cr are considered as provenance indicators as these elements have relatively low mobility in the whole extent of sedimentary processes (Mc Lennan et al. 1983). Discrimination diagram Nb/Y versus Zr/TiO2 and TiO2 versus Zr (Winchester and Floyd 1977) using Minpet software (version 4) indicate that the Garhi Chandan bentonite derived from erosion of andesite and trachyandesite extrusive rocks in the hinterland/denudation zone (Fig. 6).
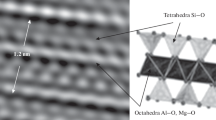
Microscopic structures and compositional analyses of bentonite were carried out using scanning electron microscope (Fig. 7). Traces of Ca2+, Mg2+, Na1+, and Ni4+ ions are present in the internal structure of bentonite. However, high content of Al+3 has also been found in the samples from the study area (Fig. 8). According to WHO, nickel level in drinking water range from 0.55 to 25 μg/l (Das et al. 2008). Bentonite powder can be used for cleaning juices, granules for medicine, pharmacy, cosmetics, household products, filtering refining, bleaching oils, and fats. The high concentration of Ni in the Garhi Chandan bentonite make the deposit prohibited to be used in the abovementioned industries because during refining of food-grade material, Ni salts also dissolved in it, which is harmful if it exceeds WHO limit. Similarly, the adsorption capacity of bentonite makes it a valuable material for a wide range of uses and applications. However, the high concentration of Ni in the Garhi Chandan deposit reduces the adsorbing capacity of the deposit (Vieira et al. 2010).
a Back-scattered SEM image of bentonite at 20 kV with magnification ×2300. b Back-scattered SEM image of bentonite at 20 kV with magnification × 2700 c Back Scattered SEM image of bentonite at 20 kV with magnification ×3700. d Back-scattered SEM image showing lamella of bentonite at high magnification (×1100). e Back-scattered SEM image of bentonite at 20 kV with magnification ×1600
Apart from the Garhi Chandan region, bentonite has also been reported from the Pliocene to Pleistocene Soan Formation, Mirpur district, Azad Kashmir (Hussain and Akhtar 2004). Chemical analyses of bentonite from Mirpur indicate that it consists of montmorillonite and beidellite with some igneous rock minerals. It is mostly Ca-bentonite in nature which suggests that the bentonite deposit is derived from alteration of volcanoclastics (Hussain and Akhtar 2004). However, the Garhi Chandan bentonite is the mixture of Ca2+ and Na1+ bentonite, Na1+ bentonite formed by alteration of digenetic materials in the sedimentary basin (Karakaya et al. 2011).
Recommendations
Type and quality are prerequisites for the selection of bentonites, and the process is usually guided by physical properties involving the chemistry of the bentonite but only to the extent that it becomes influential to physical properties. The Garhi Chandan bentonite due to its high swelling capacity can be used to reduce the permeability of soil, which is mostly used in the construction of buildings to make the porous medium water tight. It can be used in mortar cubes, concrete cylinders, and concrete beams as a partial substitute for ordinary Portland cement. It may be used as low-cost construction materials. Systematic uses of bentonite can reduce energy consumption in electrocoagulation process, preserve natural resources, solve environmental problems related to cement production and enhance the durability and life cycle of concrete structures.
Conclusions
Montmorillonite is the characteristic mineral identified from the XRD pattern of untreated (without glycolation) oriented sample. The basal spacing of montmorillonite expanded from 14.5 to 16.4 Å, and the 060-peak is around 1.49–1.50 Å after glycolation that is typical of dioctahedral smectite montmorillonite. Thermal analyses suggest that bentonite from the Garhi Chandan area has high thermal stability and can be used as a catalyst to speed up reaction in the petroleum and plastic industries. Both geochemical (XRF) and mineralogical study (SEM and XRD) indicate that the Garhi Chandan clay is a mixture of calcium and sodium. Trace elements geochemistry indicates that the deposit is formed from the erosion of intermediate volcanic rocks in the hinterland zone. Presence of Ni reduces the adsorbing capacity of bentonite.
References
Andrejkovicova S, Madejova J, Czímerova A, Galko I, Dohrmann R, Peter K (2006) Mineralogy and chemistry of Fe-rich bentonite from the Lieskovec deposit (Central Slovakia). Geol Carpath 57(5):371–378
Cara S, Carcangiu G, Padalino G, Palomba M, Tamaini M (2000) The bentonites in paleotherapy: thermal properties of clay pastes from Sardina(Italy). J. Appl Clay Sci 16:125–132
Christidis GE, Dunham AC (1993) Compositional variation in smectites. Part I. Alteration of intermediate volcanic rocks. A case study from Milos Islands, Greece. Clay Miner 28:255–273
Christidis GE, Scott PW, Dunham AC (1997) Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. J Appl Clay Sci 12:329–347
Das KK, Das SN, Dhun SA (2008) Nickel, its adverse health effects & oxidative stress. Indian J Med Res 128:412–425
Delgado A, Caballero E, Reyes E (1993) Bentonite deposits at Cabo De Gata (Almeria, Spain) 2nd Biennial SGA Meeting. Granada.Field Trip Guide Book. 81–100
Evans MA (1993) Ore geology and industrial minerals an introduction. Blackwell publishing, Third edition, pp 389
Grim RE, Rowald RA (1942) Differential thermal analysis of clay minerals and other hydrous materials part 1. Report of investigation No. 85(27): 746–761
Highley DE (1990) Britain’s industrial mineral resources and their exploitation. Erzmetall 43(1):19–28
Hussain A (1992) Working paper on the stratigraphic nomenclature of Attock- Cherat Range, Mtg. stratigraphic committee of Pakistan
Hussain A, Akhtar SS (2004) Geological map of Mirpur District AJK. Geological Survey of Pakistan Azad Jammu Kashmir Geological Map Series No 6
Hussain A, Yeats RS, Pogue K (1989) Stratigraphy and structural events around the southern margin of Peshawar Basin, Pakistan. Geol Univ Peshawar 22:45–54
Hussain A, Yeats RS, Pogue K (1990) Geological map of Attok-Cherat range and adjoining areas, N.W.F.P and Punjab Pakistan, GSP, Geological map Series,V.3
Karakaya C, Muazzez, Karakaya, Necati, Kupeli, Suayip (2011) Mineralogical and geochemical properties of the Na- and Ca-bentonites of Ordu (Ne Turkey). J. Clay Clay Miner 59(1)
Kazmi AH, Jan MQ (1997) Geology and tectonic of Pakistan. Graphic publisher Karachi 554
LeFort P (1975) Himalayas collided range present knowledge of continental arc. Am J Sci A275:1–44
Lim SC, Gomes C, Kadir MZA (2013) Characterizing of bentonite with chemical, physical and electrical perspectives for improvement of electrical grounding systems. Int J Electrochem Sci 8:11429–11447
Mc Lennan SM, Taylor SR, Korner A (1983) Geochemical evolution of Archean shales from South African: I the Swaziland and Pongola supergroup. J Precm Res 22:93–124
Odom IE (1986) Na/Ca montmorillonite: properties and uses. Soc Min Eng Trans 282
Pogue KR, Hylland MD, Yeats RS, Khattak W, Hussain A (1999) Stratigraphic and structural framework of Himalayan foothills, northern Pakistan. Geol Soc Am. Special paper. 328: 258–274
Shah SMI (2009) Stratigraphy of Pakistan, GSP, Memoir 22
Stankovic N, Logar M, Luković J, Pantić J, Miljević M, Babić B, Mihajlović AR (2011) Characterization of bentonite clay from “Greda” deposit. Process Appl Ceram 5(2):97–101
Vieira MGA, Almeida Neto AF, Gimenes ML, da Silva MGC (2010) Removal of Nickel on Bofe bentonite calcined clay in porous bed. J Hazard Mater 176:109–118
Winchester JA, Floyd PA (1977) Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem Geol 20:325–343
Yamada H, Nakazawa H, Yoshioka K, Fujita T (1991) Smectites in the montmorillonite-beidellite series. Clay Miner 206:359–369
Yeats RS, Hussain A (1987) Timing of structural events in the Himalayan foothills of North Western Pakistan. Bull Geol Soc Am 99:161–175
Yin A (2006) Cenozoic tectonic evolution of the Himalayan orogen as constrained by along-strike variation of structural geometry, exhumation history, and foreland sedimentation. Earth Sci Rev 76:1–131
Acknowledgments
Dr. Imran Khan, Director General GSP and Mr. Jawed Akhtar, Director Geoscience Advance Research Laboratories, Geological Survey of Pakistan Islamabad, are thanked for providing field and laboratory facilities. Dr. Rehan-ul-Haque Sidique, Mr. Ahmad Hussain, and Dr. A. B. Kausar of the Geological Survey of Pakistan and Dr. Shahid Assistant Professor Department of Earth science, Quaid-eAzam University are also thanked for their guidance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haider, N., Khan, S., Ali, A. et al. Mineralogical and geochemical study of bentonite deposits of Garhi Chandan area, District Nowshera, Khyber Pakhtunkhwa, Pakistan. Arab J Geosci 9, 531 (2016). https://doi.org/10.1007/s12517-016-2555-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2555-y