Abstract
Purpose of Review
We aim to discuss the diagnostic use of ultrasmall superparamagnetic iron oxide (USPIO) including ferumoxytol in targeted cardiovascular magnetic resonance imaging (MRI).
Recent Findings
Ferumoxytol is the only USPIO clinically available in the USA and is a negatively charged USPIO that has potential use for tracking and characterization of macrophage-infiltrated cardiovascular structures. As an iron supplement that is approved for treatment of iron deficiency anemia, the iron core of ferumoxytol is incorporated into the body once it is phagocytosed by macrophages. In organs or tissues with high-inflammatory cellular infiltration, such as atherosclerotic plaques and myocardial infarction, localization of iron-laden macrophages can be visualized on delayed MRI. The iron core of ferumoxytol alters the magnetic susceptibility and results in shortening of T2* and T2 relaxation rates. Areas with high concentration appear hypointense (negative contrast) on T2 and T2* MRI. Recently, in vitro findings support the potential specificity of ferumoxytol interactions with macrophage subtypes, which has implications for therapeutic interventions. With increasing concerns about gadolinium retention in the brain and other tissues, the value of ferumoxytol-enhanced MR for targeted clinical imaging is aided by its positive safety profile in patients with impaired renal function.
Summary
This paper discusses pharmacokinetic properties of USPIOs with a focus on ferumoxytol, and summarizes relevant in vitro, animal, and human studies investigating the diagnostic use of USPIOs in targeted contrast-enhanced imaging. We also discuss future directions for USPIOs as targeted imaging agents and associated challenges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of contrast agents in magnetic resonance imaging (MRI) improves the sensitivity and specificity of diagnoses by altering the magnetization properties of the tissues of interest. The measured relaxivity (r1, r2) values for each contrast agent reflect the extent to which it can alter the longitudinal (T1) or transverse (T2) relaxation time of water protons. The most widely used contrast agents in cardiovascular MRI are gadolinium-based contrast agents (GBCAs) [1]. Clinically available GBCAs contain gadolinium chelates, which are paramagnetic and generate positive T1 contrast [2]. The gadolinium ion, toxic as a stand-alone particle, is bound to a large carrier molecule that prevents it from entering cells and limits the distribution of the GBCA to the extracellular space. Despite their efficacy in contrast-enhanced MRI, GBCAs have adverse effects. In patients with advanced renal disease, gadolinium has been associated with nephrogenic systemic fibrosis, a debilitating and progressive condition without effective treatments. Even in patients with normal renal function, gadolinium retention has been detected in the central nervous system, bone, skin, and other tissues on both MRI and autopsy studies; the clinical implications of these findings are unclear [2, 3]. The presence of Gd may persist for months to years. Many regulatory agencies to date have restricted the use of GBCAs and have asked healthcare professionals to evaluate the risk/benefit ratio prior to administration of GBCAs.
Because of these limitations, there has been greater interest in alternative MR contrast agents such as iron-containing ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, MA), an ultrasmall superparamagnetic iron oxide (USPIO). While gadolinium is a rare earth metal that does not play a role in normal metabolism, iron is an essential element to human physiology. Although approved by the U. S. Food and Drug Administration for treatment of iron deficiency anemia in patients with chronic kidney disease [4•], ferumoxytol was originally developed as an MRI contrast agent. Due to ferumoxytol’s clinical availability and safety profile in patients with renal impairment, there has been a resurgence in its off-label use for MRI [5].
Within the context of cardioimmunology and inflammatory conditions, the physiologic and pharmacokinetic properties of USPIOs can be utilized for diagnostic purposes beyond those possible with GBCAs. Complex interactions exist between the immune and the cardiovascular systems in both healthy and disease states and the dynamic consequences of immune response vary from minimal myocyte loss to fibrosis, arrhythmias, and heart failure syndromes [6]. Macrophages play a key role in cardiac remodeling such that dysregulation of pro-inflammatory M1 and anti-inflammatory M2 phenotypes can result in excess inflammation and myocardial injury. Past underappreciation of these interactions has been due to challenges with detecting immune cells in the cardiovascular system. With improved technology, there is a rising focus to better characterize the role of immune cells in cardiovascular function and to develop therapeutics for the modulation of immune responses [7••, 8•, 9].
Several excellent papers have been published on the application of ferumoxytol and other USPIOs for magnetic resonance angiography and venography of large peripheral vascular structures such as atrioventricular fistulas, chronic venous occlusions, and lower extremity arteries [4•, 10•]. However, the unique properties of USPIOs, including T2-weighted tissue hypoenhancement and localization to areas of macrophage-mediated inflammation, allow for its potential use in targeted imaging of inflammatory disease states. This article provides a summary of current research on the diagnostic use of USPIOs with a focus on ferumoxytol for targeted molecular imaging.
Properties of USPIOs
Magnetic iron oxide (Fe3O4 and γ-Fe2O3) nanoparticles and more specifically small iron oxide nanoparticles (SPIOs) are composed of an outer carbohydrate shell and an iron core. SPIOs are classified using their hydrodynamic diameter: large SPIOs (300 nm to 3.5 μm), standard size SPIOs (50 to 150 nm), ultrasmall SPIO (USPIO, <50 nm), and exceedingly small SPIOs (ES-SPIOs, <5.5 nm). USPIO size has direct influence on the T1 relaxation rate, i.e., smaller particle and core size (<5 nm) tend to have greater T1 enhancement effect and suppression of T2. For the most part, SPIOs are considered T2 contrast agents whereas GBCAs are typically considered T1 agents. The surface chemistry of SPIOs can be tailored for specific biomedical applications including MRI, thermal therapy, and drug delivery. Together, surface chemistry, shape, size, and charge of the magnetic particles affect the overall biodistribution of iron oxide nanoparticles.
Table 1 summarizes the chemical and imaging properties of ferumoxytol and earlier iron-based contrast agents. Ferumoxytol is a negatively charged USPIO with an iron core and a non-reactive polyglucose sorbitol-carboxymethlether shell [11]. The compound is commercially available in an isotonic, neutral pH solution that maintains low levels of free iron in the plasma. Because of its large molecular size of 750 kDa and hydrodynamic diameter of 20–30 nm, ferumoxytol does not extravasate into the extravascular space within the first several hours of administration and is not filtered by the kidneys. Instead, ferumoxytol is eliminated by macrophage-mediated phagocytosis [4•, 12, 13]. Ferumoxytol’s dextran-derived shell slows phagocytosis and reduces its immunogenicity, which delays uptake by the surface scavenger receptors and degradation by macrophages of the reticuloendothelial system (RES). These properties result in a long intravascular half-life of 14–15 h, which guarantees a constant blood concentration at steady state, providing an excellent temporal window for repeated image acquisition and multiphase imaging based on the temporal distribution volume of ferumoxytol [4•]. Figure 1A summarizes the biodistribution of ferumoxytol and how its unique metabolism can be leveraged for multiphase MRI.
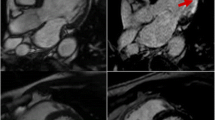
(A) Ferumoxytol biodistribution and multiphase diagnostic MRI applications are shown. Within the first 1–15 h, ferumoxytol remains within the intravascular space. Once phagocytosed by macrophages, the iron oxide core leaves the intravascular space and is metabolized body’s reticuloendothelial system and the iron is incorporated into the body. (B) Magnetic resonance images (B, top panel) of carotid atheroma and corresponding R2* (B, middle panel) and T2* (B, bottom panel) maps at baseline and at 24, 48, 72, and 96 h after ferumoxytol infusion are shown. Maximum R2* signal increase corresponding to macrophage localization in the atherosclerotic plaque is seen at 48 h. (C) Perl’s stain (C, upper panel, black arrows) and CD68 immunohistochemical (IHC) stain of macrophages (C, lower panel, black arrows) show co-localization of macrophages in areas of ferumoxytol uptake. Vessels are seen in areas with abundant macrophages (blue star). Parts B and C are adapted from Usman A, Patterson AJ, Yuan J, Cluroe A, Patterson I, Graves MJ, Gillard JH, Sadat U. Ferumoxytol-enhanced 3D magnetic resonance imaging of carotid atheroma – a feasibility and temporal dependence study. Sci Rep 10, 1808 (2020) under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Most GBCAs have r1 values ranging from 3.6 to 5.2 mM−1 s−1 and r2 values ranging from 4.3 to 6.1 mM−1 s−1 in plasma at 37 °C and 1.5T [13]. At 1.5T, ferumoxytol has an r1 of 19.0 ± 1.7 mM−1 s−1 and r2 of 64.9 ± 2.3 mM−1 s−1 in plasma at 37 °C [14]. At 3.0T, ferumoxytol has an r1 of 9.5 ± 0.2 mM−1 s−1 and r2 of 65.2 ± 1.8 mM1 s−1 in plasma at 37 °C. Ferumoxytol displays linear longitudinal and transverse relaxation in saline and plasma, but exhibits nonlinear behavior in blood [14]. At blood ferumoxytol concentrations >2.1 mM, r1 departs from its linear behavior. The high r1 facilitates bright blood imaging, but at high doses can lead to susceptibility artifacts. These attributes lead to T1 signal hyperenhancement and T2 signal hypoenhancement (signal loss) and may facilitate the characterization, localization, and staging of cardiac inflammation and infarct pathology [12, 15]. Once the dextran shell is broken down, the residual iron core is stored as ferritin or used for erythropoiesis, and the carbohydrate coating is eliminated through the kidneys or gastrointestinal tract [12].
Ferumoxytol is commercially distributed in single-use vials with 510 mg of iron. For imaging purposes, the vials are diluted with saline to a total volume of 24–60 mL for adults, with an average concentration range of 1–7.5 mg/kg. Ferumoxytol is administered as a slow infusion of 0.1–0.2 mg/kg for lymph node or steady-state imaging; in some angiographic applications, multiple, small doses are incrementally infused for a cumulative dose of 4 mg/kg [16]. For the treatment of iron deficiency anemia, the FDA has approved ferumoxytol to be given as an IV infusion over a minimum of 15 min. Post-marketing surveillance data related to its therapeutic use reported 79 anaphylactic reactions with 18 fatalities [16]. This led the FDA to issue a black-box warning and to recommend dilution, slow infusion over 15 min, and monitoring of vital signs for up to 30 min post-infusion [17]. For diagnostic MRI purposes, the ferumoxytol dose is frequently less than half the therapeutic dose and published data to date suggest a positive safety profile [18,19,20,21, 22••]. A recent multicenter registry study showed in 4240 doses of ferumoxytol given for imaging indications across a spectrum of age and disease complexity, there were no serious, life-threatening, or fatal adverse events. Of the 83 (1.9%) adverse events that were related or possibly related to diagnostic ferumoxytol infusions, seventy-five reactions were mild and eight were moderate in severity [22••].
Targeted Imaging Applications
Table 2 summarizes recent studies that have explored the use of USPIOs and ferumoxytol as targeted contrast agents. Targeted imaging with ferumoxytol is promising for inflammation because it targets macrophages directly. Other clinically available imaging techniques such as PET- or GBCA-enhanced MRI use indirect proxies of inflammation such as metabolism or enhancement of the vasa vasorum. With ferumoxytol-enhanced MRI, ultrashort echo time can be applied to improve the linearity of the MR signal intensity; high-resolution imaging can be employed in combination to improve spatial resolution with increased sensitivity for inflammation quantification.
Myocardial Infarction
While T2-weighted MRI techniques have been used to evaluate myocardial edema after myocardial infarction (MI), they do not distinguish free water from active inflammation. In contrast, USPIOs can localize areas of inflammatory cell infiltration, such as after a myocardial infarction. After acute infarction, monocytes are recruited to the infarct border zone, differentiate into macrophages, and migrate into the infarct core [18]. The MR identification of USPIO-tagged macrophages has been shown in murine studies of MI. Corresponding histopathological correlations show co-localization of both USPIO and macrophages in infarcted myocardial tissue [19].
One proof-of-concept study imaged ST elevation myocardial infarction (STEMI) patients (n = 10, age 38–65 years) within 5 days of admission at baseline, and then 24 h and 48 h after the administration of ferumoxytol. The findings were compared to the MR images of STEMI patients who did not receive ferumoxytol (n = 6, age 48–65 years) [26•]. On T2*-weighted imaging, the rate of signal decay (R2*) was assessed via USPIO uptake. Regions with higher R2* signal corresponded to areas of greater USPIO localization. At both 24 and 48 h, there was increased R2* signal in the infarcted area of myocardium, which suggested localization of macrophages and other inflammatory cells in the region of interest. To a lesser degree, increased R2* signal in non-infarcted regions was also appreciated; this latter finding suggests a potential global inflammatory process in the natural history of acute MI [20]. Similar findings were reported in another small study of post-STEMI patients (n = 14, age 45–54 years), where the administration of ferumoxytol resulted in hypoenhancement of the infarct areas on T2-weighted short-tau inversion recovery black-blood segmented turbo spin echo sequence (STIR-SE) images and signal void in the T2* images. Ex vivo experiments from this latter study showed ferumoxytol exposure led to iron deposition in macrophages but not their precursor monocytes. These findings highlight the potential use of ferumoxytol for characterizing areas of active inflammatory infiltration [29]. Although small, these studies illustrate the usefulness of ferumoxytol MRI to differentiate between active myocardial inflammation and chronic processes such as fibrosis.
A larger study imaged thirty-one patients following acute MI using T2-weighted MRI with ferumoxytol administration after the index event, and longitudinal imaging with sequential MRI scans over a 3-month period [33•]. During this time, patients received up to seven MRI scans and up to three infusions of ferumoxytol. In addition, they underwent late gadolinium enhancement (LGE) imaging at baseline and at the 3-month time point to define the distribution of infarcted myocardium. The areas of LGE served as reference for the T2 and T2* images. Relative to remote myocardium, areas of infarct showed increased R2* values marking USPIO uptake at 2–3 days after ferumoxytol administration. The R2* effect persisted until days 10–16. In contrast, there was no change in R2* values in the peri-infarct and remote myocardium over the same period of time. T2-weighted imaging of unenhanced scans showed increased signal in the infarct and peri-infarct areas at baseline and throughout the 3-month observation period. These findings support the potential use of ferumoxytol-enhanced imaging to differentiate between myocardial inflammation and myocardial edema.
Myocarditis
Cardiac MRI offers a non-invasive means for the diagnosis of myocarditis based on the Lake Louise criteria [40]. However, T2-weighted imaging cannot distinguish tissue edema from inflammation, while GBCAs are non-specific in their diffusion into areas of myocyte necrosis, fibrosis, and edema. In a murine model of autoimmune myocarditis, USPIO-enhanced MRI showed better contrast-to-noise ratios and better specificity than gadolinium-enhanced images in identifying lesions [41]. A subsequent study in mice showed T2* mapping performed 6 h after ferumoxytol infusion improved the detection of histologically significant areas of inflammation at 14 days after induction of myocarditis [38]. However, the evidence for efficacy in humans is less robust. In a cohort of patients with acute myocarditis (n = 9, age 24–34 years, 9 male), MRI was performed with T2, T2*, and LGE sequences to make an imaging diagnosis [34•]. Ferumoxytol was then administered at a dose of 4 mg/kg over 15 min, and the patients underwent repeat T2* imaging 24 h later. The same protocol was repeated in a small cohort of healthy controls (n = 10, age 45–53 years, 6 female). The administration of ferumoxytol however did not result in significant differences in the myocardial R2* values of the patients with myocarditis when compared to healthy controls. In the patients with myocarditis, the regions with high signal on T2 mapping corresponded to the regions with late gadolinium enhancement, but no such effect was seen after USPIO administration. One possible explanation is that the myocardium of these patients with myocarditis may have had a predominance of neutrophils or lymphocytes rather than macrophages, with correspondingly less phagocytosis of iron nanoparticles.
Atherosclerosis and Aneurysms
Atherosclerosis is a systemic inflammatory process that underlies many cardiovascular and cerebrovascular diseases, and involves plasma lipoprotein deposition, vascular smooth muscle cell proliferation, and inflammatory cell recruitment. Macrophages play a key role in the pathogenesis of atherosclerosis. Macrophage infiltration, activity, and apoptosis can differentiate stable lesions that cause progressive symptoms from vulnerable lesions that are prone to rupture, erosion, and thrombosis [42]. In this context, USPIOs offer a unique way to identify macrophage aggregation and localize high-risk plaques.
One of the first published studies demonstrating the role of USPIOs in atherosclerotic plaque imaging examined the effects of ferumoxtran in symptomatic patients (n = 11) referred for carotid endarterectomy [43••]. Baseline carotid MRI pulse sequences with T1-weighted gradient recalled echo, T2*-weighted gradient echo, and proton-density-weighted fast spin echo were obtained immediately prior to and at 24 and 72 h after ferumoxtran administration using a 1.5T magnet. The investigators characterized carotid plaques as stable (i.e., with an intact and thick fibrous cap), rupture-prone (i.e., with a very thin fibrous cap), or ruptured (i.e., with a disrupted fibrous cap and thrombus). The relative signal intensity in the MR regions of interest on pre- and post-contrast images was compared. Decreases in signal on T2*-weighted images at 24 h but not at 72 h were observed. Histology and electron microscopy of the excised carotid specimen confirmed correlation between visualized areas of T2* signal loss with areas of high USPIO uptake and macrophage concentration. Iron staining showed USPIO uptake in 75% of the rupture-prone and ruptured lesions, but only in 7% of the stable lesions. The latter findings support potential use of USPIO-MRI for the identification of high-risk, vulnerable plaques.
The above findings have also been supported by subsequent studies showing differences in USPIO uptake and T2*-weighted signal loss in the carotid plaques of symptomatic and asymptomatic patients with carotid artery stenosis [44], as well as the stability of USPIO burden over time in carotid artery plaques of asymptomatic patients [28]. Most recently, investigators demonstrated the use of ferumoxytol-enhanced 3D MRI at 1.5T for the characterization of carotid plaques and their temporal dependence in asymptomatic (n = 10) and symptomatic (n = 10) patients with carotid stenosis. A maximal decrease in ΔT2* (10.4 [3.5–16.2] ms, p < 0.001) and ΔT2 (13.4 [6.2–18.9] ms; p = 0.001) signal occurred at 48 h after ferumoxytol infusion (Fig. 1B) [45]. Histopathology stains are provided in Fig. 1C.
The Atorvastatin THerapy: Effects on Reduction Of Macrophage Activity (ATHEROMA) study deployed USPIO-enhanced MRI to examine the effects of statin therapy on local macrophage activity and inflammation [46••]. The investigators randomized 47 patients with atherosclerotic carotid artery disease who had baseline signal loss in carotid plaques on T2*-weighted ferumoxtran-enhanced MRI to high or low doses of atorvastatin, and then re-imaged them at 6 and 12 weeks. To identify the same area of interest, time-of-flight MR angiography was performed to identify the carotid bifurcation and the area of greatest stenosis. The investigators then manually co-registered scans before and after USPIO imaging based on plaque morphology and the distance from the carotid bifurcation. There was a significant change in the carotid plaque signal intensity (SI) in the high-intensity statin group (ΔSI 0.131 [0.065–0.198], p ≤ 0.001 at 6 weeks; ΔSI 0.203 [0.129–0.276], p < 0.001 at 12 weeks), but not in the low-intensity statin group (ΔSI 0.048 [− 0.018–0.114], p = 0.150 at 6 weeks; ΔSI − 0.038 [− 0.111–0.036], p = 0.304 at 12 weeks). The investigators showed that patients receiving high-intensity atorvastatin also had decreased rates of brain microembolization by transcranial doppler. While the findings are intriguing, one limitation of this methodology is the semi-quantitative nature of assessing signal intensity; the latter is susceptible to differences in patient positioning, coil inhomogeneity, and noise [47]. Similar results have been shown with ferumoxytol-enhanced MRI of patients with carotid artery stenosis (n = 9, age 57–77 years, all male) [32]. In this protocol, 4 mg/kg of ferumoxytol was administered and then after 72 hours, MR images of carotid vessel walls were acquired using T2*-weighted gradient echo sequences at 3.0T. Increased R2* values were present in diseased carotid arteries (24.6 ± 19.8 s−1) relative to the patient’s non-diseased vessels (7.5 ±9.3 s−1) as well as the vessels of healthy patients (2.6 ± 5.6 s−1) (p = 0.004 and 0.003, respectively).
Ferumoxytol has also been investigated in the imaging of abdominal aortic aneurysms (AAAs). The MA3RS study recruited 342 patients with ultrasound-diagnosed AAAs and obtained T2* mapping images of the abdomen before and 24–36 h after the administration of ferumoxytol using a 3.0 T MRI scanner [48••]. These patients were then followed with serial ultrasound and clinic assessments for 2 years or more. USPIO enhancement was defined as percent change in T2* on color maps. A predefined threshold of ≥71% change in T2* was used to classified color maps. Based on ≥10 contiguous voxels within the AAA wall, color maps were categorized as patients with and without USPIO enhancement of the AAA. The primary endpoint of AAA rupture or repair occurred more frequently in patients with USPIO enhancement of the AAA wall relative to patients without (35.6% vs 47.3%, respectively, p = 0.0308), although USPIO enhancement was not an independent predictor of the primary endpoint. Baseline aneurysm size and smoking status were the main predictors of rupture or repair.
Taken together, these reports demonstrate the feasibility of using USPIOs such as ferumoxytol in identifying macrophage infiltration as a marker of inflammation and vulnerable atherosclerotic plaque on T2 or T2* imaging. The clinical utility of this approach, however, has not expanded beyond experimental studies and the use of ferumoxytol-enhanced imaging of atherosclerosis remains in the research realm.
Future Directions
The conjugation of antibodies to USPIOs can allow for even more targeted imaging. This has been demonstrated in the conjugation of USPIOs to bevacizumab, a vascular endothelial growth factor (VEGF) antagonist, in localizing cancer cells [49]. Another example involves scavenger receptors, which have been proposed to be key mediators of atherosclerosis by promoting lipoprotein uptake that results in foam cell formation and inflammation. In a mouse model of atherosclerosis, USPIOs that are conjugated with scavenger receptor ligand have greater accumulation in the aortic plaques relative to unconjugated USPIOs; superior contrast-to-noise ratios on T2 MRI were observed [50]. USPIOs have also been conjugated with IL-6 antibodies to target the pro-inflammatory cytokine secreted by foamy macrophages, with similarly improved discrimination of aortic plaques of rabbits on MRI [51]. Other investigators have functionalized ferumoxytol through peptide coupling with VEGF-165 to mediate vasculogenesis and detect neoangiogenesis [52•]. The coupling of ferumoxytol to VEGF-165 is clinically viable and promising because both agents are clinically available. The functionalized ferumoxytol-VEGF165 coupling has modest payload, but retains its MR activity on T2*-weighted MRI. Further work is needed in pre-clinical animal models to demonstrate efficacy and safety.
Conjugated USPIOs have also been proposed as a means to assess response to therapeutics. Connective tissue growth factor (CTGF) is an extracellular matrix-associated protein that mediates cell adhesion, migration, and proliferation, and plays a role in atherosclerotic plaque development. Carotid artery ligation was performed to induce carotid plaques in a murine model of atherosclerosis [53]. One group of mice was given CTCF antibodies. All mice underwent anti-CTCF-conjugated USPIOs-enhanced MRI. Compared to untreated mice, the mice receiving CTCF antibodies had reduced signal change in their carotid plaques.
Challenges
The history of iron-based contrast agents is extensive [5]. To date however, ferumoxytol is the only USPIO clinically available in the USA for the treatment of iron deficiency anemia. Although interest in its off-label diagnostic use continues to increase, there are several challenges to the widespread clinical adoption of ferumoxytol-enhanced MRI, particularly for targeted imaging. From a commercial availability standpoint, several superparamagnetic iron oxide preparations have been in clinical development for MRI. Ferumoxides and ferucarbotran received regulatory approval in the USA for imaging but were withdrawn from the market because of adverse reactions, lack of superiority relative to GBCAs, or low utilization rate [54]. Second, the approved therapeutic dose of ferumoxytol is substantially higher than the diagnostic dose and poses a cost issue. Each vial of ferumoxytol is packaged as single-use. Once opened, any remaining amount is discarded. Third, due to the long-lasting effect of ferumoxytol, the diagnostic quality of subsequent MR images can be affected. According to the manufacturer’s labeling, the MRI effects of ferumoxytol can last up to 3 months, and prospective, observational studies have shown hepatic clearance can last from 3 to 11 months [55]. T1 enhancement patterns with USPIOs are different compared to gadolinium chelates and imagers will need to recognize these differences. Fourth, ferumoxytol carries a black-box warning and requires monitoring of vital signs for up to 30 min post-infusion, which could potentially alter the general outpatient imaging workflow. Last, substantial research will be necessary to improve the specificity of ferumoxytol-enhanced targeted imaging.
Conclusion
In conclusion, USPIOs such as ferumoxytol have unique contrast properties that can be leveraged for targeted cardiovascular imaging. USPIOs localize to areas of active macrophage infiltration and appear hypointense on T2 imaging, which enables nuanced characterization of pathology in patients with MI, myocarditis, and atherosclerotic vascular disease. In addition, the ability to conjugate antibodies and other moieties to ferumoxytol opens up the possibility for even more precise molecular imaging. These studies have largely been proof-of-concept, and further work is needed to validate the clinical utility of ferumoxytol and other USPIOs for targeted, contrast-enhanced imaging.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–70.
Lohrke J, Frenzel T, Endrikat J, Alves FC, Grist TM, Law M, et al. 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther. 2016;33:1–28.
Guo BJ, Yang ZL, Zhang LJ. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci. 2018;11:335.
• Finn JP, Nguyen KL, Han F, Zhou Z, Salusky I, Ayad I, et al. Cardiovascular MRI with ferumoxytol. Clin Radiol. 2016;71:796–806 The potential value of ferumoxytol as an off-label contrast agent for cardiovascular MRI is summarized.
Daldrup-Link H. Ten things you might not know about iron oxide nanoparticles. Radiology. 2017;284:616–29.
Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–44.
•• Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31 This paper showed the efficacy of the monoclonal antibody canakinumab in reducing MACE in high-risk patients, making a strong case for the inflammatory basis of cardiovascular disease.
• Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–505 This paper reported results of low-dose colchicine as a potential anti-inflammatory therapy after myocardial infarction. The results demonstrate a need for reliable imaging methods that enable inflammation detection.
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–3.
• Lehrman ED, Plotnik AN, Hope T, Saloner D. Ferumoxytol-enhanced MRI in the peripheral vasculature. Clin Radiol. 2019;74:37–50 This paper discusses the role of ferumoxytol-enhanced MRI in peripheral vascular disease.
McCormack PL. Ferumoxytol. Drugs. 2012;72:2013–22.
Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017;92:47–66.
Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2015;41:884–98.
Knobloch G, Colgan T, Wiens CN, Wang X, Schubert T, Hernando D, et al. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Invest Radiol. 2018;53:257–63.
Stoumpos S, Hennessy M, Vesey AT, Radjenovic A, Kasthuri R, Kingsmore DB, et al. Ferumoxytol magnetic resonance angiography: a dose-finding study in patients with chronic kidney disease. Eur Radiol. European Radiology. 2019;29:3543–52.
Vasanawala SS, Nguyen KL, Hope MD, Bridges MD, Hope TA, Reeder SB, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med. 2016;75:2107–11.
Finn JP, Nguyen K-L, Hu P. Ferumoxytol vs. gadolinium agents for contrast-enhanced MRI: Thoughts on evolving indications, risks, and benefits. J Magn Reson Imaging. 2017;46:919–23.
Ning P, Zucker EJ, Wong P, Vasanawala SS. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magn Reson Imaging. 2016;34:152–8.
Muehe AM, Feng D, Von Eyben R, Luna-Fineman S, Link MP, Muthig T, et al. Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Invest Radiol. 2016;51:221–7.
Varallyay CG, Toth GB, Fu R, Netto JP, Firkins J, Ambady P, et al. What does the boxed warning tell us? Safe practice of using ferumoxytol as an MRI CONTRAST AGENT. Am J Neuroradiol. 2017;38:1297–302.
Nguyen KL, Yoshida T, Han F, Ayad I, Reemtsen BL, Salusky IB, et al. MRI with ferumoxytol: a single center experience of safety across the age spectrum. J Magn Reson Imaging. 2017;45:804–12.
•• Nguyen KL, Yoshida T, Kathuria-Prakash N, Zaki IH, Varallyay CG, Semple SI, et al. Multicenter safety and practice for off-label diagnostic use of ferumoxytol in MRI. Radiology. 2019;293:554–64 This paper summarizes over 15 years of safety data across multiple centers for the use of ferumoxytol in MR imaging, and found no serious adverse events and rare moderate serverity adverse events.
Metz S, Beer AJ, Settles M, Pelisek J, Botnar RM, Rummeny EJ, et al. Characterization of carotid artery plaques with USPIO-enhanced MRI: assessment of inflammation and vascularity as in vivo imaging biomarkers for plaque vulnerability. Int J Cardiovasc Imaging. 2011 Jul;27(6):901–12.
Sadat U, Taviani V, Patterson AJ, Young VE, Graves MJ, Teng Z, et al. Ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging of abdominal aortic aneurysms-a feasibility study. Eur J Vasc Endovasc Surg. 2011;41:167–74.
Richards JMJ, Semple SI, MacGillivray TJ, Gray C, Langrish JP, Williams M, et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of Iron oxide : a pilot study. Circ Cardiovasc Imaging. 2011;4:274–81.
• Alam SR, Shah ASV, Richards J, Lang NN, Barnes G, Joshi N, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–65 This paper provides early proof-of-concept work for the off-label diagnostic use of ferumoxtyol in inflammation imaging.
Sigovan M, Kaye E, Lancelot E, Corot C, Provost N, Majd Z, et al. Anti-inflammatory drug evaluation in ApoE-/-mice by ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Invest Radiol. 2012;47:546–52.
Sadat U, Howarth SPS, Usman A, Tang TY, Graves MJ, Gillard JH. Sequential imaging of asymptomatic carotid atheroma using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging: a feasibility study. J Stroke Cerebrovasc Dis. 2013;22:E271–6.
Yilmaz A, Dengler MA, Van Der Kuip H, Yildiz H, Rösch S, Klumpp S, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34:462–75.
Qi C, Du L, Wu W, Li D, Hao J, Gong L, et al. Detection of vulnerable atherosclerotic plaques in experimental atherosclerosis with the USPIO-enhanced MRI. Cell Biochem Biophys [Internet]. 2015;73:331–7.
Kaneko C, Nitta N, Tsuchiya K, Watanabe S, Nitta-Seko A, Ohta S, et al. MRI study of atherosclerotic plaque progression using ultrasmall superparamagnetic iron oxide in Watanabe heritable hyperlipidemic rabbits. Br J Radiol. 2015;88:20150167.
Smits LP, Tiessens F, Zheng KH, Stroes ES, Nederveen AJ, Coolen BF. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis. 2017;263:211–8.
• Stirrat CG, Alam SR, MacGillivray TJ, Gray CD, Dweck MR, Raftis J, et al. Ferumoxytol-enhanced magnetic resonance imaging assessing inflammation after myocardial infarction. Heart. 2017;103:1528–35 This paper provides additional evidence to support the potential for off-label use of ferumoxytol in diagnostic MRI of inflammation.
• Stirrat CG, Alam SR, MacGillivray TJ, Gray CD, Dweck MR, Dibb K, et al. Ferumoxytol-enhanced magnetic resonance imaging in acute myocarditis. Heart. 2018;104:300–5 This paper provides proof-of-concept evidence to support the potential off-label use of ferumoxytol-enhanced MRI for inflammation detection in myocarditis.
Alam SR, Stirrat C, Spath N, Zamvar V, Pessotto R, Dweck MR, et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J Cardiothorac Surg. Journal of Cardiothoracic Surgery. 2017;12:1–10.
Usman A, Patterson AJ, Sadat U, Tang TY, Graves MJ, Gillard JH. Assessment of carotid plaque inflammation in diabetic and nondiabetic patients—an exploratory ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging study. J Stroke Cerebrovasc Dis. 2017;26:858–62.
Hedgire S, Krebill C, Wojtkiewicz GR, Oliveira I, Ghoshhajra BB, Hoffmann U, et al. Ultrasmall superparamagnetic iron oxide nanoparticle uptake as noninvasive marker of aortic wall inflammation on MRI: Proof of concept study. Br J Radiol. 2018;91:20180461.
Tada Y, Tachibana A, Heidary S, Yang PC, McConnell MV, Dash R. Ferumoxytol-enhanced cardiovascular magnetic resonance detection of early stage acute myocarditis. J Cardiovasc Magn Reson. 2019;21:77.
Zheng KH, Schoormans J, Stiekema LCA, Calcagno C, Cicha I, Alexiou C, et al. Plaque permeability assessed with DCE-MRI associates with USPIO uptake in patients with peripheral artery disease. JACC Cardiovasc Imaging. 2019;12:2081–3.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–76.
Moon H, Park HE, Kang J, Lee H, Cheong C, Lim YT, et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation. 2012;125:2603–12.
Lafont A. Basic aspects of plaque vulnerability. Heart. 2003;89:1262–7.
•• Kooi ME, Cappendijk VC, Cleutjens KBJM, Kessels AGH, Kitslaar PJEHM, Borgers M, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–8 This is one of the first published studies demonstrating the potential use of USPIOs in clinical imaging, showing ferumoxtran localizing to high-risk carotid plaques in patients referred to carotid endarterectomy and showing histopathological correlation between areas of high USPIO uptake and macrophage activity.
Howarth SPS, Tang TY, Trivedi R, Weerakkody R, U-King-Im J-M, Gaunt ME, et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur J Radiol. 2009;70:555–60.
Usman A, Patterson AJ, Yuan J, Cluroe A, Patterson I, Graves MJ, et al. Ferumoxytol-enhanced three-dimensional magnetic resonance imaging of carotid atheroma- a feasibility and temporal dependence study. Sci Rep. 2020;10:2–14.
•• Tang TY, Howarth SPS, Miller SR, Graves MJ, Patterson AJ, U-King-Im J-M, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. J Am Coll Cardiol. 2009:2039–50 This randomized controlled trial showed the ability of T2*-weighted MRI with ferumoxtran to detect changes in carotid plaque burden after high-intensity statin therapy.
Fayad ZA, Razzouk L, Briley-Saebo KC, Mani V. Iron oxide magnetic resonance imaging for atherosclerosis therapeutic evaluation. Still “rusty?”. J Am Coll Cardiol. 2009;53:2051–2.
•• Forsythe R, Mc Bride O, Robson J, Vesey A, Chalmers R, Burns P, et al. Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation. 2017;136:787–97 This large prospective trial followed patients with abdominal aortic aneurysms and found that ferumoxytol enhancement on T2 imaging was associated with the primary outcome of aneurysm rupture or repair but was not an independent predictor; the highest predictors included smoking and baseline aneurysm diameter. This suggests that markers of inflammation such as USPIOs should be interpreted in the context of traditional risk factors.
Cheng D, Zhao Y, Yao Q, Tan H, Wu B, Wu P, et al. Design and initial evaluation of USPIO modified bevacizumab as a hybrid SPECT/MRI probe to hepatoma carcinoma cells Dengfeng Cheng1, Yanzhao Zhao1. Qi. J Nucl Med. 2013;54:1169.
Segers FME, Den Adel B, Bot I, Van Der Graaf LM, Van Der Veer EP, Gonzalez W, et al. Scavenger receptor-AI-targeted iron oxide nanoparticles for in vivo MRI detection of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2013;33:1812–9.
Mo H, Fu C, Wu Z, Liu P, Wen Z, Hong Q, et al. IL-6-targeted ultrasmall superparamagnetic iron oxide nanoparticles for optimized MRI detection of atherosclerotic vulnerable plaques in rabbits. RSC Adv. Royal Society of Chemistry. 2020;10:15346–53.
• Bietenbeck M, Engel S, Lamping S, Hansen U, Faber C, Ravoo BJ, et al. Functionalization of clinically approved MRI contrast agents for the delivery of VEGF. Bioconjug Chem [Internet]. American Chemical Society. 2019;30:1042–7 This paper describes the functionalization of ferumoxytol with VEGF and provides a strong example for targeted MRI using ferumoxytol.
Yao Y, Li B, Fu C, Teng G, Ma G, Liu N. Anti-connective tissue growth factor detects and reduces plaque inflammation in early-stage carotid atherosclerotic lesions. Nanomedicine Nanotechnology, Biol Med. 2017;13:2385–94.
Wang YJ. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J Gastroenterol. 2015;21:13400–2.
Wáng YXJ, Idée JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg. 2017;7:88–122.
Funding
Dr. Nguyen receives grant support from the American Heart Association (18TPA34170049), the National Heart, Lung, and Blood Institute (R01HL148182), and the Veterans Health Administration (VA-MERIT, I01-CX001901).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No original experiments were completed in the context of this review paper. Where appropriate, the authors have referenced prior published work and obtain permission for reprint and re-use of figures.
Conflict of Interest
The authors have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Imaging
Rights and permissions
About this article
Cite this article
Lu, Y., Huang, J., Neverova, N.V. et al. USPIOs as Targeted Contrast Agents in Cardiovascular Magnetic Resonance Imaging. Curr Cardiovasc Imaging Rep 14, 2 (2021). https://doi.org/10.1007/s12410-021-09552-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s12410-021-09552-8