Abstract
The present study was conducted to investigate the concentrations of heavy metals (HMs) in deep groundwater from coal mining area, including cadmium (Cd), chromium (Cr), copper (Cu), zinc (Zn), lead (Pb), and nickel (Ni). The samples were collected from different aquifers in four coal mines of northern Anhui province, China, which were unconsolidated formation (UF), coal measure aquifer (CA), Taiyuan limestone aquifer (TA), and Ordovician limestone aquifer (OA), respectively. HM concentrations from the four different sources were analyzed by atomic absorption spectrometer, and were found in the order of Zn>Ni>Pb>Cu>Cd>Cr (in UF), Ni>Zn>Pb>Cu>Cr>Cd (in CA), Ni>Zn>Pb>Cu>Cd>Cr (in TA), and Zn>Ni>Pb>Cu>Cr>Cd (in OA), respectively. Concentrations of Cu, Zn, and Cr were found within the quality guidelines set by Bureau of Quality and Technical Supervision of China (GB/T 14848-93) and World Health Organization, while the concentrations of Cd, Pb, and Ni were higher than their respective permissible limits. The enhanced concentrations of Ni, together with Cr, were considered to be affected by anthropogenic sources, since they both had high variable coefficient. Moreover, the inter-dependence of HMs and their pollution sources were further discussed using statistical techniques, including one-factor analysis of variance, Pearson correlation analysis, and principle component analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although some HMs are the essential micronutrients for living beings, higher concentrations of HMs can lead to severe poisoning, which has been considered as deadly pollutants owing to their toxicity, persistence, and bioaccumulative nature in the environment (Pekey et al. 2004). Contamination with HMs such as Cd, Cr, Cu, Zn, Pb, and Ni is a worldwide environmental problem (Muhammad et al. 2011), and has drawn great attention in recent decades. Investigation of water contamination in terms of HMs has become the prime focus of environmental research in recent years (Muhammad et al. 2011). The HMs in water could be derived from both natural (i.e., weathering, erosion of bed rocks, ore deposits, and volcanic activities) and anthropogenic sources (i.e., mining, smelting, industrial influx, wastewater irrigation, and agriculture activities) (Demirak et al. 2006; Chanpiwat et al. 2010; Muhammad et al. 2010; Khan et al. 2013), among which mining process is also an important source of trace metals (Cantor 1997).
In China, water shortage has become an important water resource issue. Particularly, the shallow groundwater pollution is becoming increasingly serious. It was reported that fully 90 % of China’s shallow groundwater is polluted, according to the Ministry of Land and Resources, and an alarming 37 % is so foul that it cannot be treated for use as drinking water (Qiu 2011). For coal mining area, the water resource is also facing big challenges in terms of water quality and water quantity. On one hand, 71 % of 96 key state-owned mines are somewhat short of water, and 40 % of them suffer from serious water shortages, because Chinese water resources are largely located in South China, while most coal lies in the north (Wang et al. 2013). On the other hand, the water quality of the mining areas is deteriorating, and excavation works has become a key factor causing groundwater pollution. So, quality- and quantity-induced water shortage has come to be the prime factor, restricting the sustainable development of the coal mine areas.
From the current research situation, the majority of the studies on HMs in groundwater of coal mine areas focus on shallow groundwater (Wang et al. 2008; Liu 2010), while the study of deep groundwater is rare. Moreover, the study focusing on different aquifers in deep groundwater is much rarer. For the study area, some trace metals in deep groundwater collected from different aquifers were measured to discuss hydrogeochemical characteristics (Gui 2005), hydrological implications (Sun et al. 2014), quality and usability (Lin et al. 2014a; Sun et al. 2013a), risk assessment (Lin et al. 2014b; Hu et al. 2015), and background (Sun et al. 2013b). But, no study was conducted to research the HMs’ relationship and sources in deep groundwater. So, the present study is undertaken to address this critical knowledge gap, since the relationship between trace metals plays an important role to further understand the mechanism of groundwater. Overall, the specific goals of this study were as follows: (1) to investigate the concentrations of HMs in deep groundwater from different aquifers, which were sampled from four typical coal mines located at northern Anhui coal mining area, and (2) to better understand HMs’ relationships and sources using various multivariate techniques, including one-way ANOVA procedure, Pearson correlation analysis (CA), and principal components analysis (PCA). The ambitious target of this paper is to provide reference for the utilization and protection of groundwater resources in coal mining area.
Materials and methods
Study area description
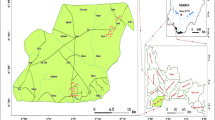
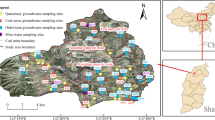
Northern Anhui province (32°25′–34°35′N, 114°55′–118°10′E) is an important production base for coal mine, agriculture, grain, and cotton in Anhui province and East China, which is located in the middle/east of China (Fig.1a), with a total area of 30,000 km2. The study area borders Shandong province in the north, Jiangsu province to the east, and Henan province to the west, mainly including Fuyang, Bozhou, Suzhou, and Huaibei city (Gui 2005). As an important coal base in China, the mine area has rich coal resources, and there are two big mining groups including more than 30 coal mines. The climate of the study area is warm temperate monsoon, where the average temperature is 14.6 °C, the average annual precipitation and evaporation are about 867.0 and 832.4 mm, respectively, and more than 50 % of the total precipitation falls during June to September (Yin et al. 2010; Chen et al. 2013).
The study area is also a part of Huaibei coalfield, one of the major coalfields in China, which is located in the central and southern area of the XueSu arcuate thrust nappe structure, east of the Henan-Huai depression at the southeastern margin of North China ancient plate (Jiang et al. 2010). The geological and hydrogeological backgrounds including stratum, regional tectonics, lithology, aquifer, aquiclude, etc. had been well described previously (Zheng et al. 2008; Jiang et al. 2010; Chen et al. 2013; Guo et al. 2014). According to the Huaibei coalfield integrated hydrogeological histograms (thickness, burial conditions, and water-bearing features), the main groundwater-inrushing aquifers can be divided into four categories from top to bottom (Fig. 1b), with the order of UF, CA, TA, and OA. More specifically, there are four sub-aquifers in QA as shown in Fig. 1b, the first aquifer, the second aquifer, the third aquifer, and the fourth aquifer in top-down order, and their average thickness are 27.92, 19.45, 33.84, and 17.5 m, respectively.
Sampling collection
The representative groundwater samples were selected from four locations (mine 1, mine 2, mine 3, and mine 4) of the study area shown in Fig. 1a. Hydrogeological types of the four mines are all at medium-complexity level, so they can represent the common conditions of the coal mines in this study area as a whole. A total of 59 groundwater samples were collected from four aquifers in the study area in October 2012. The four aquifers were UF (16 samples, primarily sampled from the third aquifer and the four aquifer), CA (26 samples), TA (12 samples), and OA (5 samples), and their sampling depth were 223.8~349.95, 250~649.5, 430~538.49, and 200~440 m, respectively. Clean and unmixed water samples were wanted in this study. The samples were selected preferentially under the coal mine, for example, the water samples from TA and OA could be sampled through drain holes. When the sampling under coal mine was not convenient, a tailor-made sampler was used to collect groundwater samples from observation wells above ground, which were constructed for pumping test and groundwater regime observation of different aquifers. Any obviously mixed or contaminative water samples were abandoned. Overall, samples were collected under coal mine in this study while only a small number were pulled out directly from observation well by hand pump on the ground. The sampling process had been described previously (Lin et al. 2014a; Lin et al. 2014b). Groundwater samples were collected in polyethylene bottles (2.5 L). Before sampling, the bottles were washed three times with double-deionized water in the laboratory and with the water at the sampling site during field sampling. Two bottles of groundwater (5 L totally) were collected at each sampling site; one bottle was analyzed for major ions, while the other was used for trace elements analysis. To analyze trace elements, each water sample was filtered through a 0.45-μm filter paper and acidified with ultrapure HNO3 (2 ml/L) to keep pH < 2. All the samples were transported to laboratory within 24 h and stored in a refrigerator at 4 °C for further analyses.
Chemical analysis procedures
The main physicochemical parameters were measured on the spot, including pH, temperature, and total dissolved solids. The six HMs (Cd, Cr, Cu, Zn, Pb, and Ni) in acidified water samples were analyzed using atomic absorption spectrometer (TAS-990 Purkinje General, China). Zn was detected by flame method, while the other HMs were determined by graphite furnace method. Standard samples (GSB 04-1767-2004) were used to fit standard curve with external standard method (all r values >0.999), and each heavy metal’s recovery was measured to confirm the accuracy, which were 93.64, 105.84, 101.83, 111.92, 95.50, and 96.96 % for Cd, Cr, Cu, Pb, Ni, and Zn, respectively. All the acids and reagents used were of analytical grade, and all these analyses were performed in research center of coal mine exploration engineering and technology in Anhui province, Suzhou University. In view of the data quality assurance, each sample was analyzed in triplicate, and average value of each water sample was used for further interpretation.
Statistical analysis
Descriptive statistics were calculated with Excel 2007 (Microsoft Office). The multivariate statistical analyses such as one-factor analysis of variance (one-way ANOVA), Pearson correlation analysis (CA), and principle component analysis (PCA) were performed by using the statistical product and service solutions software ver. 19.
Results and discussions
Heavy metal concentrations
Selected parameters in groundwater samples collected from the four aquifers (UF, CA, TA, and OA) are summarized in Table 1 . In this study, the detection rate of HMs were all 100 %, and their concentrations in the groundwater were found in the order of Zn>Ni>Pb>Cu>Cd>Cr (in UF), Ni>Zn>Pb>Cu>Cr>Cd (in CA), Ni>Zn>Pb>Cu>Cd>Cr (in TA), and Zn>Ni>Pb>Cu>Cr>Cd (in OA), respectively, in the four locations of the study area. Except Cd and Cr, the others selected HMs (Cu, Zn, Pb, and Ni) in groundwater from UF and OA had the same concentrations order of Zn>Ni>Pb>Cu, while the same concentrations order of Ni>Zn>Pb>Cu from CA and TA. In 2008–2009, Liu (2010) collected and detected 151 shallow groundwater samples (8–60 m) from Huaibei plain, where this study area was located in, and the results showed that the selected HMs (Zn, Cu, Pb, Ni, and Cd) in the order of Zn>Cu>Pb>Ni>Cd. It was suggested that the HMs concentrations are different between shallow and deep groundwater, furthermore, it is also different in deep groundwater with aquifers change. Moreover, the selected HMs in groundwater also had different concentration orders from the surface water, for example, Wang et al. (2015) found that HM concentrations in the water of subsidence pools were in the order of Cu>Zn>Pb>Cd (middle aged and old coal mine) and Zn>Cu>Pb>Cd (new coal mine), which were different from the four aquifers of groundwater in this study. Predecessors’ researches have shown that aquifers and crustal rock had the same curve shape in microelement abundance (Shen et al. 1993; Gui 2005), and the crustal rock is the final source for microelement in groundwater (Gui 2005). Thus, HMs had different concentrations with the change of hydrogeological condition, lithology of aquifer, and water-resisting layers in the deep groundwater.
The concentrations of Cu, Zn, and Cr in all groundwater samples were found within their respective permissible limits (GB/T 14848-93, Grade III) set by Bureau of Quality and Technical Supervision of China (BQTS, 1994) and World Health Organization (WHO, 2008) (Table 1). However, Cd, Pb, and Ni concentrations in some samples were higher than their respective permissible limits, for example, there was only one groundwater sample collected from TA, where Cd and Pb concentrations exceeded the permissible limit in 1.70 % of the water samples, while 6.25, 76.92, and 66.67 % groundwater samples in Ni concentrations were exceeded the permissible limit in UF, CA, and TA, respectively. The mean concentrations for Ni in CA and TA had also exceeded GB/T 14848-93 (Fig. 2). So, in this whole study area, Cd, Pb, and Ni should be paid much attention seriously, especially when the groundwater was considered as the water source for domestic water in drinking way. Moreover, the study area is also noteworthy with respect to Cr, although its single and mean concentrations were all within the permissible limit in four aquifers, the maximal concentration of Cr (47.35 μg/L) was recorded in CA (Table 1), which almost exceeded the permissible limit (50 μg/L).
Previous researches showed that high Cr and Ni concentrations in water samples could be attributed to the erosion of mafic and ultramafic rocks (Miller et al. 1991) and mining and industrial activities (Kavcar et al. 2009; Shah et al. 2010; Arif et al. 2011). According to the Huaibei coalfield integrated hydrogeological histogram, the bottom of Plx (including 4, 5, 6, 7, 8 coal bed) was aluminum mudstone in study area. Thus, it was recommended that Cr and Ni got the maximal value in coal measure aquifer mainly due to the etching effects of parent rock and bed rock. However, Cr and Ni both had bigger value of variable coefficient (CV), 173.77 and 82.24 % (Table 1), respectively. So, it was not hard to find that the enhanced concentration of Cr in groundwater samples, together with and Ni, might also be ascribed to anthropogenic sources, especially the coal mining activities in the study area.
Relationship analysis of heavy metals
Variation trend
One-way ANOVA procedure was conducted for the statistical comparison of selected HMs’ contaminations in the four different sampling aquifers, using least significant difference (LSD). Results showed significant variation (P = 0.015) between these four aquifers, which indicated that different sampling aquifers contribute differently to the mean metal concentrations in the deep groundwater (Table 2). However, each heavy metal also had a different variation value (LSD method) for an individual sampling aquifer in the study area, as given in Table 3 and Fig. 3. Cd, Pb, and Ni concentration in deep groundwater had significant variation (P < 0.01) from the four aquifers, which is shown in Table 2 and Fig. 3a, e, f. However, no significant variation was found for Cr, Cu, and Zn in groundwater samples collected from different aquifers as shown in Table 2 and Fig. 3b–d. Figure 3 also suggested the distributions of HMs. Cr, Cu, and Ni got the maximal value in CA, Cd, and Pb obtained the maximal value in TA, while Zn got the maximal value in OA.
Boxplots comparison for Cd (a), Cr (b), Cu (c), Zn (d), Pb(e), and Ni (f). (In this box plot, the square line shows the mean values, the horizontal line means the 50 % of the variable, the top and bottom of the box mark the limits of ±25 % of the variable, while the lines extending from the top and bottom of each box mark the minimum and maximum values)
Inter-metal relationship in different aquifers
The inter-metal relationships provided interesting information on metal sources and pathways (Noorain, et al. 2014; Muhammad et al. 2011). The physio-chemical parameters correlation matrices in deep groundwater from the four aquifers (UF, CA, TA, and OA) are summarized in Table 4. In deep groundwater from UF, the correlation matrices show that various physio-chemical parameters pairs have significant positive correlations, such as Cd–Cr (r = 0.508), Cd–Cu (r = 0.850), Cd–Pb (r = 0.903), Cd–Ni (r = 0.729), Cr–Zn (r = 0.683), Cr–Pb (r = 0.671), Cu–Pb (r = 0.827), Cu–Ni (r = 0.869), and Pb–Ni (r = 0.644), suggesting a major common origin. In deep groundwater from CA, the correlation matrices also show that various physio-chemical parameters pairs have significant positive correlations at the 0.01 level, such as Cd–Cr (r = 0.540), Cd–Cu (r = 0.519), Cd–Pb (r = 0.908), Cd–Ni (r = 0.671), Cr–Cu (r = 0.947), Cr–Pb (r = 0.752), Cr–Ni (r = 0.825), Cu-Pb (r = 0.731), Cu–Ni (r = 0.764), and Pb–Ni (r = 0.755). Moreover, significant positive correlations were also observed between most physio-chemical parameters pairs in deep groundwater from TA, such as Cd–Cr (r = 0.606), Cd–Cu (r = 0.632), Cd–Zn (r = 0.716), Cd–Pb (r = 0.980), Cd–Ni (r = 0.958), Cr–Cu (r = 0.721), Cr–Zn (r = 0.878), Cr–Pb (r = 0.659), Cu–Zn (r = 0.834), Cu–Pb (r = 0.624), Zn–Pb (r = 0.747), Zn–Ni (r = 0.607), and Pb–Ni (r = 0.968).
However, in the case of groundwater samples from OA, correlation matrices show that there is no physio-chemical parameter pair having significant positive correlations at the 0.05 level or at the 0.01 level. But Cd–Cu (r = 0.678), Cr–Zn (r = 0.656), Cr–Ni (r = 0.664), Zn–Pb (r = 0.636), Zn–Ni (r = 0.629), and Pb–Ni (r = 0.805) also have better positive correlations. However, Cd–Zn (r = −0.838) shows negative correlation, indicating their different behaviors, sources or migration features in OA. The result was also very different from the other groundwater samples collected from UF, CA, and TA. From Table 4, the correlation matrix of HMs shows that Cd–Pb (r = 0.903, 0.908, and 0.980 in UF, CA, and TA, respectively) and Cu–Pb (r = 0.827, 0.731, and 0.624 in UF, CA, and TA, respectively) both have significant positive correlations in the deep groundwater collected from UF, CA, and TA; however, these two parameters pairs do not have significant positive correlations in OA, and in other words, a low correlation (r = 0.349) and negative correlation (r = −0.150) were even evident for Cd–Pb and Cu–Pb pairs, respectively. Previous studies revealed that Cd–Pb have significant positive correlation in groundwater sampled from Kohistan region (r = 0.925) (Muhammad et al. 2011). Similarly, Cu–Pb also show significant positive correlation in groundwater collected from Dabaoshan tailing zone and its surrounding areas (r = 0. 0.993) (Zhang et al. 2013). Except for OA, the correlation analysis conclusions of Cd–Pb and Cu–Pb in groundwater were consistent with previous studies.
The correlation coefficients of Cd and Pb concentrations in groundwater collected from UF, CA, TA, and OA were 0.138, 0.825, 0.534, and 0.664, respectively. As suggested by previous studies, the co-variation of Cr and Ni in magmatic or clastic rocks can be attributed to the existence of dark-colored minerals (e.g., biotite, hornblend) (Miller et al. 1991), and therefore, during water-rock interaction, the significant correlation between Cr and Ni concentrations in this study might be an indication of the participation of dark-colored minerals. This hypothesis can be demonstrated by the hydro-geological conditions that the main rock types in the CA is sandstone with enrichment of dark-colored minerals, and the correlation coefficient between Cr and Ni in this aquifer is highest relative to other aquifers. Alternatively, this phenomenon might be an indication of the hydro-dynamic conditions that the CA is close relative to other aquifers, because of the high degrees of participation of dark-colored minerals implying that water-rock interaction is the dominant process controlling the hydrochemical characteristics of the groundwater in the CA. However, the groundwater samples in UF have the lowest correlation coefficient of Cr–Ni (r = 0.138), which might be an indication of low degrees of participation of dark-colored minerals or the open condition of the aquifer. Similar conclusions can also be obtained for other aquifers (TA and OA).
Source apportionment of heavy metals
To analyze the source of HMs in the deep groundwater, PCA was performed on the normalized data of HM concentration in the deep groundwater to describe the association and the qualitative behavior of clustering. Kaiser–Meyer–Olkin (KMO) and Bartlett’s results were 0.656 and 350 (df = 15, p < 0.001), respectively, which indicated that PCA would be effective in the data set. In this study, two principal components with eigenvalues >1 that explained about 79.61 % of the total variance were obtained. The corresponding principal components, percentage of variance, and cumulative percentage are presented in Table 5.
The first principal component (PC1), accounting for 60.49 % of the total variance, had high positive loadings on Cd (0.793), Cr (0.818), Cu (0.894), Zn (0.868), and Pb (0.847) and a low loading on Zn (0.249). However, the second principal component (PC2) accounted for 19.12 % of the total variance, and only had strong positive loadings on Zn (0.743). As mentioned above, although Cr and Ni contaminations could result from the erosion of mafic and ultramafic rocks (Miller et al. 1991) and mining and industrial activities (Kavcar et al. 2009; Shah et al. 2010; Arif et al. 2011), their enhanced concentration (Ni) and high value of variable coefficient (Ni and Cr) may also be ascribed to anthropogenic sources. For example, coal mining activity may be the most probable origin in this study area. Cu could be influenced more by agricultural activities (Khan et al. 2013). As to Cd and Pb, Cd is usually present in all soils and rocks, and its level elevation could be caused by the erosion of schistose rocks, while Pb level could be influenced by plumbing systems (Khan et al. 2013). So, the results suggested that PC1 may represent both geologic and anthropogenic sources. Zn could come from the schistose rocks with sulfide seams (Khan et al. 2013). Moreover, our previously published study had found that Zn and Fe had close correlation (Sun et al. 2013a), and as we all know, Fe is mainly from natural weathering processes, usually abundant on the earth and selected as the normalizing element due to its conservative nature during diagenesis; thus, the PC2 may have only geologic contributions.
Conclusions
HMs in shallow groundwater were well-recognized, especially when it was taken as drinking water source for urban areas. The present study focused on the deep groundwater, and described the relationships and sources of HMs in the four different aquifers for the first time. HM concentrations in the groundwater from UF, CA, TA, and OA were investigated in the order of Zn>Ni>Pb>Cu>Cd>Cr, Ni>Zn>Pb>Cu>Cr>Cd, Ni>Zn>Pb>Cu>Cd>Cr, and Zn>Ni>Pb>Cu>Cr>Cd, respectively. Concentrations of Cu, Zn, and Cr were found within the permissible limits set by GB/T 14848-93 and WHO, while Cd, Pb, and Ni concentrations exceeded their respective permissible limits. The ANOVA analysis showed that heavy metal concentration in the four different sampling aquifers varied significantly (P < 0.05). Various physio-chemical parameters pairs in deep groundwater had a strong correlation, which varied with the different sampling aquifers. Moreover, except for Zn, the PCA results revealed that both geologic and anthropogenic sources were responsible for the HM contamination of the deep groundwater in the coal mining area, northern Anhui province. Furthermore, this study will give references for water pollution control to local government, as well as to the study on HMs from deep groundwater in the other coal mining areas.
References
Arif M, Henry DJ, Moon CJ (2011) Host rock characteristics and source of chromium and beryllium for emerald mineralization in the ophiolitic rocks of the Indus Suture Zone in Swat, NW Pakistan. Ore Geol Rev 39:1–20
BQTS (Bureau of Quality and Technical Supervision of China) (1994) National Standard of the People’s Republic of China: Quality Standard for Ground water. GB/T 14848-93. Standards Press of China, Beijing
Cantor KP (1997) Drinking water and cancer. Cancer Causes Control 8:292–308
Chanpiwat P, Sthiannopkao S, Kim K-W (2010) Metal content variation in wastewater and biosludge from Bangkok’s central wastewater treatment plants. Microchem J 95:326–332
Chen L, Yin X, Xie W, Feng X (2013) Calculating groundwater mixing ratios in groundwater-inrushing aquifers based on environmental stable isotopes (D, 18O) and hydrogeochemistry. Nat Hazards 71:937–953
Demirak A, Yilmaz F, Levent Tuna A, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458
Gui HR (2005) Hydrogeochemical characteristics discrimination of groundwater in mining district of north Anhui province. University of Science and Technology of China, Hefei, PhD Dissertation
Guo P, Cheng Y, Jin K, Liu Y (2014) The impact of faults on the occurrence of coal bed methane in Renlou coal mine, Huaibei coalfield, China. Journal of Natural Gas Science and Engineering 17:151–158
Hu YH, Wang XM, Dong ZB, Liu GJ (2015) Determination of heavy metals in the groundwater of the Huaibei Plain, China, to characterize potential effects on human health. Anal Lett 48:349–359
Jiang B, Qu Z, Wang GGX, Li M (2010) Effects of structural deformation on formation of coalbed methane reservoirs in Huaibei coalfield, China. Int J Coal Geol 82:175–183
Kavcar P, Sofuoglu SC, Sofuoglu A (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212:216–227
Khan K, Lu Y, Khan H, Zakir S, Khan S, Khan AA, Wei L, Wang T (2013) Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J Environ Sci 25:2003–2013
Lin ML, Gin HR, Peng WH (2014a) Study on content characteristics and water quality assessment of heavy metals in deep groundwater from northern Anhui mining areas. J Saf Environ 14:266–271
Lin ML, Gin HR, Peng WH, Sun LH, Chen S, Li ZC (2014b) Health risk assessment of heavy metals in deep groundwater from different aquifers of a typical coal mining area: a case study of a coal mining area in northern Anhui province. Acta Geosci Sin 35:589–598
Liu J (2010) Research on the hydrogeoechemical characteristics of shallow groundwater of Huaibei plainin Anhui province. AnHui University of Science and Technology, Huainan, MS Dissertation
Miller DJ, Loucks RR, Ashraf M (1991) Platinum-group element mineralization in the Jijal layered ultramafic-mafic complex, Pakistani Himalayas. Econ Geol 86:1093–1102
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98:334–343
Muhammad S, Tahir Shah M, Khan S (2010) Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, northern Pakistan. Food Chem Toxicol 48:2855–2864
Noorain MI, Ahmad ZA, Wan YL, Wan NAWS, Sarva MP (2014) Evaluation of heavy metal contamination in groundwater samples from Kapas Island, Terengganu, Malaysia. Arab J Geosci 7:1087–1100
Pekey H, Karakaş D, Bakoğlu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar Pollut Bull 49:809–818
Qiu J (2011) China to spend billions cleaning up groundwater. Science 334:745
Shah HU, Simpson TJ, Alam S, Khattak KF, Perveen S (2010) Mould incidence and mycotoxin contamination in maize kernels from Swat Valley, North West Frontier Province of Pakistan. Food Chem Toxicol 48:1111–1116
Shen ZL, Zhu WH, Zhong ZS (1993) Hydrogeochemical basis. Geological Publishing House, Beijng
Sun LH, Gui HR, Peng WH (2013a) Heavy metals in groundwater from the coal bearing aquifer in northern Anhui province, China: concentrations and usability. J Chem Pharm Res 5:1349–1353
Sun LH, Gui HR, Peng WH (2014) Heavy metals in groundwater from the Wolonghu coal mine, northern Anhui province, China and their hydrological implications. Water Practice & Technology 9:80–87
Sun LH, Gui HR, Peng WH, Lin ML (2013b) Heavy metals in deep seated groundwater in Northern Anhui province, China: quality and background. Nature Environment And Pollution Technology 12:533–536
Wang J, Feng L, Davidsson S, Höök M (2013) Chinese coal supply and future production outlooks. Energy 60:204–214
Wang TJ, Zha XF, Xiong WN, Guo XH, Lai KD, Yang GZ (2008) Primary study of health risk assessment of heavy metals in karst groundwater in Gaoping area in Zunyi city, Guizhou province. Research of Environmental Sciences 21:46–50
Wang X, Chu Z, Zha F, Liu S, Liu G, Dong Z (2015) Determination of heavy metals in water and tissues of crucian carp (Carassius auratus Gibelio) collected from subsidence pools in Huainan coal fields (China). Anal Lett 48:861–877
WHO (World Health Organization) (2008) Guidelines for drinking-water quality 3rd edition, vol 1, Recommendations. World Health Organisation, Geneva
Yin D, Shu L, Chen X, Wang Z, Mohammed ME (2010) Assessment of sustainable yield of karst water in Huaibei, China. Water Resour Manag 25:287–300
Zhang Y, Li Z, Chen Z, Peng X, Feng H, Lei G, Ding Z, Zhao S (2013) Assessment of health risk from heavy metals in groundwater of the Dabaoshan tailing zone and its surrounding areas, Guangdong province, China. Journal of Agro-Environment Science 32:587–594
Zheng L, Liu G, Wang L, Chou C-L (2008) Composition and quality of coals in the Huaibei coalfield, Anhui, China. J Geochem Explor 97:59–68
Acknowledgments
This work was financially supported by State Natural Science Fund Projects (41373095, 41173106), the Project for Outstanding Young Talent of Suzhou University (2013XQRL05), the Foundation for Suzhou Regional Development Cooperation and Creatively Center (2013szxtcx001), the Program for Innovative Research Team of Suzhou University (2013kytd01), and the Talents Project of Suzhou University (2014LJ01) together. The authors thank Prof. Zhang from Zhongshan University for his help in the technical guidance and English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, M., Peng, W. & Gui, H. Heavy metals in deep groundwater within coal mining area, northern Anhui province, China: concentration, relationship, and source apportionment. Arab J Geosci 9, 319 (2016). https://doi.org/10.1007/s12517-016-2354-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2354-5