Abstract
A combined study of both geoelectrical and geochemical methods was used to find the quality of groundwater in the Agastheeswaram Taluk of Kanyakumari District, Tamil Nadu, India. The vertical electrical sounding (VES) method was applied to identify the variations in electrical resistivity of the subsurface. Three to five geoelectric layers are interpreted in the study area from the observed VES data. The aquifer was mostly found in the second or third layer. Analytical results from the 14 groundwater samples collected near the VES stations are in good correlation with the VES outcomes. Also, the interpreted results of VES data collected from the field were compared with the existing subsurface data and correlation was made, which proved the consistency of the VES method. Iso-resistivity contour maps for different depths and pseudo cross sections showing the resistivity distribution with depth are prepared for detailed analysis. More than 50 % of the area is having aquifer resistivity value less than 10 Ωm. This indicates that the aquifer is found to get polluted by saline water intrusion or some other anthropogenic activities, like intense agricultural practices and poor sewage management. From the geochemical analysis of the groundwater samples, it is found that the Na+ and Cl− are the dominant cation and anion in the study area. Evaporation and rock-water interaction are found to be the major processes controlling the ion concentration in the groundwater. The results obtained from geochemical analysis are in good correlation with the VES results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater exploration is important worldwide due to the ever increasing demand of water for agriculture and drinking purposes. Rapid urbanization, increased industrial and agricultural activities, which depend greatly on groundwater, have led to significant degradation in groundwater quality. Once contamination has occurred, it is usually very difficult and sometimes impossible to successfully clean the aquifers. So, maintaining the existing quality of groundwater remains the best option. Early detection of contamination can be achieved by regular monitoring of groundwater quality (Zulfiqar et al. 2010). Such monitoring programmes can only be achieved if water wells are available. However, in locations where water wells are not available, the geoelectrical techniques can be used to assess the extent of groundwater contamination (Gnanasundar and Elango 1999). Groundwater investigations using electrical resistivity method have been successfully carried out by several researchers worldwide (Sree Devi et al. 2001; Umar et al. 2007; Pervaiz et al. 2010; Asfahani 2011). The geoelectrical method is one of the geophysical methods that can be used to assess the presence of aquifers, water tables, impermeable formations, depths to the bedrock and groundwater salinity (Todd 1980).
The geoelectrical resistivity survey may aid in planning efficient and economic test drilling programmes for groundwater explorations (Lusczynski and Swarzenski 1966). The thickness and resistivity of different horizontal or low dipping subsurface layers, including aquifer zones, can be determined from the vertical electrical sounding data (Choudury and Saha 2004). Different formations with nearly similar resistivities such as saline clay and saline sand cannot be distinguished clearly with the electrical sounding. In such cases, integration of geochemical data with geophysical data can be used to resolve such problems (Sang-ho et al. 2002; Cimino et al. 2008; Gurunandha Rao et al. 2011). Results observed from the analyses of geochemical data can be used to gain insight into hydrogeological conditions and source of contamination in a particular area (Krishna Kumar et al. 2009; Tirumalesh et al. 2010; Srinivasamoorthy et al. 2011a). In earlier years, the groundwater quality was not very poor in the study area because of good precipitation (Perumal and Thamarai 2008). But nowadays, due to over exploitation, increase in population, rapid urbanization and unrestrained agricultural practices, the quality of groundwater is in a declining trend. The aim of this study is to correlate the results of both the geoelectrical and geochemical investigations for delineating the groundwater quality. The present study is also aimed to detect the dominant ionic sources and also to outline the areas suitable for groundwater development.
Study area
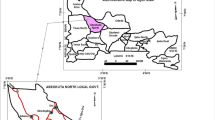
Agastheeswaram Taluk of Kanyakumari District lies in the southern tip of peninsular India and covers an estimated area of about 272 km2. It is located between latitudes 8° 33′ 45″ N and 8° 12′ 45″ N and longitudes 77° 18′ 45″ E to 77° 34′ 15″ E (Fig. 1). The average elevation of the study area is about 13 m above mean sea level. The study area is surrounded by hills in the northern sides, lush green paddy fields in the central regions and sandy beaches on its southern and eastern sides. A gentle slope can be noticed towards south and southwest directions. It receives both the northeast monsoon during the month of July and southwest monsoon during the months October to December. Average maximum temperature is about 30 °C, and minimum temperature is about 19 °C. In the northern part of the study area is the Thovalai Taluk while the Kalkulam Taluk borders its western boundary. The southern and southeastern regions are surrounded by the Indian Ocean and Bay of Bengal, respectively, while on the northeast side is the Tirunelveli District.
Geology and hydrogeology
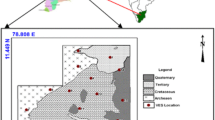
The study area was underlain by charnockites, leptinites, leptinite gneisses, granite gneisses, laterites, sandstones, variegated clay, river alluvium and so on. The northwestern region of the district is completely occupied by Western Ghats Mountain with a maximum elevation of 1658 m. The coastal region in the south is a thin strip of plain with a width that varies from ~1–2.5 km. The coastline has narrow stretches of beaches and sand dunes. The area adjoining the coast is characterized by laterite capping. The geology map of the study area is shown in Fig. 2.
Soil types within the study area are classified into red loams and pale reddish coloured lateritic soils. The mixed red and alluvial soil occurs commonly within the study area. The thickness of the overburden materials in the mountainous areas is almost negligible and is around 2 m in the valleys. The Charnockites group consists mainly of charnockites, pyroxene granulites and their associated migmatites. Banded and reticular gneisses are also exposed in the gneissic rocks. The groundwater can be seen in all the regions of the study area. Almost all of the geological formations in the district are saturated, and the level of saturation is more in the weathered mantle of the hard rock. Rock weathering is more predominant in the upper 10–35 m depth below the surface. The alluvial formations are highly permeable, and the groundwater occurs under water table conditions (PWD 2005).
The Kanyakumari District has a different type of drainage pattern having perennial streams flowing towards south and southwest directions. All the major rivers draining in the Kanyakumari District originate from the Western Ghats and flow towards the southwest directions. The major river flowing in the study area, the Agastheeswaram Taluk, is the Pazhayar River. The Pazhayar River originates from the Mahendragiri Hills north to the Arumanallur village at Thovalai Taluk and drains through the Agastheeswaram Taluk and confluences with the Indian Ocean near Manakudy (PWD 2005). The drainage pattern of the study area is given in Fig. 3.
Mostly, on hard-rock regions, the occurrence of weathered overburden materials is discontinuous both in space and depth. Hence, the groundwater recharge is influenced by the intensity of weathering. Static water level usually fluctuates throughout the year and attains maximum levels from October to December due to the southwest monsoon. It reaches its minimum levels during February to September because of summer, but a minor increasing trend is seen in the water level during the month of July because of southwestern monsoon. However, the water level studies in the study area reveal a general decreasing trend in water level for the past 10 years (PWD 2005).
Methodology
To assess the quality of the groundwater circulating inside the aquifers and to delineate the saline and industrial waste contaminated areas from the fresh water zones in the study area, two methods were employed. These techniques are (1) geoelectrical survey and (2) geochemical analysis.
Geoelectrical survey and data analysis
An electrical resistivity survey involving vertical electrical soundings (VES) was carried out at 38 locations randomly cited within the study area (Fig. 1). Even though the Wenner and Schlumberger electrode configurations are commonly employed in such investigations, the Schlumberger electrode configuration is most suited for attaining better results. Since Schlumberger method has practical, operational, and interpretational advantages over the Wenner method of electrode arrangement (Zohdy et al. 1974; Bhimasankaram and Gaur 1977; Ward 1990). The soundings were performed using a DC resistivity meter. Maximum half current electrode spacing (AB/2) used in field data acquisition was up to 100 m. The VES data obtained from the field were inverted using the IPI2Win software, which carry out an automated approximation of the initial resistivity model with the observed data (Bobachev 2002). IPI2Win works in an iterative mode by calculating at the end of each step: (a) a simplified model of layer thicknesses and resistivities and (b) the misfit function involving both the observed and calculated data sets. All resulting models produced low root mean square errors that were below 5 %. From the preliminary analysis, the resistivities and thicknesses of the different geoelectric layers at each VES locations were acquired.
Geochemical analysis
Groundwater samples were collected randomly from shallow wells and deep tube wells of static water level depth varying from 3 m to more than 30 m at different places of Agastheeswaram Taluk in the end of the month January 2013. Fourteen samples were collected from different areas close to the VES stations. Groundwater samples for chemical analysis were collected in acid washed polyethylene bottles cleaned with distilled water. In the field, the sample bottles were washed at least three times by the groundwater from respective groundwater sources in the study area. During the collection and transportation of water samples to the laboratory, all essential precautions were observed as suggested by Brown et al. (1974).
The collected samples were analysed for several chemical parameters using the standard procedures enumerated in the American Public Health Association (APHA 1995). The pH and EC were measured by means of pH and conductivity meters, respectively, in the field. The major cations such as calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+) and the major anions such as bicarbonate (HCO3 −), chloride (Cl−) and sulphate (SO4 2−) were analysed in the laboratory. TDS were computed from the EC by calculation method. The formula used for determining the TDS was
Na+ and K+ were determined by flame photometer. Ca2+ and Mg2+ were determined titrimetrically using standard EDTA titration. AgNO3 titration was adapted to estimate chloride (Cl−). The ion balance error (IBE) technique was used to assess the accuracy of the observed results by comparing the total cations with the total anions for complete analyses of water sample. The IBE was normally found to be within the range of acceptability (±10 %) (Mandel and Shiftan 1981).
Results and discussion
Geoelectrical survey
Vertical electrical soundings
A total of 38 VES were carried out to study the groundwater conditions in the study area. The data obtained from the field were processed by using the one-dimensional inversion programme IPI2WIN (Bobachev 2002). The obtained layer parameters viz. resistivity and thickness are shown in Table 1, and these results are correlated with the nearby known lithlog data to obtain better results. Some of the selected VES curves along with the layer parameters are given in Fig. 4.
Three to five geoelectric layers were interpreted from the observed VES data. The first layer was interpreted as top soil layer or hardened clay with resistivity that varies from 6.3 to 854 Ωm and thickness that ranges between 0.4 and 6 m. Resistivity values of the materials in the second layer were interpreted in the range of 1.2 to 237 Ωm. The thickness of the materials in the second layer varies between 1 to 28.4 m. In most of the sounding stations, the shallow aquifers were present in this layer. The major lithological compositions found in this layer are highly weathered charnockite, clay, clay with interbed of sand, fine sand or highly weathered gneiss.
The water quality in the aquifer varies from potable to very saline. In the present study, the aquifers with the resistivity value less than 10 Ωm is considered as saline, and if the resistivity of the aquifer is in the range 10 to 100 Ωm, then there is a possibility of potable water. The third layer is characterized by resistivity values that vary from less than 1.2 to 4545 Ωm. In majority of the stations, this layer attributed to fractured hard rock, hard rock or other materials. In some stations, the other lithological compositions include the clayey and sandy materials with saline water that are characterized by low resistivities. The resistivity values observed in some stations were less than 10 Ωm which probably indicates salinity problem in such areas. The reliability of the vertical electrical sounding is checked by correlating the collected VES data with the existing lithological information. The subsurface data from Varioor and Mylaudy areas were correlated with stations S1 and S21 (Fig. 5), and good correlation was obtained.
Pseudo resistivity cross sections (Fig. 6) were prepared along three profiles. The resistivity cross sections enable us to decipher subsurface resistivity distribution in both vertical and horizontal directions. The pseudo cross sections (profile-1, profile-2 and profile-3) were prepared from the results of the VES data inversion using IPI2WIN software.
From Fig. 6, in the pseudo cross section along profile-1, it can be seen that there are no layers with resistivity less than 10 Ωm, which shows these stations are free from saline water intrusion. It seems that the water quality near the VES stations S27 and S32 are found to be good. In the profile-2, the subsurface near the VES stations S23, S9 and S11 are found to have lower resistivity zones. At the VES station S11, the lower resistivity may be because of the contamination of groundwater due to saline water intrusion from the nearby estuary. The areas around the stations S9 and S23 show lower resistivity because of the pollution by some anthropogenic activities. In profile-3, it can be seen that two zones with resistivity are less than 10 Ωm, one is near VES station S6, which may be because of the saline water incursion; eventually, the aquifer is found to be a coastal aquifer. Second one near the station S20, we can find a low resistivity zone which may be because of the local contamination mainly from the effluent water from the surrounding agricultural fields and sewages.
Spatial and vertical distribution of resistivity
The spatial distribution maps of the thicknesses and resistivities (Fig. 7) of the obtained geoelectric layers were prepared using Arc GIS 9.3 software. As all the soundings are conducted away from the coastal region, the resistivity values depicted in the resistivity distribution maps are extrapolated. Figure 7a shows the distribution of the top layer resistivity in the study area. Figure 7a shows that the resistivity of the top layer in a greater part of the study area is less than 150 Ωm. The resistivity values of the top layer were observed to be higher at VES stations S12, S13, S27, S31 and S35. Compacted clay formations increase the resistivity in these stations. High resistivity values of the second layer depicted in the spatial map (Fig. 7b) was observed in the southern and western sides of the study area. Based on the interpretation of the VES curves and considering the resistivity values, the second layer was identified as aquifer at VES stations S1, S2, S4, S5, S6, S8, S9, S11, S17, S18, S20-S23, S31 and S32. Within the saturated aquifers, the resistivity values at VES stations S4, S6, S8–S11, S20, S22 and S23 were observed as very low and were attributed to contamination by saline water intrusion and other anthropogenic activities. These activities include increased soil moisture content due to irrigation farming and increased salinity caused by excessive evaporation and use of inorganic fertilizers (Elwaseif et al. 2012).
The resistivity distribution pattern of the third geoelectric layer (Fig. 7c) shows that the northern and eastern parts of the Agastheeswaram Taluk is characterized by high resistivity values compared to the other parts. The dominant materials at VES stations S3, S7, S10, S13–S16, S19, S25, S26, S33 and S37 were suspected to be saturated aquifers. But at VES stations S7, S14–S16 and S37, the resistivity values of the aquifers were observed to be less than 10 Ωm suggesting that this aquifer environment may be contaminated by saline water. The distribution of resistivity in delineated aquifers is given in the figure (Fig. 7d). This show the central regions of the study area fall along the Palayar River which was contaminated by saline intrusion.
The iso-resistivity spatial distribution maps (Fig. 8) were prepared using the same ArcGIS software for half the distance between the current electrodes (AB/2) spacings of 5, 10, 25 and 50 m. Figure 8a, b is nearly similar in resistivity distribution, suggesting that there is not much significant change in the shallow subsurface materials in the study area. The northern and eastern parts of the study area is characterized by low resistivity values compared to the other regions. But from Fig. 8c, d, resistivity values were observed to be lower in
the south and central parts of the Agastheeswaram Taluk suggesting that these regions are contaminated by saline water at deeper depth. From the iso-resistivity contour maps, we can depict that the overall resistivity distribution is suitable for potable aquifers in the west and southwestern parts of the study area, and the groundwater quality in these areas is good for all purposes.
Groundwater chemistry
Drinking water quality
If the groundwater is free from toxic constituents and contains low quantity of dissolved solids, then it is good for drinking purposes (Srinivas et al. 2013a). Drinking water standards set by the World Health Organization (WHO 1997) were used to assess the groundwater quality in the study area (Table 2). The pH of the groundwater is the measure of its acidity or alkalinity (Sherif et al. 2006). The pH values of the groundwater samples ranged from 6.5 to 7.7, which show that the groundwater quality is slightly acidic to slightly alkaline in nature. All solid materials in the water solution whether ionized or not were measured as the total dissolved solids (TDS) content of that solution (Sherif et al. 2006). According to WHO (1997) standards, the TDS of two water samples from wells W5 and W11 were higher than the permissible limit of 1500 mg/l for drinking water purpose. The electrical conductivity of water depends on the water temperature, types of ions present in the water and their concentration (Sherif et al. 2006). The electrical conductivity in the study area ranges from 207 to 5806 μS/cm, with a mean value of 1879 μS/cm. The total hardness of the groundwater samples ranges from 165 to 1158 mg/l with an average value of 564 mg/l. According to the classification based on WHO (1997) standards, 50 % of samples exceeds the maximum permissible limit of 500 mg/l. Hard water has no recognized adverse effect on humans for a short period consumption, but long-term usage of very hard water might lead to some health disorders and may also cause corrosion on water supply systems (Agrawal and Jagetia 1997).
The dominance of major cations in the area was observed to be in the order Na+ > Ca2+ > Mg2+ > K+. Na+ was found to be the most dominant cation in the study area. The concentration of Na+ in the study area ranges from 53 to 424 mg/l with an average of 221 mg/l. Fifty-seven percent of all the samples from the study area was in excess of the permissible limits of 200 mg/l as prescribed by WHO (1997) guidelines. The concentration of K+ is between 2 and 117 mg/l with an average of 28 mg/l. The concentration of K+ in majority of the samples exceeds the permissible limit of 12 mg/l in the study area (Table. 2). Increase in the concentration of Na+ and K+ may be due to the dilution of water in the monsoon season by infiltration and runoff (Edet and Worden 2009). The Ca2+ content in the groundwater lies between 60 and 196 mg/l with an average of 107 mg/l. A total of ten samples had Ca2+ values in excess of the desired limit of 75 mg/l. The Mg2+ concentration in the study area lies between 3 and 221 mg/l with a mean value of 72 mg/l. Fourteen percent of samples are exceeding the permissible limit 150 mg/l for magnesium. The rainwater, dissolution of several minerals like calcite (CaCO3) and dolomite (CaMg(CO3)2) in soils, bedrocks, and weathering of calcium- and magnesium-enriched rocks in the aquifer environment are some of the sources which changes the concentration of Ca2+ and Mg2+ in the groundwater (Akpan et al. 2013).
The dominance of anion in the groundwater was observed to be in the order Cl−>HCO3 − >SO4 2−. The concentration of Cl−, HCO3 − and SO4 2− ranges from 14 to 799 mg/l, 98 to 378 mg/l and 8 to 146 mg/l, respectively. The increased concentration of Cl− and HCO3 − indicates the diverse sources of salinization problems in the study area. Some of these sources include precipitation, natural saline groundwater, halite dissolution, agricultural and domestic sources and natural pollution from iron-enriched clayey sediments (Hounslow 1995; Inoubli et al. 2006; Edet and Worden 2009; Bahar and Reza 2010; Akpan et al. 2013).
Irrigation water quality
The suitability of the groundwater for irrigation purposes was studied by using the parameters percent sodium (%Na), sodium absorption ratio (SAR) and by plotting Wilcox diagram (Wilcox 1955) and USSL plots. The mechanism controlling the groundwater quality was studied by using Gibbs plots. Standard classification of groundwater for irrigation usage on the basis of some techniques such as percent sodium (%Na), sodium absorption ratio (SAR), electrical conductivity (EC), total hardness (TH) and total dissolved solids (TDS) is given in Table 3.
According to these classifications, the samples from stations 9, 13 and 14 were found to be best suitable for irrigation. The Wilcox diagram (Fig. 9) shows two samples from stations W5 and W11 which were found to be unsuitable for irrigation usage. The USSL plot (Fig. 10) shows that
majority of the samples fall in the C3S2 region of high salinity and medium alkalinity. Two samples from wells W5 and W11 have very high salinity hazard and were found to be unsuitable for irrigation. Seventy-nine percent of the samples falls in the medium to high salinity region, which shows that the study area is good for salt tolerant crops (Srinivasamoorthy et al. 2011b) like coconut.
The Gibbs plots (Gibbs 1970) were drawn between (i) Na+/(Na+ + Ca2+) versus TDS and (ii) Cl−/(Cl− + HCO3 −) versus TDS (Fig. 11a, b). The Gibbs plots show that evaporation is the major process controlling cationic concentration in the study area, whereas rock weathering and evaporation are the major processes controlling cationic dominance. No sample was observed in the rainfall dominance regions of the Gibbs plots, which confirms that rainfall has no major contribution for the enrichment of ions in the groundwater.
Integration of VES and geochemical results
Information generated from VES and geochemical analyses have been used in identifying the aquifer horizons and assessing the quality of groundwater that circulates in the aquifers. Since resistivity is inversely proportional to EC, the aquifer resistivity must be low for the water with high EC to be present in an aquifer (Srinivas et al. 2013b). The relationship between the water resistivity (ρ w) and electrical conductivity is given as (Vouillamoz et al. 2007),
The aquifer resistivity, EC and water resistivity for the collected groundwater samples are given in Table 4. From Table 4, it is observed that the water samples with high EC values have low aquifer resistivity values. Some minor deviations exist because of the distance between the water wells and the VES stations. The statistical correlation between apparent resistivity and water resistivity values was plotted (Fig. 12). The overall correlation of aquifer resistivity with water resistivity and other geochemical parameters was done using Pearson correlation matrix, and it is depicted in Table. 5.
The overall correlation between the resistivity of the aquifers and EC was found to be satisfactory in the study area thus attesting to the reliability of both methods in hydrogeological investigations. Water wells W9, W13 and W14 located at Ramanathichanputhoor, Ethamoli and Gandhipuram stations, respectively, were found to have low EC values, and the nearby aquifers show considerably high aquifer resistivity values, which show the presence of potable groundwater in the aquifers. But at VES stations S12 and S23 that were stationed close to water wells W5 and W11 in Koilvilai and Nalloor areas, respectively, low-quality groundwater was suspected. The groundwater is characterized by high EC values while the aquifers have low resistivity values. The low aquifer resistivity along with high EC and Cl− content are connected with saline water intrusion (Choudury and Saha 2004). The areas surrounding VES stations S6, S12, S15, S16 and S23 are demarcated as stations that are challenged by problems of saline water intrusion.
Conclusions
The integrated surface geophysical and geochemical techniques provide a promising tool for assessing groundwater quality in the Agastheeswaram Taluk in Kanyakumari District. The information obtained from the combined methods was used to demarcate various contaminated water zones. The geochemical analysis shows that evaporation was the dominant process controlling ionic concentration in the study area. A good correlation between the geophysical apparent resistivity values of the aquifer and the EC values proves the importance of both methods as a useful guide for groundwater quality assessment. Also, the geochemical investigations carried out in this research prove the consistency of the geoelectrical methods in solving the hydrogeological problems like saline water intrusion. The aquifers at VES stations S6, S12, S15, S16 and S23 were found to have low resistivity, and the water from nearby wells shows high EC showing that the area surrounding these stations are polluted by saline water intrusion. From both the geophysical and geochemical studies, we can conclude that 45 % of the stations are having groundwater with appreciably good quality. But salt water intrusion may become a problem in the future. So it is important to keep up monitoring the fresh saline water interface by avoiding overexploitation of the groundwater resources.
References
Agrawal V, Jagetia M (1997) Hydrogeochemical assessment of ground water quality in Udaipur city, Rajasthan, India. In: Proceedings of national conference on “Dimensions of Environmental Stress in India”, Department of Geology, M S University, Baroda, India, pp: 151–154
Akpan AE, Ugbaja AN, George NJ (2013) Integrated geophysical, geochemical and hydrogeological investigation of shallow groundwater resources in parts of the Ikom-Mamfe Embayment and the adjoining areas in Cross River State, Nigeria. J Environ Earth Sci. doi:10.1007/s12665-013-2232-3
APHA (American Public Health Association) (1995) Standard methods for the examination of water and wastewater Washington, DE 19th ed, p. 1467
Asfahani J (2011) Electrical resistivity investigations for guiding and controlling fresh water well drilling in semi-arid region in Khanasser Valley, Northern Syria. J Acta Geophys 59(1):139–154
Bahar M, Reza S (2010) Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ Earth Sci 61:1065–1073. doi:10.1007/s12665-009-0427-4
Bhimasankaram VLS, Gaur VK (1977) Lectures on exploration geophysics for geologists and engineers. Assoc Explor Geo-physicists Centre Explor Geophys, Hyderabad
Bobachev C (2002) IPI2Win: Windows software for automatic interpretation of resistivity sounding data, Ph.D. thesis, Moscow State University
Brown E, Skougslad MW, Fishman MJ (1974) Methods for collection and analysis of water samples for dissolved minerals and gases. US Geological survey, Techniques for water resources investigations, Book 5, Chapter A1
Choudury K, Saha DK (2004) Integrated geophysical and chemical study of saline water intrusion. GroundWater 42(5):671–677
Cimino A, Cosentino C, Oieni A, Tranchina L (2008) A geophysical and geochemical approach for seawater intrusion assessment in the Acquedolci coastal aquifer (Northern Sicily). J Environ Geol 55:1473–1482
Edet AE, Worden RH (2009) Monitoring of physical parameters and evaluation of the chemical composition of river and groundwater in Calabar (Southeastern Nigeria). J Environ Monit Assess 157:243–258. doi:10.1007/s10661-008-0532-y
Elwaseif M, IsmailA AM, Abdel-Rahman M, Hafez MA (2012) Geophysical and hydrological investigations at the west bank of Nile River (Luxor, Egypt). J Environ Earth Sci 67:911–921
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Gnanasundar D, Elango L (1999) Groundwater quality assessment of a coastal aquifer using geoelectrical techniques. J Environ Hydrol 7:2
Gurunandha Rao VVS, Tamma Rao G, Surinaidu L, Rajesh R, Mahesh J (2011) Geophysical and Geochemical Approach for Seawater Intrusion Assessment in the Godavari Delta Basin, A.P., India. J Water Air Soil Pollut 217:503–514
Hounslow AW (1995) Water quality data: analysis and interpretation. Oklahoma State University, Lewis, pp. 24–90
Inoubli N, Gouasmia M, Gasmi M, Mhamdi A, Dhia HB (2006) Integration of geological, hydrochemical and geophysical methods for prospecting thermal water resources: the case of the Hmeı¨ma region (Central–Western Tunisia). J Afr Earth Sci 46:180–186. doi:10.1016/j.jafrearsci.2006.04.009
Krishna Kumar S, Rammohan V, Dajkumar Sahayam J, Jeevanandam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. J Environ Monit Assess 159:341–351
Lusczynski NJ and Swarzenski WV (1966) Salt-water encroachment in Southern Nassau and Southeastern Queens Counties, Long Island, New York, U,S. Geological Survey Water-Supply Paper 1613-F: 76
Mandel S, Shiftan ZL (1981) Groundwater resource. Academic, New York, p. 269
Perumal BS, Thamarai P (2008) Groundwater level before and after Tsunami in coastal area of Kanyakumari, South Tamilnadu, India. Int J Appl Environ Sci 3(2):139–147
Pervaiz S, Bakhsh A, Arshad M, Rana T (2010) The use of vertical electrical sounding resistivity method for the location of low salinity groundwater for irrigation in Chaj and Rachna Doabs. J Environ Earth Sci 60:1113–1129
PWD (2005) Groundwater perspectives: a profile of Kanyakumari district, Tamil Nadu. Tamil Nadu Public Works Department, India
Richards LA (1954) Diagnosis and improvement of saline and alkalisoils. US department of agriculture handbook 60, Washington
Lee S-H, Kim K-W, Ko I, Iee S-G, Hwang H-S (2002) Geochemical and geophysical monitoring of saline water intrusion in Korean paddy fields J. Environ Geochem Health 24:277–291
Sawyer CN, Mccarty PL, Parkin GF (2003) Chemistry for environmental engineering and science(5th ed.). McGraw-Hill, New York, p. 752
Sherif M, El Mahmoudi A, Garamoon H, Kacimov A, Akram S, Ebraheem A, Shetty A (2006) Geoeletrical and hydrogeochemical studies for delineating seawater intrusion in the outlet of Wadi Ham, UAE. J Environ Geol 49:536–551
Sree Devi PD, Srinivasulu S, Raju KK (2001) Delineation of groundwater potential zones and electrical studies for groundwater exploration. J Environ Geol 40:1252–1264
Srinivas Y, Hudson Oliver D, Stanley Raj A, Chandrasekar N (2013a) Evaluation of groundwater quality in and around Nagercoil town, Tamilnadu, India: an integrated geochemical and GIS approach. J Appl Water Sci 3:631–651
Srinivas Y, Muthuraj D, Hudson Oliver D, Stanley Raj A, Chandrasekar N (2013b) Environmental applications of geophysical and geochemical methods to map groundwater quality at Tuticorin, Tamilnadu, India. J Environ Earth Sci 70(5):2143–2152
Srinivasamoorthy K, Vijayaraghavan K, Vasanthavigar M, Rajivgandhi R, Sarma VS (2011a) Integrated techniques to identify groundwater vulnerability to pollution in a highly industrialized terrain, Tamilnadu, India. J Environ Monit Assess 182:47–60
Srinivasamoorthy K, Nandha Kumar C, Vijayaraghavan K, Vasanthavigar M, Rajiv Gandhi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2011b) Groundwater quality assessment from a hard rock terrain, Salem district of Tamilnadu, India. Arab J Geol Sci 4:91–102
Tirumalesh K, Shivanna K, Sriraman AK, Tyagi AK (2010) Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. J Environ Monit Assess 163:171–184
Todd DK (1980) Ground water hydrology. JohnWiley & Sons, New York, p. 419
Umar H, Samsudin AR, Malim EP (2007) Groundwater investigation in Kuala Selangor using vertical electrical sounding (VES) surveys. J Environ Geol 51:1349–1359
USGS (2000) Classification of natural ponds and lakes. U.S Department of the Interior, U.S. Geological Survey, Washington DC
Vouillamoz JM, Chatenoux B, Mathieu F, Baltassat JM, Legchenko A (2007) Efficiency of joint use of MRS and VES to characterize coastal aquifer in Myanmar. J Appl Geophys 61:142–154
Ward SH (1990) Resistivity and induced polarization methods. Geotechnical and Environmental Geophysics, vol 1. Society of Exploration Geophysics
WHO (1997) Guideline for drinking water quality. Recommendations. vol 1, 2nd Edn. Geneva, WHO
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington, DC, USA
Zohdy A, Eaton GP, Mabey DR (1974) Application of surface geophysics to ground-water investigations: techniques of water-resources investigations of the United States Geological Survey, chap D1, book 2, pp.116
Zulfiqar A, Akhter G, Ashraf A, Fryar A (2010) Implications and concerns of deep-seated disposal of hydrocarbon exploration produced water using three-dimensional contaminant transport model in Bhit Area, Dadu District of Southern Pakistan. J Environ Monit Assess 170:395–406
Acknowledgments
The corresponding author acknowledges the Central Groundwater Board (CGWB), Chennai, for providing the lithological information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivas, Y., Hudson Oliver, D., Stanley Raj, A. et al. Geophysical and geochemical approach to identify the groundwater quality in Agastheeswaram Taluk of Kanyakumari District, Tamil Nadu, India. Arab J Geosci 8, 10647–10663 (2015). https://doi.org/10.1007/s12517-015-1989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-015-1989-y