Abstract
Purpose of Review
Intravascular imaging provides improved diagnostic accuracy and optimization of percutaneous coronary interventions (PCI) compared with angiography alone. We review the latest literature on the predominant intravascular imaging modalities, intravascular ultrasound (IVUS), and optical coherence tomography (OCT).
Recent Findings
A number of recently published clinical studies evaluating OCT and IVUS use have demonstrated improved procedural and clinical outcomes over angiography. Recent literature also reports on novel potential applications of these technologies.
Summary
Intravascular imaging is an important diagnostic tool that augments angiography. IVUS has been the primary adjunctive intravascular imaging modality in interventional cardiology over the past three decades, while OCT is a newer modality of growing clinical importance. Both modalities augment angiography alone while having their own specific advantages and disadvantages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Angiography has been guiding percutaneous coronary intervention (PCI) over the past four decades allowing visual estimations of lesion length, severity, and vessel size. Unfortunately, measurements of these parameters critical to PCI are highly variable between operators. Intravascular imaging (IVI) not only overcomes these limitations by providing precise measurements facilitating accurate stent sizing but also guides the need for lesion preparation by visualizing plaque morphology, while also decreasing the likelihood of geographic miss, and detecting procedural issues including malapposition, underexpansion, edge dissection, and tissue protrusion. The two predominant IVI modalities are intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Despite a large evidence base of registries, randomized trials, and meta-analyses reporting that IVI decreases major adverse cardiovascular events (MACE), including mortality, their utilization in clinical practice remains very low [1, 2]. In this review, we outline the evidence base supporting IVI and describe the advantages and disadvantages of IVUS vs OCT, the latest applications of IVI, as well as future directions of these technologies.
Background
Intravascular Imaging Modalities
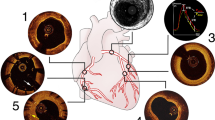
IVUS utilizes back-reflection of ultrasound waves (~ 40um wavelength) to construct intravascular images with a resolution of 50–150 um at a penetration depth of 3–8 mm. In comparison, OCT uses back-reflection of light waves (~ 1.3um wavelength) to generate images with a 10-fold higher resolution but at a shorter penetration depth of 1–2 mm (Fig. 1) [3]. With a greater resolution, OCT allows for more detailed imaging of luminal features including thrombus formation, stent malapposition, coronary dissection, intimal tears, thin-cap fibroatheroma, and fibrous plaques [4]. However, the greater depth with IVUS provides imaging of the entire vessel structure, including media and adventitia, which is often missed by OCT especially in the presence of lipidic or calcific plaque. With an acquisition speed of 25 mm/s for OCT compared to maximum 10 mm/s for IVUS, OCT allows for more rapid assessment of vessel morphology during PCI. The utility of light wave back-reflection for OCT comes with the need to clear blood from the lumen prior to imaging. The most widely used flushing agent has been radiographic contrast, resulting in optimal clearing of lumen, but with the potential for increased total contrast burden. This is one reason often cited for the limited use of OCT, particularly in patients with compromised renal function. While this may theoretically raise concerns, published data suggests no clinically significant difference in imaging-related complications with either imaging modality [5].
Incorporating Intravascular Imaging into PCI
When incorporated into PCI both IVUS and OCT significantly influence operator strategy. Table 1 summarizes the guidelines and society recommendations. In the ILUMIEN I study, OCT imaging changed management prior to PCI in 57% and post-PCI in 27% [11]. Intravascular imaging can be seamlessly incorporated into a standard intervention by separating the procedure into three components: (1) pre-intervention assessment, (2) stent deployment, and (3) complication and post-procedural assessment (Fig. 2).
Pre-intervention Assessment
In the pre-intervention assessment, IVI provides assessment of plaque composition which may guide lesion preparation. While lipidic and fibrous plaques may be amenable to direct stenting approaches, highly calcified lesions may require upfront plaque modification with specialty balloons or debulking with atherectomy respectively [12]. Moreover, there is a high rate of detection of abnormal findings by OCT in patients with suspected coronary disease and angiographically non-obstructive lesions that would otherwise go undiagnosed [11]. While the clinical significance of such findings are currently unclear, multiple studies investigating these findings in specific sub-populations (women, smokers, substance abuse) are underway. Further details on pre-intervention assessment are described in the following section on lesion subtypes.
Stent Deployment
When the decision to stent has been made, IVI can be used to precisely size and guide the implantation of stents, in particular using angiographic co-registration, minimizing geographic miss. Identifying “normal” reference vessel segments by angiography alone can frequently lead to inadvertently implanting stent edges in diseased segments [13]. Therefore, intravascular imaging may minimize longitudinal geographic miss which can be seen in as much as half of patients undergoing PCI guided by angiography alone [14, 15]. Geographic miss, leading to residual reference segment disease, may lead to increased rates of target vessel failure [16, 17]. More accurate anatomical positioning is possible through co-registration of angiography and IVI. The DOCTOR fusion trial from 2014 demonstrated the feasibility of a computer-based online co-registration of angiography and OCT [18]. Landmarks were used for full co-registration during the procedure with any inaccuracies being identified and corrected through matching of a second landmark. Without co-registration, there was a higher rate of incorrect matching by the operator resulting in OCT-identified lesion areas being only partially covered with stent in 70% of the lesions. Improved outcomes with co-registration were thus demonstrated. In the OPTICO-integration study, OCT-angiographic co-registration led to additional changes in PCI strategy in 40.7% of lesions in comparison to OCT imaging alone [14]. The integration of co-registration to minimize the protrusion of stent struts into the main branch while ensuring full coverage of the ostium in the side branch has been reported in bifurcation lesions [19]. Similarly, real-time co-registration of IVUS and angiography is feasible and available from third-party vendors such as Pie Medical (Netherlands) [20]. With the SyncVision system (Philips, The Netherlands), not only IVUS can be co-registered but also physiologic lesion significance co-registration as well [21].
Stent sizing protocols differ between IVUS and OCT. In the OPUS-CLASS study, OCT was shown to accurately size the lumen of a phantom model of a vessel while IVUS oversized the lumen by approximately 10% [22]. This difference partly explains why early trials suggested the minimum stent area (MSA) of IVUS-implanted stents were larger than OCT-implanted stents, when using lumen-based sizing for OCT. Furthermore, earlier trials frequently sized the OCT arm based on lumen while sizing the IVUS group by the external elastic lamina (EEL) or media. Recently, EEL-based stent sizing with OCT has been demonstrated to be safe and feasible, without perforation, matching post-PCI IVUS luminal dimensions for the first time [23••]. Figure 3 demonstrates the influence that measuring EEL to EEL has on final stent area compared with a lumen-based measurement. Maximizing stent expansion is an important factor in optimizing outcomes following PCI and can be achieved with IVI [24]. Smaller stent areas have been shown to be a predictor for increased risk of stent thrombosis, myocardial infarction, and in-stent restenosis [25,26,27]. There have been a number of stent strategies that have been suggested and studied with IVI. The Multicenter Ultrasound Stenting in Coronaries study (MUSIC) criteria, based on IVUS, sought an in-stent minimum lumen area (MLA) greater than or equal to 80% of the average reference lumen area with symmetric stent expansion [28]. IVUS-XPL by contrast used a goal of MSA equal to or exceeding the distal reference lumen area [29••]. ILUMIEN III recommended dividing the stented segment into a proximal and distal half and having a goal of ≥ 90% expansion compared with the respective reference segment. Table 2 summarizes the recommended assessment for stent expansion.
a, b EEL to EEL stent sizing vs lumen-based stent sizing. OCT image of the reference segment of a vessel. Measurements based on the lumen of the vessel (outlined in green) would result in selection of a 2.5-mm stent and a theoretical cross-sectional area of 4.9 mm2. Measurements based on EEL to EEL measurements (B and C) would result in selection of a 3.0-mm stent and a theoretical cross-sectional area of 7.1 mm2. EEL to EEL stent sizing therefore results in a 2.2-mm2 increase in cross-sectional area
Complications and Post-Procedural Assessments
After stent implantation, a post-PCI imaging run is performed to assess expansion and complications. In addition to identifying underexpansion, intravascular imaging can diagnose intra-procedural thrombosis, major dissections, malapposition, and tissue protrusion, all of which have been shown to impact PCI outcomes (Fig. 4) [16, 17]. While irregular tissue protrusion has been associated with target vessel failure [31] and untreated edge dissections associated with target lesion revascularization [32], it is unclear whether malapposition in the absence of an underexpansion is associated with adverse outcomes [33]. Identifying and treating these complications can theoretically lead to long-term improved outcomes. Given the increased resolution, OCT is able to more frequently diagnose these complications. In ILUMIEN III, OCT guidance led to significantly less malapposition (11% vs 21%) and untreated major dissections (14% vs 26%) than IVUS guidance [23••]. Applying an algorithmic approach to IVI can standardize the process and ensure a comprehensive approach to baseline lesion assessment, stent selection, and stent optimization [34].
Intravascular Image-Guided PCI to Improve Outcomes
Multiple registries, clinical trials, and meta-analyses have consistently shown intravascular imaging improves both clinical and procedural outcomes in patients undergoing PCI. This has been summarized and appraised in recent publications [35, 36]. Since IVUS is an older technology compared to OCT, a more extensive evidence base exists for IVUS-guided PCI. However, emerging OCT data and head-to-head trials suggest that both technologies represent vast improvement over angiography-alone PCI.
In the prospective nonrandomized ADAPT-DES trial with 8582 patients in 11 international centers, IVUS guidance was shown to reduce 1 year rates of stent thrombosis, myocardial infarction (MI), and major adverse cardiac events (MACE) [16]. In the recently published ULTIMATE trial, 1448 all-comer patients were randomized to IVUS-guided PCI vs angiography alone [30••]. IVUS-guided PCI significantly reduced the primary endpoint of target vessel failure at 12 months (2.9% vs 5.4%, p = 0.019). The signal was strongest in acute coronary syndrome and complex lesions. In the IVUS-XPL trial, 1400 patients with lesions > 28 mm were randomized to angiography alone or IVUS-guided PCI with a significant improvement in MACE primarily driven by reduced target vessel revascularization (2.9% vs 5.8%, p = 0.007) [29••]. In the most recent meta-analysis, the risks of all-cause death, MI, target lesion revascularization (TLR), and stent thrombosis were significantly reduced by IVUS guidance [30••].
OCT has been shown to improve clinical outcomes as well, in retrospective and registry studies. The CLI-OPCI trial was a retrospective propensity-matched cohort study, OCT use was associated with a reduced 12 month rate of cardiac death or non-fatal MI (odds ratio = 0.37 [0.10–0.90], p = 0.05) [38]. The Pan-London PCI registry included 123,764 patients who underwent PCI. Rates of imaging use were low with only 1.3% of interventions using OCT and 12.6% using IVUS [1]. In the propensity-matched cohort analysis, OCT-guided PCI was associated with significantly reduced mortality rates compared to angiography guidance (hazards ratio = 0.39 [0.21–0.77], p = 0.0008), but not when compared to IVUS guidance (HR = 0.88, [0.61–1.38, p = 0.43). Overall, intravascular imaging (OCT + IVUS) was associated with a survival advantage when compared to angiography (hazards ratio = 0.55 [0.38–0.82]; p < 0.0001).
Randomized trials directly comparing OCT and IVUS have primarily had procedural outcomes as the primary endpoint. The only prospective randomized trial comparing IVUS and OCT with a clinical outcome as a primary endpoint was the OPINION trial. In this trial, target vessel failure was not significantly different between OCT and IVUS at 1 year follow-up [39]. In a prospective randomized trial, Habara et al. reported OCT guidance was associated with a smaller minimum stent area (7.1 ± 2.1 mm2 vs 6.1 ± 2.2 mm2) [40]. This was likely secondary to the stent sizing for OCT being based on reference vessel lumen measurements as opposed to vessel wall measurements in the IVUS arm. In fact, 40% of patients in the OCT arm had stents sized according to angiography due to an inability to visualize satisfactory lumen measurements. The ILUMIEN III trial incorporated an EEL-based stent sizing algorithm [23••]. This was a randomized non-inferiority trial comparing OCT versus IVUS versus angiography-guided PCI. The trial met its primary endpoint, with no difference in the OCT evaluated minimum stent area between IVUS and OCT (5.89 mm2 vs 5.79 mm2, respectively).
Use of Intravascular Imaging in Specific Clinical Scenarios
As detailed above, one of the consistent findings from prior randomized studies and registries is imaging that has greater benefit for longer and more complex lesions. A meta-analysis of IVUS-guided versus angiography-guided drug-eluting stent (DES) implantation in patients with complex lesions showed that IVUS-guided PCI had lower rates of major adverse cardiac events (RR = 0.64, [0.51–0.80], p = 0.0001), target lesion revascularization (RR = 0.62, [0.45–0.86], p = 0.004), and target vessel revascularization (RR = 0.60, [0.42–0.87], p = 0.007) [41].
The ongoing ILUMIEN IV: OPTIMAL PCI trial (NCT03507777) is an international, multicenter randomized controlled clinical study that plans to enroll up to 3500 patients to OCT-guided vs angiography-guided coronary stent implantation. Patients in the angiography group will undergo blinded post-PCI OCT by an independent core laboratory. The primary outcomes include an imaging endpoint (MSA) as well as a clinical endpoint (target vessel failure) between 1 and 2 years. This landmark trial will comprehensively evaluate the role of IVI specifically in high-risk patients (diabetes, end-stage renal disease) and high-risk angiographic lesions (non-ST-elevation MI, 2-stent bifurcation, long lesion, severe calcification, antegrade wire escalation chronic total occlusion, and in-stent restenosis).
Calcified Lesions
Encountered increasingly in clinical practice, severely calcified lesions represent an especially challenging subset of lesions. Calcified lesions are associated with overall increased plaque burden, stent underexpansion, malapposition, and procedural failure [42,42,44]. Both IVUS and OCT can assist in pre-procedure planning by (1) diagnosing the presence of calcium and (2) predicting the need for upfront calcium debulking or modification strategies including laser angioplasty, atherectomy, specialty balloons, or intravascular lithotripsy.
OCT imaging offers several advantages over IVUS when assessing calcified lesions. Since calcium is a reflector of sound, IVUS images depict calcified lesions as a hyperechoic deposits with shadowing making it impossible to evaluate calcium thickness and depth. Light is not reflected by calcium, therefore allowing delineation of calcium thickness, arc, and length by producing a signal poor well-defined area with sharp borders (Fig. 5).
IVUS vs OCT imaging of calcium. a IVUS image of a highly calcified coronary artery. Ultrasound waves are absorbed by calcium creating a hyperechoic shadowing effect making it impossible to determine the extent of calcification. b OCT image of a highly calcified coronary artery. OCT imaging allows delineation of calcium thickness, arc, and length by producing a signal poor area with sharp well-defined borders (arrowhead)
Mintz et al. demonstrated that IVUS detected calcium in 73% of lesions, significantly more often than standard angiography (38%) [45]. Wang et al. corroborated these findings, when they compared the ability of IVUS, OCT, and angiography to diagnose calcium [46]. A total of 440 lesions were evaluated and calcium was detected by angiography in 40.2%, IVUS in 82.7%, and OCT in 76.8%. Notably, in 21.6% of lesions with calcium angle greater than 180°, no angiographically visible calcium was noted. However, only angiographically visible calcium was associated with stent underexpansion.
Using IVUS or OCT to delineate calcium characteristics can help guide selection of calcium debulking strategies. Calcium arc and thickness are predictors of calcium cracking with balloon angioplasty [43, 47]. Fujino et al. recently created an OCT-based scoring system using a retrospective cohort and an external cohort for validation [12]. A multivariable model showed that calcium thickness, calcium length, and calcium angle were independent predictors of stent expansion. A scoring system defined as 2 points for maximum angle > 180°, 1 point for maximum thickness > 0.5 mm, and 1 point for length > 5 mm was developed and in a validation cohort scores > 4 were highly predictive of poor stent expansion suggesting that these lesions may benefit from upfront aggressive vessel preparation strategies such as specialty balloons, atherectomy, or intravascular lithotripsy.
Left Main and Bifurcation Lesions
Bifurcation lesions represent up to 15–20% of percutaneous coronary interventions. Expert opinion favors liberal use of imaging due to a combination of technical procedural benefits and meta-analyses suggesting improved outcomes. A recent meta-analysis by Fan et al. including 15 clinical trials and 8084 patients demonstrated significant reductions in all-cause mortality, MI, and target vessel revascularization in the subgroup of patients with bifurcation lesions using imaging guidance [48]. In a prospective propensity-matched cohort study by Chen et al., IVUS guidance for the treatment of complex bifurcation lesions was associated with decreased MACE at 1 year follow-up compared to angiography only-guided PCI (10.0% vs 15.0% p = 0.036) [49].
Due to the higher rates of restenosis and target vessel revascularization, stent optimization is critical in treating bifurcation lesions and imaging can facilitate the finer technical aspects of these interventions. Ensuring adequate coverage of the ostial side branch lesions is a common technical challenge when stenting bifurcation disease. Imaging guidance can also facilitate appropriate guidewire crossing. When performing kissing balloon inflation, guidewire recrossing in the distal cell optimizes results and decreases incomplete stent apposition at the side branch. In a feasibility study of 150 bifurcation lesions treated using provisional stenting followed by final kissing balloon inflations, three-dimensional OCT facilitated distal guidewire recrossing, known to improve carina coverage without strut jailing of the side branch, in 91.7% of lesions [50].
The left main represents a special type of bifurcation lesion. While no randomized trials exist, expert opinion overwhelmingly favors image-guided interventions given the high stakes of left main PCI and thus the need for stent optimization to achieve the largest minimal stent area possible [9]. Imaging guidance was strongly recommended in randomized clinical trials comparing PCI vs CABG in left main disease. IVUS guidance was utilized in 80% of patients in the PCI arm of EXCEL and 47% of patients pre-PCI and 74% of patients post-PCI in the NOBLE trial [51, 52]. In propensity-matched analyses, registry studies, and meta-analysis imaging guidance for left main intervention was associated with reduced MACE and all-cause mortality [48, 53,53,55].
While OCT may be used for left main interventions, limited data is available. Given the need to clear blood with contrast to obtain clear images, IVUS is preferred for left main lesions especially those involving the aorto-ostial junction.
Chronic Total Occlusions
Intravascular imaging has become an indispensable tool during chronic total occlusion (CTO) intervention. For example, IVUS guidance can increase technical success of both anterograde and retrograde dissection and reentry into the true lumen. Likewise, IVUS can help facilitate guidewire crossing in both anterograde and retrograde manner (Fig. 6) [56, 57]. The increased technical success is coupled with improved clinical outcomes. In the CTO-IVUS study Kim et al. randomized 402 CTO lesions to IVUS-guided vs angiography-guided intervention [58••]. At 12 months follow-up, MACE (composite of cardiac death, MI, or TVR) was significantly lower in the IVUS group (2.6% vs 7.1%; p = 0.035). In the AIR-CTO trial 230 patients were randomized to IVUS-guided vs angiography-guided intervention [59]. The rate of stent thrombosis at 2 years was significantly lower in the IVUS group (0.9% vs 6.1% p = 0.043).
IVUS-guided CTO revascularization. a CTO of the first diagonal with ambiguous proximal cap (arrow). Late retrograde filling can be seen on dual injection. b, c IVUS was used to identify proximal cap. Arrow identifies proximal cap (denoted by an asterisk) with soft lipidic plaque with no calcium. c Based on plaque morphology a Miracle 3 wire (Asahi-Intecc, Japan) was selected to probe the proximal cap position identified by IVUS (arrow). d The lesion was wired and intervened upon. e Final angiogram post stenting
Given the risk of restenosis with long stents often placed into small distally remodeled vessels, imaging guidance to optimize stent expansion and minimum stent area is critical in CTO PCI. The need for multiple imaging runs, and avoidance of antegrade contrast injection to limit propagation of dissection, makes IVUS more desirable than OCT.
Coronary Artery Vasculopathy
In transplanted patients, coronary artery vasculopathy (CAV) represents a rapidly developing form of coronary artery disease that is associated with poor prognosis long term and due to its diffuse and concentric nature is difficult to detect early by conventional angiography [60, 61]. Despite these drawbacks, angiography is still recommended for routine surveillance post-transplant due to its widespread availability and prognostic significance [60]. Intravascular imaging offers the ability to detect early CAV and thus potentially modify immunosuppressive regimens to prevent progression. IVUS studies have demonstrated that maximal intimal thickness (MIT) > 0.3 mm at 1 year post-transplant reduced 4-year survival (73% vs 96% [55]. Increased intimal thickening defined by an increase in MIT > 0.5 mm in 1 year has also been associated with increased MACE and the development of angiographic CAV [62, 63].
OCT measurements of intimal thickness correlate well with IVUS measurements. While not as widely used as IVUS post-transplant, OCT offers several promising features. The increased resolution and plaque characterization, including ability to image macrophage infiltration, may theoretically offer the ability to better understand the pathophysiology of CAV as well as make an earlier diagnosis of CAV compared with IVUS [64,64,65,67]. In the international pediatric OCT registry, 17% of patients receiving routine surveillance had medical management changes based solely on OCT findings [68].
Bioresorbable Vascular Scaffold
Bioresorbable vascular scaffolds (BVS) were designed to provide the theoretical benefit of drug-eluting stents during the first year after implantation but resorb over time, allowing restoration of vasomotion, unjailing side branches, and restoring cyclic strain [69]. The ABSORB BVS was the most widely studied and clinically available bioresorbable scaffold and was removed from the market due to concerning safety data. In the ABSORB II and ABSORB III 3-year follow-up, there was an increased incidence of target vessel MI and stent thrombosis compared to metallic drug-eluting stents [70, 71]. An explanation for this increased signal was the implantation technique of the BVS. In a retrospective analysis of the major ABSORB studies, the pre-dilation, sizing, and post-dilation (PSP) technique and vessel sizing were significantly associated with improved outcomes [72]. In OCT and IVUS studies malapposition, underexpansion, and smaller minimal scaffold diameter were all associated with adverse outcomes in patients with BVS implantation [73, 74]. While no randomized trials exist, imaging guidance represents an opportunity to further optimize BVS implantation [75]. In a complex cohort undergoing BVS implantation using the AVIO algorithm and PSP, 24.5% of lesions required additional optimization after imaging [76]. The ABSORB IV trial attempts to test the importance of implantation technique and lesion selection by mandating the use of PSP implantation technique and limits lesions with a reference vessel diameter of ≥ 2.5 mm and ≤ 3.75 mm. In this study, intravascular imaging was not mandated to optimize implantation. BVS was non-inferior to everolimus-eluting DES (Xience, Abbott Vascular, Santa Clara, CA) with respect to the primary endpoint of target lesion failure (5.0% vs 3.7% Pnon-inferiority = 0.02) at 30-day follow-up. However, there was a trend towards increased incidence of stent thrombosis and higher rates of target vessel revascularization in the BVS group [77].
Stent Thrombosis
In the PESTO registry, OCT identified the likely underlying etiology of stent thrombosis in 97% of cases. Patients with stent thrombosis had an increased incidence of neointimal hyperplasia (4%), uncovered struts (8%) edge-related disease progression (8%), major stent underexpansion (11%), neoatherosclerotic lesions (22%), and strut malapposition (24%) [78]. Similarly, in a single-center, observational, prospective study, patients presenting with late stent thrombosis 50% presented with OCT-defined malapposition [79]. In ADAPT-DES IVUS guidance was associated with a significant reduction in stent thrombosis compared to angiography alone at 12 months of follow-up (0.6% vs 1.0% hazard ratio 0.53 [0.31–0.90] p = 0.02) [16]. OCT can more readily identify both neoatherosclerosis and malapposition than IVUS. This information gained can be important to elucidate if the stent thrombosis is more likely to be due to stent failure, or more likely secondary to pharmacology.
Contemporary Technology and Future Directions
The growing popularity of IVUS and OCT amongst clinicians has prompted research studies which further characterize the imaging modalities as well as analyze their novel application to current PCI-related challenges.
The Dual Imaging study (NCT02984891) aims to directly compare high-definition IVUS images to OCT in the same patients to determine the differences between each modality as they relate to imaging coronary pathology, with the goal of determining which modality is most appropriate in particular clinical scenarios. Patients will undergo IVUS and OCT before and after stent placement assessing MLA and MSA with follow-up limited to the index hospitalization.
Patients with advanced kidney disease are increasingly being considered for revascularization with PCI. Contrast administration to patients with kidney disease can often be a precipitant that results in initiation of dialysis. Intravascular imaging plays a central role in these patients, allowing for the reduction or even elimination of contrast during the PCI. While contrast avoidance has its benefit for renal preservation, it comes at the added cost of risk of potential complications including perforation that are not as easily detected without contrast angiography. Increased surveillance of the patient is paramount during zero-contrast procedures.
IVUS-guided PCI without contrast administration is feasible, safe, and readily available for routine use [80, 81•] as well as with complex PCI [82] (Fig. 7). In the event of suspected complications, minimal contrast can be used as needed. Limiting the ratio of total contrast administration to GFR ratio below 1 is suggested [83].
IVUS guidance to perform a zero contrast PCI. a Significant prox/mid LAD and distal left main disease found on ultra-low contrast diagnostic angiogram (9.1 cc). Patient returned at a later date for zero contrast PCI. b Dynamic Coronary Roadmap (Philips Healthcare, The Netherlands) was used to wire LAD and D1. c IVUS was used to size and mark (arrow) distal landing zone of prox/mid LAD lesion and d stent was deployed at the mark (arrow). e, f IVUS (arrow) was used to mark ostial left main and stent was deployed (g) with 100% stent expansion (f)
Contrast is traditionally used to achieve blood clearing from the lumen to allow for image acquisition with OCT. The use of alternative agents including dextran have been reported [84]; however, due to the high osmolar content of dextran, the risk of nephropathy may be similar to contrast. Saline flush OCT has been reported and allows for good image acquisition and is effective at identifying factors including plaque composition, edge dissection, and stent apposition [80]. Measurement calculations with saline flush however are not yet reliable with commercially available software due to differences in refraction through contrast media as compared with saline. Pending commercial availability of software calibrated for saline flush OCT, this may offer an attractive alternative for image acquisition in the future.
Outlook
While there are specific scenarios favoring each modality, the focus should be improved integration of IVI into routine practice rather than on which modality is better. At a minimum, a clinician should gain comfort with at least one imaging modality to give them the tools needed to optimize revascularization for their patients.
Conclusion
Intravascular imaging is essential and offers numerous benefits beyond angiography. There are multiple registries, meta-analyses, and randomized trials to support improved procedural and clinical outcomes with image-guided PCI. Despite this, usage rates remain low secondary to an unfamiliarity with the technology and lack of reimbursement. A familiarity with both IVUS and OCT can allow one to leverage their respective strengths and weakness to optimally treat a wide variety of lesion subsets. The future of PCI is trending towards more complex and high-risk disease for which imaging will be an indispensable tool for safe and successful intervention.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jones DA, Rathod KS, Koganti S, Hamshere S, Astroulakis Z, Lim P, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the Pan-London PCI Cohort. JACC Cardiovasc Interv. 2018;11(14):1313–21. https://doi.org/10.1016/j.jcin.2018.01.274.
Smilowitz NR, Mohananey D, Razzouk L, Weisz G, Slater JN. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc Interv. 2018. https://doi.org/10.1002/ccd.27673.
Rathod KS, Hamshere SM, Jones DA, Mathur A. Intravascular ultrasound versus optical coherence tomography for coronary artery imaging—apples and oranges? Interv Cardiol. 2015;10(1):8–15. https://doi.org/10.15420/icr.2015.10.1.8.
Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33(20):2513–20. https://doi.org/10.1093/eurheartj/ehs095.
van der Sijde JN, Karanasos A, van Ditzhuijzen NS, Okamura T, van Geuns RJ, Valgimigli M, et al. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imaging. 2017;18(4):467–74. https://doi.org/10.1093/ehjci/jew037.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574–651. https://doi.org/10.1161/CIR.0b013e31823ba622.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart diseasE, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123–55. https://doi.org/10.1161/CIR.0000000000000404.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018. https://doi.org/10.1093/eurheartj/ehy394.
Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14(6):656–77. https://doi.org/10.4244/EIJY18M06_01.
Lotfi A, Davies JE, Fearon WF, Grines CL, Kern MJ, Klein LW. Focused update of expert consensus statement: use of invasive assessments of coronary physiology and structure: a position statement of the society of cardiac angiography and interventions. Catheter Cardiovasc Interv. 2018;92:336–47. https://doi.org/10.1002/ccd.27672.
Yamamoto MH, Maehara A, Song L, Matsumura M, Chin CY, Losquadro M, et al. Optical coherence tomography assessment of morphological characteristics in suspected coronary artery disease, but angiographically nonobstructive lesions. Cardiovasc Revasc Med. 2018. https://doi.org/10.1016/j.carrev.2018.07.011.
Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13(18):e2182–e9. https://doi.org/10.4244/EIJ-D-17-00962.
Mintz GS, Painter JA, Pichard AD, Kent KM, Satler LF, Popma JJ, et al. Atherosclerosis in angiographically “normal” coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25(7):1479–85.
Leistner DM, Riedel M, Steinbeck L, Stahli BE, Frohlich GM, Lauten A, et al. Real-time optical coherence tomography coregistration with angiography in percutaneous coronary intervention-impact on physician decision-making: the OPTICO-integration study. Catheter Cardiovasc Interv. 2017;92:30–7. https://doi.org/10.1002/ccd.27313.
Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101(12):1704–11. https://doi.org/10.1016/j.amjcard.2008.02.053.
Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129(4):463–70. https://doi.org/10.1161/CIRCULATIONAHA.113.003942.
Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. 2015;8(11):1297–305. https://doi.org/10.1016/j.jcmg.2015.08.013.
Hebsgaard L, Nielsen TM, Tu S, Krusell LR, Maeng M, Veien KT, et al. Co-registration of optical coherence tomography and X-ray angiography in percutaneous coronary intervention. The Does Optical Coherence Tomography Optimize Revascularization (DOCTOR) fusion study. Int J Cardiol. 2015;182:272–8. https://doi.org/10.1016/j.ijcard.2014.12.088.
Shlofmitz E, Sosa F, Goldberg A, Maehara A, Ali ZA, Mintz GS, et al. Bifurcation and ostial optical coherence tomography mapping (BOOM)—case description of a novel bifurcation stent technique. Cardiovasc Revasc Med. 2018. https://doi.org/10.1016/j.carrev.2018.05.005.
Frimerman A, Abergel E, Blondheim DS, Shotan A, Meisel S, Shochat M, et al. Novel method for real time co-registration of IVUS and coronary angiography. J Interv Cardiol. 2016;29(2):225–31. https://doi.org/10.1111/joic.12279.
Sacha J, Lipski P, Feusette P. Angiographic co-registration of instantaneous wave-free ratio and intravascular ultrasound improves functional assessment of borderline lesions in the coronary artery. Postepy Kardiol Interwencyjnej. 2018;14(1):107–8. https://doi.org/10.5114/aic.2018.74366.
Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging. 2013;6(10):1095–104. https://doi.org/10.1016/j.jcmg.2013.04.014.
•• Ali ZA, Maehara A, Genereux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618–28. https://doi.org/10.1016/S0140-6736(16)31922-5 The largest randomized trial comparing OCT, IVUS, and angiograpghy.
Fitzgerald PJ, Oshima A, Hayase M, Metz JA, Bailey SR, Baim DS, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000;102(5):523–30.
Choi SY, Witzenbichler B, Maehara A, Lansky AJ, Guagliumi G, Brodie B, et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ Cardiovasc Interv. 2011;4(3):239–47. https://doi.org/10.1161/CIRCINTERVENTIONS.110.959791.
Song HG, Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2014;83(6):873–8. https://doi.org/10.1002/ccd.24560.
Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009;2(12):1269–75. https://doi.org/10.1016/j.jcin.2009.10.005.
de Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J. 1998;19(8):1214–23.
•• Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL Randomized Clinical Trial. JAMA. 2015;314(20):2155–63. https://doi.org/10.1001/jama.2015.15454 A randomized clinical trial demonstrating improved outcomes with IVUS-guided stent implantation in complex disease.
•• Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S et al. Intravascular Ultrasound-Guided Versus Angiography-Guided Implantation of Drug-Eluting Stent in All-Comers: The ULTIMATE trial. J Am Coll Cardiol. 2018. https://doi.org/10.1016/j.jacc.2018.09.013. This is one of the largest randomized trials to compare image guided PCI and angiography
Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, et al. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation. 2015;132(11):1020–9. https://doi.org/10.1161/CIRCULATIONAHA.114.014704.
Kobayashi N, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, et al. Prevalence, features, and prognostic importance of edge dissection after drug-eluting stent implantation: an ADAPT-DES intravascular ultrasound substudy. Circ Cardiovasc Interv. 2016;9(7):e003553. https://doi.org/10.1161/CIRCINTERVENTIONS.115.003553.
Romagnoli E, Gatto L, La Manna A, Burzotta F, Taglieri N, Saia F, et al. Role of residual acute stent malapposition in percutaneous coronary interventions. Catheter Cardiovasc Interv. 2017;90(4):566–75. https://doi.org/10.1002/ccd.26974.
Shlofmitz E, Shlofmitz RA, Galougahi KK, Rahim HM, Virmani R, Hill JM, et al. Algorithmic approach for optical coherence tomography-guided stent implantation during percutaneous coronary intervention. Interv Cardiol Clin. 2018;7(3):329–44. https://doi.org/10.1016/j.iccl.2018.03.001.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10(12):1487–503. https://doi.org/10.1016/j.jcmg.2017.09.008.
Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet. 2017;390(10096):793–809. https://doi.org/10.1016/S0140-6736(17)31957-8.
•• Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D'Arrigo P, et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10(24):2488–98. https://doi.org/10.1016/j.jcin.2017.08.051 A large meta-analysis demonstrating improved outcomes with IVI.
Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8(7):823–9. https://doi.org/10.4244/EIJV8I7A125.
Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38(42):3139–47. https://doi.org/10.1093/eurheartj/ehx351.
Habara M, Nasu K, Terashima M, Kaneda H, Yokota D, Ko E, et al. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5(2):193–201. https://doi.org/10.1161/CIRCINTERVENTIONS.111.965111.
Bavishi C, Sardar P, Chatterjee S, Khan AR, Shah A, Ather S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. https://doi.org/10.1016/j.ahj.2016.10.008.
Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y, Fukuhara K, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78(9):2209–14.
Maejima N, Hibi K, Saka K, Akiyama E, Konishi M, Endo M, et al. Relationship between thickness of calcium on optical coherence tomography and crack formation after balloon dilatation in calcified plaque requiring rotational atherectomy. Circ J. 2016;80(6):1413–9. https://doi.org/10.1253/circj.CJ-15-1059.
Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–33.
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91(7):1959–65.
Wang X, Matsumura M, Mintz GS, Lee T, Zhang W, Cao Y, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10(8):869–79. https://doi.org/10.1016/j.jcmg.2017.05.014.
Fujino A, Mintz GS, Lee T, Hoshino M, Usui E, Kanaji Y, et al. Predictors of calcium fracture derived from balloon angioplasty and its effect on stent expansion assessed by optical coherence tomography. JACC Cardiovasc Interv. 2018;11(10):1015–7. https://doi.org/10.1016/j.jcin.2018.02.004.
Fan ZG, Gao XF, Li XB, Shao MX, Gao YL, Chen SL, et al. The outcomes of intravascular ultrasound-guided drug-eluting stent implantation among patients with complex coronary lesions: a comprehensive meta-analysis of 15 clinical trials and 8,084 patients. Anatol J Cardiol. 2017;17(4):258–68. https://doi.org/10.14744/AnatolJCardiol.2016.7461.
Chen L, Xu T, Xue XJ, Zhang JJ, Ye F, Tian NL, et al. Intravascular ultrasound-guided drug-eluting stent implantation is associated with improved clinical outcomes in patients with unstable angina and complex coronary artery true bifurcation lesions. Int J Cardiovasc Imaging. 2018. https://doi.org/10.1007/s10554-018-1393-2.
Nagoshi R, Okamura T, Murasato Y, Fujimura T, Yamawaki M, Ono S, et al. Feasibility and usefulness of three-dimensional optical coherence tomography guidance for optimal side branch treatment in coronary bifurcation stenting. Int J Cardiol. 2018;250:270–4. https://doi.org/10.1016/j.ijcard.2017.09.197.
Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375(23):2223–35. https://doi.org/10.1056/NEJMoa1610227.
Makikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388(10061):2743–52. https://doi.org/10.1016/S0140-6736(16)32052-9.
Ye Y, Yang M, Zhang S, Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta-analysis. PLoS One. 2017;12(6):e0179756. https://doi.org/10.1371/journal.pone.0179756.
Mintz GS, Lefevre T, Lassen JF, Testa L, Pan M, Singh J, et al. Intravascular ultrasound in the evaluation and treatment of left main coronary artery disease: a consensus statement from the European Bifurcation Club. EuroIntervention. 2018;14(4):e467–e74. https://doi.org/10.4244/EIJ-D-18-00194.
Andell P, Karlsson S, Mohammad MA, Gotberg M, James S, Jensen J et al. Intravascular ultrasound guidance is associated with better outcome in patients undergoing unprotected left main coronary artery stenting compared with angiography guidance alone. Circ Cardiovasc Interv. 2017;10(5). doi:https://doi.org/10.1161/CIRCINTERVENTIONS.116.004813.
Mohandes M, Vinhas H, Fernandez F, Moreno C, Torres M, Guarinos J. When intravascular ultrasound becomes indispensable in percutaneous coronary intervention of a chronic total occlusion. Cardiovasc Revasc Med. 2018;19(3 Pt A):292–7. https://doi.org/10.1016/j.carrev.2017.10.004.
Galassi AR, Sumitsuji S, Boukhris M, Brilakis ES, Di Mario C, Garbo R, et al. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: an overview. JACC Cardiovasc Interv. 2016;9(19):1979–91. https://doi.org/10.1016/j.jcin.2016.06.057.
•• Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7):e002592. https://doi.org/10.1161/CIRCINTERVENTIONS.115.002592 A randomized clinical trial demonstrating superior outcomes with IVUS guidance in CTO interventions.
Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10(12):1409–17. https://doi.org/10.4244/EIJV10I12A245.
Dipchand AI, Rossano JW, Edwards LB, Kucheryavaya AY, Benden C, Goldfarb S, et al. The Registry of the International Society for Heart and Lung Transplantation: Eighteenth official pediatric heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34(10):1233–43. https://doi.org/10.1016/j.healun.2015.08.002.
Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol. 2016;68(1):80–91. https://doi.org/10.1016/j.jacc.2016.04.033.
Mehra MR, Ventura HO, Stapleton DD, Smart FW, Collins TC, Ramee SR. Presence of severe intimal thickening by intravascular ultrasonography predicts cardiac events in cardiac allograft vasculopathy. J Heart Lung Transplant. 1995;14(4):632–9.
Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45(9):1532–7. https://doi.org/10.1016/j.jacc.2005.02.035.
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, et al. Assessment of coronary intima-media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69(8):903–7.
Hou J, Lv H, Jia H, Zhang S, Xing L, Liu H, et al. OCT assessment of allograft vasculopathy in heart transplant recipients. JACC Cardiovasc Imaging. 2012;5(6):662–3. https://doi.org/10.1016/j.jcmg.2012.01.018.
Garrido IP, Garcia-Lara J, Pinar E, Pastor-Perez F, Sanchez-Mas J, Valdes-Chavarri M, et al. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012;110(5):655–61. https://doi.org/10.1016/j.amjcard.2012.04.047.
Clemmensen TS, Holm NR, Eiskjaer H, Logstrup BB, Christiansen EH, Dijkstra J, et al. Layered fibrotic plaques are the predominant component in cardiac allograft vasculopathy: systematic findings and risk stratification by OCT. JACC Cardiovasc Imaging. 2017;10(7):773–84. https://doi.org/10.1016/j.jcmg.2016.10.021.
McGovern E, Hosking MCK, Balbacid E, Voss C, Berger F, Schubert S, et al. Optical coherence tomography for the early detection of coronary vascular changes in children and adolescents after cardiac transplantation: findings from the international pediatric OCT registry. JACC Cardiovasc Imaging. 2018. https://doi.org/10.1016/j.jcmg.2018.04.025.
Sotomi Y, Suwannasom P, Tenekecioglu E, Collet C, Nakatani S, Okamura T, et al. Imaging assessment of bioresorbable vascular scaffolds. Cardiovasc Interv Ther. 2018;33(1):11–22. https://doi.org/10.1007/s12928-017-0486-5.
Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, et al. 2-year outcomes with the absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390(10096):760–72. https://doi.org/10.1016/S0140-6736(17)31470-8.
Ali ZA, Gao R, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. 2018;137(5):464–79. https://doi.org/10.1161/CIRCULATIONAHA.117.031843.
Stone GW, Abizaid A, Onuma Y, Seth A, Gao R, Ormiston J, et al. Effect of technique on outcomes following bioresorbable vascular scaffold implantation: analysis from the ABSORB trials. J Am Coll Cardiol. 2017;70(23):2863–74. https://doi.org/10.1016/j.jacc.2017.09.1106.
Kraak RP, Kajita AH, Garcia-Garcia HM, Henriques JPS, Piek JJ, Arkenbout EK, et al. Scaffold thrombosis following implantation of the ABSORB BVS in routine clinical practice: insight into possible mechanisms from optical coherence tomography. Catheter Cardiovasc Interv. 2018;92:E106–14. https://doi.org/10.1002/ccd.27475.
Okada K, Honda Y, Kitahara H, Otagiri K, Tanaka S, Hollak MB, et al. Bioresorbable scaffold for treatment of coronary artery lesions: intravascular ultrasound results from the ABSORB Japan Trial. JACC Cardiovasc Interv. 2018;11(7):648–61. https://doi.org/10.1016/j.jcin.2017.11.034.
Ali ZA, Karimi Galougahi K, Shlofmitz R, Maehara A, Mintz GS, Abizaid A, et al. Imaging-guided pre-dilatation, stenting, post-dilatation: a protocolized approach highlighting the importance of intravascular imaging for implantation of bioresorbable scaffolds. Expert Rev Cardiovasc Ther. 2018;16(6):431–40. https://doi.org/10.1080/14779072.2018.1473034.
Tanaka A, Latib A, Kawamoto H, Jabbour RJ, Sato K, Miyazaki T, et al. Clinical outcomes of a real-world cohort following bioresorbable vascular scaffold implantation utilising an optimised implantation strategy. EuroIntervention. 2017;12(14):1730–7. https://doi.org/10.4244/EIJ-D-16-00247.
Stone GW. Outcomes of absorb bioresorbable scaffolds with improved technique in an expanded patient population: the ABSORB IV Randomized Trial. Presented at Transcatheter Cardiovascular Therapeutics Conference 2017.
Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37(15):1208–16. https://doi.org/10.1093/eurheartj/ehv711.
Nato M, Gomez-Lara J, Romaguera R, Roura G, Ferreiro JL, Teruel L, et al. One-year optical coherence tomography findings in patients with late and very-late stent thrombosis treated with intravascular imaging guided percutaneous coronary intervention. Int J Cardiovasc Imaging. 2018;34:1511–20. https://doi.org/10.1007/s10554-018-1372-7.
Karimi Galougahi K, Zalewski A, Leon MB, Karmpaliotis D, Ali ZA. Optical coherence tomography-guided percutaneous coronary intervention in pre-terminal chronic kidney disease with no radio-contrast administration. Eur Heart J. 2016;37(13):1059. https://doi.org/10.1093/eurheartj/ehv667.
• Ali ZA, Karimi Galougahi K, Nazif T, Maehara A, Hardy MA, Cohen DJ, et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016;37(40):3090–5. https://doi.org/10.1093/eurheartj/ehw078 Feasability of zero contrast PCI.
Karimi Galougahi K, Mintz GS, Karmpaliotis D, Ali ZA. Zero-contrast percutaneous coronary intervention on calcified lesions facilitated by rotational atherectomy. Catheter Cardiovasc Interv. 2017;90(4):E85–E9. https://doi.org/10.1002/ccd.26999.
Nyman U, Bjork J, Aspelin P, Marenzi G. Contrast medium dose-to-GFR ratio: a measure of systemic exposure to predict contrast-induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008;49(6):658–67. https://doi.org/10.1080/02841850802050762.
Azzalini L, Mitomo S, Hachinohe D, Regazzoli D, Colombo A. Zero-contrast percutaneous coronary intervention guided by dextran-based optical coherence tomography. Can J Cardiol. 2018;34(3):342 e1–3. https://doi.org/10.1016/j.cjca.2017.11.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Mintz reports grants and personal fees from BostonScientific, grants and personal fees from Philips, grants from Abbott, and personal fees from Infraredx, outside the submitted work.
Dr. Maehara reports grants from Boston Scientific and grants from Abbott Vascular, outside the submitted work.
Dr. Jeremias reports grants and personal fees from Abbott Vascular and grants and personal fees from Philips/Volcano, outside the submitted work.
Dr. Stone reports personal fees from Claret, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Shockwave, personal fees from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, other from MedFocus family of funds, other from Ancora, other from Cagent, other from Qool Therapeutics, other from Aria, other from Caliber, other from SpectraWave, and other from Biostar family of funds, outside the submitted work.
Dr. Shlofmitz reports personal fees from CSI outside the submitted work.
Dr. Ali reports grants from St Jude Medical, personal fees from St Jude Medical, personal fees from Acist Medical, and personal fees from Cardiovascular Systems Inc, outside the submitted work.
All other authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Rahim, H.M., Shlofmitz, E., Gore, A. et al. IVUS- Versus OCT-Guided Coronary Stent Implantation: a Comparison of Intravascular Imaging for Stent Optimization. Curr Cardiovasc Imaging Rep 11, 34 (2018). https://doi.org/10.1007/s12410-018-9475-z
Published:
DOI: https://doi.org/10.1007/s12410-018-9475-z