Abstract
Coronary near-infrared spectroscopy (NIRS) is a catheter-based imaging technique that can reliably detect lipid core plaques in the coronary artery wall. NIRS has now been combined with intravascular ultrasound (IVUS) in a single catheter. The combined NIRS/IVUS instrument provides all the information obtained by IVUS and adds lipid detection by NIRS. The instrument can detect large lipid core plaques that are at increased risk of causing periprocedural myocardial infarction during stenting. Preliminary data indicate that NIRS/IVUS imaging can identify vulnerable patients and vulnerable plaques associated with increased risk for spontaneous adverse cardiovascular events. Multiple ongoing studies are in progress to determine if NIRS/IVUS imaging can enhance the prediction of coronary events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease is currently the world’s leading cause of death, accounting for 7.4 million deaths in 2012 [1]. The disease is a dynamic, multifocal process that often causes an unexpected clinical event due to rapid progression of a single culprit lesion [2]. The initial process begins with lipid deposition leading to endothelial dysfunction and vessel remodeling. With time, a fibroatheroma often forms, creating a vulnerable plaque that is susceptible to disruption and thrombosis. While clinical and angiographic characteristics have some value as predictors of future acute coronary syndromes (ACS), a high proportion of events occur in patients classified as low to intermediate risk; hence, improved predictive methods are needed [3].
Intravascular imaging modalities such as intravascular ultrasound (IVUS), optical coherence tomography (OCT), and near-infrared spectroscopy (NIRS) have provided novel insights into the development, progression, and treatment of coronary artery disease. NIRS can reliably [4, 5] and reproducibly [6, 7] detect lipid core plaques (LCPs). Since its approval by the FDA in 2008, NIRS has now been utilized in over 16,000 patients. This usage has demonstrated clinical utility of NIRS for prediction during the stenting procedure and stimulated large outcome trials to test its ability to identify vulnerable plaques and vulnerable patients.
Principles, Design, and Interpretation
Principles of Diffuse Reflectance NIRS
NIRS is widely used across the physical and biomedical sciences due to its ability to distinguish between chemically different substances. Unlike other commonly used analytical methods, it is also non-destructive, it is amenable to remote sensing over fiber optics, and it is practical to perform in water-based media. The principle behind NIRS is the interaction of light with different functional groups of molecules, which produces a unique “chemical fingerprint” that can be analyzed to yield qualitative and quantitative information [8] about material composition. NIRS measurements are taken by shining specific wavelengths of light onto a sample and measuring the ratio of reflected to incident light [8]. Sample preparation is generally not required, nor is the sample altered by the measurement. While these features make NIRS well suited for intravascular imaging, over a decade of development was required to design and validate a NIRS instrument suitable for use in the human coronary artery where it was necessary to solve the problems of access, motion, penetration through blood, the need to scan wide areas, and automatic algorithmic prediction of lipid core presence.
Combined NIRS–IVUS System
NIRS for coronary imaging is currently available as a combined NIRS/IVUS imaging system (TVC Imaging System TM, InfraRedx Inc, Burlington, MA) that consists of a 3.2-Fr catheter, a pullback and rotation device, and a console, which houses the scanning laser, the computer that processes the spectral signals, and two monitors [4]. The catheter tip emits and collects light, which is transmitted back to the system for processing. The catheter imaging core is able to collect data rapidly by rotating at 960 rpm as it is pulled back at an automated speed of 0.5 mm/s. IVUS images are simultaneously acquired and inherently co-registered with NIRS measurements and are available in various combined and individual displays. The IVUS transducer operates at 40 MHz. A NIRS/IVUS catheter that acquires “high-definition” IVUS images using a 50 MHz transducer is in clinical use at several sites in the USA.
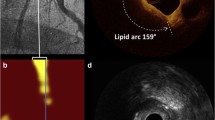
Spectral data are automatically analyzed by the system to produce a digital color map of the vessel wall, called a chemogram, which represents the probability of the presence of LCP over the scanned segment of vessel (Fig. 1). The NIRS algorithm that generates the chemogram was trained using histopathology as the gold standard for lipid identification and a rigorous experimental methodology that included blinded, prospective validation under simulated clinical use conditions [5]. It is notable that despite widespread claims of lipid core detection capability by other methods (such as OCT, grayscale IVUS, and radiofrequency IVUS), to date, no other technology has been subjected to a rigorous validation in this manner, and none has an associated FDA label claim as NIRS does. The algorithm for LCP detection in humans was constructed defining LCP as a fibroatheroma containing a lipid core ≥200 μ thick and >60° angular extent on histologic analysis. The algorithm could reliably identify LCPs with an area under the curve of 0.80 [5]. The NIRS algorithm was prospectively validated in humans in the SPECTACL study, in which chemograms obtained in vivo were similar to those obtained in histology controls [4]. The results of these studies led to FDA approval of the NIRS imaging system in 2008 for LCP detection. In 2010, the FDA approved the addition of IVUS to the NIRS system.
a Three cross-sections demonstrating a chemogram superimposed on the IVUS image at different pullback locations. b Longitudinal chemogram, with the pullback location on the x-axis (mm), and the circumferential position on the y-axis (°). c Block chemogram, where each block shows the 90th percentile of pixel values for its corresponding 2-mm chemogram segment. d Block chemogram superimposed on longitudinal IVUS image
Interpretation of NIRS Findings
On the chemogram, each pixel is assigned a color (128-color scale) based on the probability that lipid is present at that site, with the color scale transitioning smoothly from red (zero probability of LCP) to yellow (100 % probability of LCP). The color scale transition from red to yellow occurs near a probability of 0.60. Pixels with indeterminate data, such as those interfered with by the guidewire, may appear black. On a longitudinal chemogram, the pullback location in the vessel is denoted on the x-axis and the circumferential position on the y-axis. The lipid core burden index (LCBI) is a measure of lipid burden for a given vessel segment, calculated by dividing the number of yellow pixels (p > 0.6) by the total number of valid pixels in that segment, multiplied by a factor of 1000 (LCBI range 0–1000) [6]. The maxLCBI4 mm is defined as the LCBI of the 4-mm region with the highest lipid burden within the segment of interest [9].
The block chemogram is a summary measure that allows rapid interpretation as well as potential traceability back to the autopsy validation. Each block corresponds to a 2-mm segment of the pullback, and the color corresponds to the 90th percentile probability for the pixels within that segment. Thus, the block color represents probability of lipid within the segment, as follows: yellow P > 0.98, tan 0.84 ≤ P ≤ 0.98, orange 0.57 ≤ P < 0.84, and red P < 0.57 [9]. Other intuitive and potentially important parameters can be computed, such as the LCBI for selected regions of interest, or the lipid arc, which is the number of degrees spanned circumferentially by the lipid at a given longitudinal location.
Basic NIRS parameter measurements are fully automated, quantitative, and generated in real time during catheter pullback, allowing ready incorporation into the workflow of the catheterization laboratory and clinical decision making [10•]. Major providers of offline analysis software for intravascular imaging also now handle NIRS-IVUS data with versatile tools that can be used to compute quantities already used in the literature, to devise novel trial endpoints, or to create new clinical decision-making parameters.
Identification of LCP and Comparison with Other Intravascular Imaging Modalities
In contrast to IVUS and OCT, which collect structural information, NIRS is unique for its ability to directly identify the chemical composition of the vessel wall. NIRS alone does not provide information about structural anatomic parameters such as vessel remodeling, plaque thickness, lumen area, and calcification [11]. However, the combined NIRS/IVUS imaging catheter allows co-acquisition and co-registration of NIRS with IVUS, such that both structure and composition are available.
Since an important defining characteristic of fibroatheromas is plaque burden, grayscale IVUS can contribute to their detection in vivo. False positives are frequent, however, since many large plaques do not have a lipid core. The combined measures of plaque burden and LCBI improve the accuracy of fibroatheroma detection as compared with plaque burden alone. In a study of 116 coronary arteries from 51 autopsied hearts, the accuracy of fibroatheroma detection was 67.5 % using plaque burden, 71.2 % using NIRS, and 77 % using both plaque burden and NIRS [12]. NIRS identifies the LCP component of fibroatheromas, causing fewer false negatives as compared with IVUS alone. Calcification can complicate more sophisticated subjective methods of fibroatheroma detection using IVUS, such as attenuated plaque, since the patterns of echo-attenuation being analyzed can be quite similar for lipid versus various forms of calcification. Calcification can even sometimes completely preclude IVUS analyses altogether due to loss of IVUS signal. In an autopsy study, 52 % of IVUS-detected calcified plaques were shown by the histology gold standard to contain fibroatheromas that would have remained undetected by IVUS alone [13]. In calcified plaques and also in plaques with small plaque burden, NIRS improves LCP detection beyond that provided by IVUS alone [14].
OCT is an established modality for plaque characterization [15] and identification of the risk that complications will occur during a stenting procedure. OCT and NIRS are both optical techniques and both employ rapidly scanning lasers; however, the use of photons by the techniques is fundamentally different. OCT is the optical analogue of IVUS, using photons to overcome some of the near-field resolution limitations of sound waves and challenges with high-frequency ultrasound transducers. OCT achieves striking near-field spatial resolution at the expense of the necessity to clear the vessel of blood and very limited imaging depth. As with ultrasound, there is essentially no fundamental basis for OCT to determine composition. Composition assessments are made by subjective assessment of structural information and inferences regarding causes for dropout of usable imaging signal.
Nevertheless, the two techniques have often been compared for LCP detection, and a comparison of NIRS and OCT for LCP detection demonstrated modest correlation between the two modalities [16], possibly due to the higher sensitivity of NIRS for detecting small amounts of lipid or due to false-negative OCT findings. Yellow blocks on the chemogram correlated with OCT features suggesting plaque vulnerability, such as longer lipid length, greater lipid arc, and thinner fibrous cap. Use of OCT and NIRS can complement each other by combining structure and composition information [17].
In a retrospective study of 110 patients who underwent IVUS, OCT, and NIRS before PCI, cap thickness as assessed by OCT was the only variable independently associated with periprocedural myocardial infarction (MI) [18]. However, OCT requires significant training and experience for interpretation. When OCT was excluded from the analysis, both plaque burden as detected by IVUS and maxLCBI4 mm as measured by NIRS were associated with periprocedural MI.
Pathophysiologic Insights Gained with NIRS
Endothelial dysfunction has an important role in the development of atherosclerosis. Choi et al. performed coronary endothelial function assessment using intracoronary acetylcholine infusion and NIRS of the proximal left anterior descending artery and found a significant association between LCBI (and maxLCBI4 mm) and the degree of epicardial endothelial dysfunction [19]. These findings support the hypothesis that endothelial dysfunction is associated with the pathogenesis of early atherosclerosis.
Positive vessel remodeling has been associated with ACS [20, 21], while negative vessel remodeling is more often seen in stable coronary artery disease [22]. Ota et al. reported a positive correlation between remodeling index and maxLCBI4 mm (0.58; p < 0.001) [23], both in obstructive and in non-obstructive lesions, supporting the potential role of NIRS for predicting future coronary events [23]. Townsend et al. showed that lipid burden as assessed by NIRS was similar between non-bifurcation sites and sites within 10 mm of a bifurcation [24]. Zynda et al. demonstrated only a weak correlation between SYNTAX score and LCBI [25], indicating that NIRS might be able to improve prediction of outcomes by providing an additional type of information.
Ali et al. used NIRS and OCT to assess the development of neoatherosclerosis in patients with in-stent restenosis. The prevalence of LCP within neointimal hyperplasia segments was 89 % using NIRS versus 62 % using OCT [26]; however, LCP identified by NIRS alone was not associated with periprocedural MI during treatment for ISR. This finding may reflect the limited ability of NIRS to differentiate lipid located within neointimal tissue (i.e., neoatherosclerosis) from a lipid core located in the wall of the vessel underneath the stent struts. The ability of NIRS to correctly characterize lipid despite the presence of metallic stent struts was also confirmed by postmortem imaging followed by histology [26].
Clinical Applications of NIRS/IVUS
Several clinical applications of NIRS/IVUS have been demonstrated, such as identifying the culprit lesion for ACS, optimizing stent implantation, identifying plaques at high risk for distal embolization and periprocedural MI during PCI, and identifying stents placed over LCPs which have a higher rate of stent failure [27]. Early identification of coronary lesions at risk for complications may allow early implementation of preventive and treatment strategies. In addition to the aforementioned clinical uses, preliminary data suggest that NIRS/IVUS imaging will be useful in identifying vulnerable patients and vulnerable plaques, the uses for which the technology was developed. Multiple large-scale studies are in progress to test this hypothesis.
Identify Culprit Lesions in ACS
Several studies have evaluated NIRS signs of LCP (increased LCBI) at the sites of culprit lesions associated with coronary events. Madder et al. performed NIRS within the culprit vessels of 20 patients with acute ST-segment elevation acute myocardial infarction (STEMI). The maxLCBI4 mm was 5.8-fold higher in STEMI culprit segments than in 87 non-culprit segments of the STEMI culprit vessel (median [interquartile range (IQR)] 523 [445 to 821] vs. 90 [6 to 265]; p < 0.001) (Fig. 2) [28••]. An even more striking difference was observed when the STEMI culprit sites were compared with 279 coronary autopsy segments free of large LCP by histology (median [IQR] 523 [445 to 821] vs. 6 [0 to 88]; p < 0.001). These significant NIRS findings at culprit sites led to the proposal that maxLCBI4 mm >400 is a signature of plaques causing STEMI.
Angiographic and NIRS findings in acute STEMI. a A patient 56 years of age with acute chest pain and inferior-posterior injury (a) was referred for primary PCI. Angiography of the right coronary artery revealed complete occlusion (b). Aspiration yielded a thrombus characteristic of STEMI (c) and resulted in TIMI flow grade 3 (d). NIRS performed after TIMI flow grade 3 was established revealed a prominent, nearly circumferential LCP concentrated at the culprit site (e). b Angiographic and NIRS findings in eight patients with STEMI. In each case, the initial angiogram demonstrated a culprit lesion (block arrow) with impaired flow. Following treatment, TIMI flow grade 3 is established. NIRS shows prominent signs of LCP at culprit sites (asterisks denote culprit segment located between green lines), often in a circumferential pattern. LCP lipid core plaque, NIRS near-infrared spectroscopy, PCI percutaneous coronary intervention, STEMI ST-segment-elevation myocardial infarction, TIMI thrombolysis in myocardial infarction. Reprinted from Madder et al. [28••] with permission from Journal of The American College of Cardiology: Cardiovascular Interventions/Elsevier
In a small number of STEMI cases, the culprit lesion did not contain LCP. In one of these cases, the culprit lesion was a calcified nodule, and in another, it was a dissection, non-lipid causes of a coronary event well documented by pathologic studies [29]. Similar, albeit less pronounced, evidence of lipid in culprit lesion NIRS findings was observed in patients with non-ST-segment-elevation ACS [11, 30]. Among 81 patients (53.1 % with non-STEMI and 46.9 % with unstable angina), non-STEMI culprit segments had a 3.4-fold greater maxLCBI4 mm than non-culprits (448 ± 229 vs. 132 ± 154, p < 0.001) and unstable angina culprit segments had a 2.6-fold higher maxLCBI4 mm than non-culprits (381 ± 239 vs. 146 ± 175, p < 0.001). A large LCP was identified within the culprit lesion of all five cases of resuscitated out-of-hospital cardiac arrest that subsequently underwent coronary angiography (Fig. 3) [31].
Angiographic and NIRS findings in five victims of sudden cardiac arrest. Cases I–IV were out-of-hospital cardiac arrests, whereas in case V, the cardiac arrest occurred in the emergency room while awaiting transfer to the catheterization laboratory. All patients were successfully resuscitated and taken to the catheterization laboratory. In each case, initial angiography demonstrated an obvious culprit lesion (arrow) and NIRS revealed the presence of a large lipid core plaque within the culprit segment (white box). The culprit vessel was the left anterior descending artery in four cases and the right coronary artery in one case. In all cases, the maxLCBI4 mm was >400. Reproduced from Madder et al. [31] with permission from The Journal of Invasive Cardiology
In aggregate, these studies report a stepwise increase in lipid content (maxLCBI4 mm) from non-culprit segments (0–130), to unstable angina (∼380), to NSTEMI (∼450), and finally to STEMI and cardiac arrest (∼550). These NIRS imaging results are in accord with pathologic data indicating that fibrotic lesions are more frequent in stable angina, while lipid-rich plaques are more frequently observed as causes of NSTEMI, STEMI, and sudden death [29].
The close association of LCP with culprit lesions described above can be helpful in cases of ACS or cardiac arrest in which it is difficult to identify a culprit lesion. NIRS/IVUS evidence of a LCP with a large plaque burden suggests that the lesion is a culprit. This information can be useful in patient management (Fig. 4) [29]. In some patients, the culprit lesion may not be the most stenotic lesion detected by angiography. This can occur, for example, when thrombus forms in a proximal, relatively non-stenotic location and embolizes to a more distal location.
Cases demonstrating the ability of NIRS/IVUS to identify the culprit plaque and to discriminate between different causes of ACS. a A typical case of ST-elevation myocardial infarction (STEMI) with a circular lipid-rich plaque (LPR) in the proximal right coronary artery causing thrombotic occlusion. b A calcified nodule causing STEMI (approximately 5 % of STEMI cases). No lipid is detected at the culprit segment. c A stent thrombosis caused by an under-expanded stent. This is a purely thrombotic occlusion, and as expected, the NIRS/IVUS chemogram is red (no LRP). d A STEMI caused by neoatherosclerosis in a vein graft with a circular LRP with a high maximum lipid core burden index demonstrating novel lipid accumulation in the graft. e An inferior STEMI with typical acute chest pain that disappeared during transport to the percutaneous coronary intervention center. Normal angiography, but a circular ulcerated plaque rich in lipid was detected in the proximal right coronary artery (RCA), which probably caused a thrombotic occlusion that was later dissolved by spontaneous thrombolysis. f A patient with cardiac arrest and STEMI with normal angiogram but LRP in the proximal left anterior descending artery (LAD), which may explain his cardiac arrest. g Stent neoatherosclerosis causing restenosis and non-ST-elevation myocardial infarction. h A case of Takotsubo cardiomyopathy. As expected, no major LRPs are detected. i An embolic thrombus was detected in RPD and PLA and aspirated with no residual stenosis. NIRS/IVUS revealed LRP in the proximal RCA as the probable source of the embolus. j A 36-year-old woman with a dissection of the LAD. As expected, no LRP was detected. Reproduced from Erlinge [29] with permission from Journal of Internal Medicine/John Wiley and Sons
OCT can also be used to identify the culprit lesion in ACS patients by identifying plaque rupture and thrombus formation [32]. OCT also has some ability, with expert interpretation, to identify LCP, but it was developed primarily to identify structures and not chemical composition.
Optimize Stent Implantation
Given that NIRS is currently available as a combined catheter with IVUS, use of NIRS/IVUS can provide all benefits of IVUS, such as accurate measurement of the proximal and distal reference diameter and lesion length and accurate estimation of the presence and extent of coronary calcification. In the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES), “all-comers” registry of 8583 consecutive patient IVUS was utilized in 39 % and was associated with lower risk of major adverse cardiac events at 1 year (4.7 vs. 3.1 %, adjusted hazard ratio 0.70, p = 0.002) as compared with angiographically guided PCI [33]. The greatest benefit of IVUS-guided PCI was observed in patients who had ACS and complex lesions, but all patient subgroups benefited. A meta-analysis of 24,849 patients demonstrated significant reduction in MACE (odds ratio 0.079, p = 0.001), all-cause mortality, MI, and stent thrombosis with IVUS-guided PCI as compared with angiographically guided PCI [34]. Similar results were observed in another large meta-analysis by Ahn et al. [35].
Use of NIRS may facilitate optimal stenting by more accurate identification of the coronary lesion margins. Dixon et al. analyzed 75 lesions with NIRS and found that the LCP extended beyond the angiographic margins of the lesion in 16 % of cases [36]. Hanson et al. analyzed 58 lesions with NIRS/IVUS showing that atheroma (defined as plaque burden >40 % or LCP) extended beyond the angiographic margins of 52 (90 %) lesions and LCP extended beyond the angiographic border in 30 (52 %) lesions [37]. Moreover, the mean lesion length was significantly longer by NIRS/IVUS as compared with angiography alone (19.8 ± 7.0 vs. 13.4 ± 5.9 mm, p < 0.0001) [37]. Complete lesion coverage (“red to red” stenting) could reduce the risk for edge dissection and subsequent adverse outcomes [38], such as stent thrombosis [39], but this needs to be confirmed in prospective studies.
Prevent Distal Embolization and Periprocedural MI
Several studies have demonstrated an association between NIRS-defined LCP and periprocedural MI [40–43]. Goldstein et al. analyzed 62 PCI patients from the Chemometric Observation of LCP of Interest in Native Coronary Arteries Registry (COLOR) registry without pre-procedural biomarker elevation or suspected intracoronary thrombus [44]. Periprocedural MI (defined as a post-procedural biomarker elevation above 3× upper limit of normal (ULN) for either CK-MB or cTnI measured 4 to 24 h after PCI) occurred in nine patients (14.5 %) and was more common among patients with maxLCBI4 mm ≥500 (7 of 14 patients, 50 %) versus patients with maxLCBI4 mm < 500 (2 of 48, 4.2 %) [44]. Raghunathan et al. analyzed 30 patients with pre-procedure NIRS imaging from a single-center registry, demonstrating post-PCI CK-MB level elevation >3× the ULN in 27 % of patients with two or more yellow blocks (n = 11) versus none of the 19 patients with 0–1 yellow blocks within the stented lesion (p = 0.02) [45].
Distal embolization and side branch occlusion are the most likely mechanisms underlying the increased incidence of periprocedural MI in coronary lesions with large LCPs. In a pilot study, an embolic protection device (EPD) was used in nine patients who underwent PCI of lesions with large LCPs (Fig. 5). Embolized material was captured in the EPD in eight of the nine lesions: the captured material mainly consisted of fibrin and platelet aggregates, suggesting that a major mechanism of periprocedural MI might be distal embolization of thrombi associated with exposure of blood to LCP in the lesion. Post-PCI MI occurred in two patients (22 %), in one of whom, two filters were required because the first one filled with debris during PCI. Intra-stent thrombus formation is supported by another study in which stenting large LCPs often led to in-stent thrombus formation as detected by OCT [17]. Two of three patients with a large LCP (66 %) developed intra-stent thrombus post-stent implantation (Fig. 6) versus none of six patients without large LCPs (0 %, p = 0.02). The importance of embolization as a dominant mechanism of periprocedural MI is further supported by several studies that have demonstrated a significant decrease in the size of LCP after stenting [6, 46, 47].
Angiographic and near-infrared imaging before (a, b) and after (c, d) stenting and image of the filter that was used during stenting (e). Reproduced from Brilakis et al. [46] with permission from Catheterization and Cardiovascular Interventions/John Wiley and Sons
Coronary angiography and NIRS imaging before (a, b) and after (c) stenting of a proximal LAD lesion demonstrated a large circumferential lipid core plaque. Debris was captured in a filter (d) that was used during stenting. Optical coherence tomography after stenting demonstrated intra-stent thrombus formation (e). Reproduced from Papayannis et al. [17] with permission from Catheterization and Cardiovascular Interventions/John Wiley and Sons
The ability to identify the lipid-rich lesions that are prone to embolization during stenting suggests several strategies to prevent or at least mitigate the risk of periprocedural MI during stenting. These treatments include aspiration thrombectomy [48•], use of EPDs, vasodilators, or intensive anticoagulation and antiplatelet regimens.
There is evidence that aspiration thrombectomy can remove lipid-rich plaque. Erlinge et al. performed NIRS before and after aspiration thrombectomy in patients presenting with STEMI. The technique produced a 28 % reduction in culprit lesion lipid content (pre-aspiration LCBI 466 ± 141 vs. post-aspiration 335 ± 117, p = 0.0001) [48•]. Histological analysis of the aspirates revealed lipids, calcium, and macrophages, indicating that fragments of atherosclerotic plaques had been aspirated [48•]. Even though routine aspiration thrombectomy failed to provide clinical benefit in large randomized controlled trials of STEMI patients [49, 50], targeted application in large LCP coronary lesions might be useful.
Given the near universal capture of embolic debris with the use of EPDs during stenting of large LCP lesions [46], the Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow (CANARY) trial randomized patients undergoing stent implantation of a single native coronary lesion with a maxLCBI4 mm ≥600 to PCI with versus without a distal protection filter [10•]. The study was stopped prematurely due to difficulty in identifying patients suitable for randomization to EPD and lack of signs of benefit. Among 31 randomized lesions with maxLCBI4 mm ≥600, there was no difference in the rates of periprocedural MI with versus without the use of a distal protection filter (35.7 vs. 23.5 %, respectively; relative risk 1.52; 95 % confidence interval 0.50 to 4.60, p = 0.69) [10•]. However, the study used a broad definition of MI (troponin or creatine kinase-myocardial band increase to three or more times the ULN within 72 h), which could have diluted a potential benefit with EPDs.
A second study of prevention of periprocedural MI has been designed, taking into account the obstacles to enrollment encountered in CANARY. In the on-going CONCERTO (Randomized-controlled Trial of a Combined versus Conventional Percutaneous Intervention for Near-Infrared Spectroscopy Defined High-Risk Native Coronary Artery Lesions) trial (NCT02601664), patients undergoing PCI of a high-risk native coronary artery lesion (defined as ≥2 contiguous yellow blocks on the block chemogram) are randomized to “combined” preventive measures versus conventional PCI. Combined preventive measures consist of pre-PCI administration of an intracoronary vasodilator and a glycoprotein IIb/IIIa inhibitor, use of an EPD if technically feasible, and complete coverage of the LCP, if technically feasible. Although NIRS-guided prevention of periprocedural MI is a promising approach, whether the above treatments or other novel approaches (such as selective use of a laser for ablation of lipid-rich plaques) will protect the myocardium at risk for embolization remains to be proven.
Reduce Lipid-Core Plaque with Pharmacologic Therapy
The ability of NIRS/IVUS imaging to detect large LCP in the coronary arteries has led to studies of attempts to reduce coronary lipid with high-dose statin therapy. The Reduction in YELlow Plaque by Aggressive Lipid LOWering Therapy (YELLOW) trial randomized 87 patients with multivessel coronary artery disease to standard of care versus rosuvastatin 40 mg daily for 6–8 weeks. Patients then underwent repeat coronary angiography and NIRS imaging of the non-target lesion. In spite of the limited duration of treatment, a significant reduction in the lesion LCBI was observed [51•]. Interpretation of this result has been difficult because there was a baseline difference in the amount of yellow observed in the two arms of the study. This difference could have led to a regression to the mean in the rosuvastatin group, which might account for the observed reduction in yellow.
A similar study of rosuvastatin treatment of LCP, the Integrated Biomarker and Imaging Study 3 (IBIS 3, presented at EuroPCR 2015), did not demonstrate a significant decrease in LCBI after 1 year of high-dose rosuvastatin therapy. This finding is compatible with the observation that statins do not prevent all events and that the mechanism of action of a statin suggested by angioscopy studies is to thicken the cap over a lipid core [52, 53]. More aggressive low-density lipoprotein cholesterol-reducing therapies are currently available, such as proprotein convertase subtilisin kexin 9 (PCSK-9) inhibitors [54, 55], which might be effective in reducing the NIRS/IVUS signs of LCP in coronary patients.
Identify and Treat High-Risk Coronary Lesions and Vulnerable Patients
Detection of the vulnerable plaque—a plaque at increased risk of disruption and thrombosis, which was introduced as a concept over 25 years ago [56]—has unfortunately remained an elusive goal. For over two decades, multiple attempts have been made to identify such structures. The most ambitious and successful trial was PROSPECT One, which utilized routine IVUS and Virtual Histology to detect vulnerable plaques [57]. During a median follow-up time of 3.4 years, 11.6 % of patients experienced major cardiovascular events due to an initially untreated lesion. Most of those lesions were either thin-cap fibroatheromas, as determined by virtual histology IVUS, or were characterized by a large plaque burden, a small luminal area, or some combination of these characteristics, as determined by gray-scale and radiofrequency IVUS [57]. The study demonstrated that lesions with plaque burden >70 % had a 9.6 % risk of causing a coronary event in the subsequent 3.4 years. While this finding validated the vulnerable plaque concept, the risk was more modest than many expected, and the means of detection—imaging and contouring significant portions of the vasculature searching for regions meeting risk definitions—was time-consuming and not clinically feasible in real time. The combination of lack of specificity (nine false positives for each true positive) and difficulty of making the measurement (hours were required in a core laboratory) prevented the results of the PROSPECT study from changing clinical practice.
Given the massive expenditures of effort and funds for projects that have failed to identify vulnerable plaque in a practical manner that provides benefits to large numbers of patients, the opinion has been expressed that the search for a single vulnerable plaque will not succeed and should be abandoned. The argument has been advanced that atherosclerosis is a systemic disease with multiple foci, and priority should be given to systemic diagnostics and treatment [58, 59].
While the multiple failed efforts to find vulnerable plaque support considerable skepticism about the pursuit, the outcome of vulnerable plaque detection by the advanced and promising technology of NIRS/IVUS imaging is not yet known. NIRS/IVUS imaging was developed for the specific task of identifying vulnerable coronary plaques and provides an automated readout of LCP presence. These features give NIRS/IVUS imaging a special position in the field of vulnerable plaque study. Preliminary data from cross-sectional studies have shown a strong association between NIRS signs of LCP and coronary culprit lesions, and multiple prospective outcome studies are in progress.
While detection of vulnerable plaque was the primary impetus for the development of NIRS/IVUS imaging, the technology has also been tested for its ability to detect vulnerable patients.
Oemrawsingh et al. utilized NIRS to detect signs of lipid in a non-culprit artery in 203 patients undergoing PCI for an index event. After 1 year, the incidence of a composite endpoint of all-cause mortality, nonfatal ACS, stroke, unplanned coronary revascularization in CAD patients was 4-fold higher in the group with LCBI at or above the median (LCBI = 43), as compared with the group with LCBI below the median [60••] (Fig. 7).
Time to event curves for the various composite endpoints exclusive of definite culprit lesion-related events in 203 patients referred for angiography due to stable angina pectoris or acute coronary syndrome. A 4-fold increase in major adverse cardiac and cerebrovascular events during 1-year follow-up was observed in patients with a lipid core burden index (LCBI) above the median, as assessed by near-infrared spectroscopy of a non-stenotic non-culprit coronary artery segment (16.7 vs. 4.0 % event rate [adjusted hazard ratio 4.04; 95 % confidence interval 1.33 to 12.29; p = 0.01]). ACS acute coronary syndrome, PCI percutaneous coronary intervention. Reproduced from Oemrawsingh et al. [60••], with permission from Journal of the American College of Cardiology/Elsevier
A similar study by Madder et al. also demonstrated that NIRS imaging can detect vulnerable patients [61]. NIRS imaging was performed in 121 patients in a single culprit artery during stenting of the lesion causing the index event. The NIRS results from the index culprit were excluded, and the NIRS findings in the remaining portions of the culprit artery were compared to the incidence of MACCE events 1.5 years later. Patients with a maxLCBI4 mm ≥500 had a 58.3 % event rate compared to a rate of 6.4 % for those without the finding (p < 0.001).
While the results of Oemrawsingh et al. and Madder et al. are promising, the number of events in each study is small, and validation of vulnerable patient detection is required in studies with larger numbers of events. Multiple outcome studies are in progress to test the ability of NIRS/IVUS imaging to detect vulnerable plaques. The largest of these and the nearest completion is the Lipid-rich Plaque Study (LRP, NCT02033694) led by Dr. Ron Waksman. The LRP Study is conducted in patients undergoing a planned PCI. NIRS/IVUS imaging is performed in at least 50-mm total length from two or more native imaged arteries. The study will compare NIRS/IVUS findings in 30-mm segments of the coronary artery to the appearance of a new culprit lesion in that segment in 9000 patients. Over 1400 patients have been enrolled in the study as of December 1, 2015, with results expected in mid-2016.
A second major study of NIRS/IVUS imaging is being conducted in Scandinavia. The PROSPECT II ABSORB Study (NCT02171065), which is led by Dr. Gregg Stone and Dr. David Erlinge, is studying the relationship between LCP determined by NIRS and clinical events as is the LRP Study. PROSPECT II ABSORB also includes the ABSORB sub-study of treatment of non-flow-limiting, presumably vulnerable plaques (plaque burden by IVUS >65 %) with an ABSORB bioresorbable vascular scaffold (Abbott Vascular, Santa Clara, California). Over 350 patients have been enrolled in the study as of December 1, 2015, which will eventually include 900 patients, 300 of whom will be enrolled in the treatment sub-study.
A third study is the Lipid cORe Plaque Association With CLinical Events: a Near-InfraRed Spectroscopy Study (ORACLE-NIRS, NCT02265146), which now has >350 patients enrolled. Results are also expected from numerous databases of patients who have undergone NIRS/IVUS imaging in research hospitals throughout the world.
Conclusions
NIRS/IVUS can reliably, reproducibly, and efficiently identify intracoronary LCPs as documented by autopsy and clinical studies and supported by a specific FDA approval for that purpose. Plaques identified as lipid rich by NIRS/IVUS imaging complicate stenting and are associated with culprit lesions in cross-sectional studies of patients experiencing a coronary event. Preliminary data indicate that NIRS/IVUS imaging can identify vulnerable patients. Multiple prospective outcome studies are in progress to determine if NIRS/IVUS imaging can detect vulnerable plaques. If detection of vulnerable plaques and vulnerable patients is demonstrated, many studies of preventive treatment with devices and pharmacologic agents will begin. Success in the treatment studies would be a major advance in the struggle against coronary artery disease.
Abbreviations
- IVUS:
-
Intravascular ultrasound
- LCP:
-
Lipid core plaque
- MI:
-
Myocardial infarction
- NIRS:
-
Near-infrared spectroscopy
- PCI:
-
Percutaneous coronary intervention
- LCBI:
-
Lipid core burden index
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. Cardiovascular diseases. 2015: Fact sheet No317.
Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–22.
Bourantas CV, Garcia-Garcia HM, Farooq V, et al. Clinical and angiographic characteristics of patients likely to have vulnerable plaques: analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2013;6:1263–72.
Waxman S, Dixon SR, L’Allier P, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. J Am Coll Cardiol Img. 2009;2:858–68.
Gardner CM, Tan H, Hull EL, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. J Am Coll Cardiol Img. 2008;1:638–48.
Garcia BA, Wood F, Cipher D, Banerjee S, Brilakis ES. Reproducibility of near-infrared spectroscopy for the detection of lipid core coronary plaques and observed changes after coronary stent implantation. Catheter Cardiovasc Interv. 2010;76:359–65.
Abdel-Karim AR, Rangan BV, Banerjee S, Brilakis ES. Intercatheter reproducibility of near-infrared spectroscopy for the in vivo detection of coronary lipid core plaques. Catheter Cardiovasc Interv. 2011;77:657–61.
Siesler HW, Ozaki Y, Kawata S, Heise HM. Near-infrared spectroscopy: principles, instruments, applications. Weinheim: Wiley-VCH; 2008.
Kilic ID, Caiazzo G, Fabris E et al. Near-infrared spectroscopy-intravascular ultrasound: scientific basis and clinical applications. Eur Heart J Cardiovasc Imaging. 2015.
Stone GW, Maehara A, Muller JE, et al. Plaque characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary intervention: the CANARY Trial (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow). J Am Coll Cardiol Intv. 2015;8:927–36. CANARY is the first and only trial to date assessing the prophylactic use of embolic protection devices in lesions with large lipid core plaques. The study did not demonstrate any reduction in periprocedural myocardial infarction with use of embolic protection devices, but used a broad definition of myocardial infarction.
Madder RD, Smith JL, Dixon SR, Goldstein JA. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv. 2012;5:55–61.
Puri R, Madder RD, Madden SP et al. Near-infrared spectroscopy enhances intravascular ultrasound assessment of vulnerable coronary plaque: a combined pathological and in vivo study. Arterioscler Thromb Vasc Biol. 2015.
Kang SJ, Mintz GS, Pu J, et al. Combined IVUS and NIRS detection of fibroatheromas: histopathological validation in human coronary arteries. JACC Cardiovasc Imaging. 2015;8:184–94.
Brilakis ES, Banerjee S. How to detect and treat coronary fibroatheromas: the synergy between IVUS and NIRS. J Am Coll Cardiol Img. 2015;8:195–7.
Lee T, Yonetsu T, Koura K, et al. Impact of coronary plaque morphology assessed by optical coherence tomography on cardiac troponin elevation in patients with elective stent implantation. Circ Cardiovasc Interv. 2011;4:378–86.
Yonetsu T, Suh W, Abtahian F, et al. Comparison of near-infrared spectroscopy and optical coherence tomography for detection of lipid. Catheter Cardiovasc Interv. 2014;84:710–7.
Papayannis AC, Abdel-Karim AR, Mahmood A, et al. Association of coronary lipid core plaque with intrastent thrombus formation: a near-infrared spectroscopy and optical coherence tomography study. Catheter Cardiovasc Interv. 2013;81:488–93.
Kini AS, Motoyama S, Vengrenyuk Y, et al. Multimodality intravascular imaging to predict periprocedural myocardial infarction during percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:937–45.
Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J. 2013;34:2047–54.
Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–43.
Inaba S, Mintz GS, Farhat NZ, et al. Impact of positive and negative lesion site remodeling on clinical outcomes: insights from PROSPECT. JACC Cardiovasc Imaging. 2014;7:70–8.
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101:598–603.
Ota H, Magalhaes MA, Torguson R et al. The influence of lipid-containing plaque composition assessed by near-infrared spectroscopy on coronary lesion remodelling. Eur Heart J Cardiovasc Imaging. 2015.
Townsend JC, Steinberg DH, Nielsen CD, et al. Comparison of lipid deposition at coronary bifurcations versus at nonbifurcation portions of coronary arteries as determined by near-infrared spectroscopy. Am J Cardiol. 2013;112:369–72.
Zynda TK, Thompson CD, Hoang KC, et al. Disparity between angiographic coronary lesion complexity and lipid core plaques assessed by near-infrared spectroscopy. Catheter Cardiovasc Interv. 2013;81:529–37.
Ali ZA, Roleder T, Narula J, et al. Increased thin-cap neoatheroma and periprocedural myocardial infarction in drug-eluting stent restenosis: multimodality intravascular imaging of drug-eluting and bare-metal stents. Circ Cardiovasc Interv. 2013;6:507–17.
Dohi T, Maehara A, Moreno PR, et al. The relationship among extent of lipid-rich plaque, lesion characteristics, and plaque progression/regression in patients with coronary artery disease: a serial near-infrared spectroscopy and intravascular ultrasound study. Eur Heart J Cardiovasc Imaging. 2015;16:81–7.
Madder RD, Goldstein JA, Madden SP, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol Intv. 2013;6:838–46. This study demonstrated that coronary lesions causing STEMI have large lipid core plaques and proposed a maxLCBI 4mm >400 as a signature of plaques causing STEMI.
Erlinge D. Near-infrared spectroscopy for intracoronary detection of lipid-rich plaques to understand atherosclerotic plaque biology in man and guide clinical therapy. J Intern Med. 2015;278:110–25.
Madder RD, Husaini M, Davis AT, et al. Detection by near-infrared spectroscopy of large lipid cores at culprit sites in patients with non-ST-segment elevation myocardial infarction and unstable angina. Catheter Cardiovasc Interv. 2015;86:1014–21.
Madder RD, Wohns DH, Muller JE. Detection by intracoronary near-infrared spectroscopy of lipid core plaque at culprit sites in survivors of cardiac arrest. J Invasive Cardiol. 2014;26:78–9.
Wang L, Parodi G, Maehara A, et al. Variable underlying morphology of culprit plaques associated with ST-elevation myocardial infarction: an optical coherence tomography analysis from the SMART trial. Eur Heart J Cardiovasc Imaging. 2015;16:1381–9.
Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–70.
Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7:233–43.
Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–47.
Dixon SR, Grines CL, Munir A, et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012;109:60–6.
Hanson ID, Goldstein JA, Dixon SR, Stone GW. Comparison of coronary artery lesion length by NIRS-IVUS versus angiography alone. Coron Artery Dis. 2015;26:484–9.
Dohi T, Weisz G, Powers ER, et al. TCT-583 the extent of lipid-rich plaque assessed by near-infrared spectroscopy may predict DES failure: a COLOR Registry analysis. J Am Coll Cardiol. 2013;62:B176–7.
Sakhuja R, Suh WM, Jaffer FA, Jang IK. Residual thrombogenic substrate after rupture of a lipid-rich plaque: possible mechanism of acute stent thrombosis? Circulation. 2010;122:2349–50.
Fernandez-Friera L, Garcia-Alvarez A, Romero A, et al. Lipid-rich obstructive coronary lesions is plaque characterization any important? J Am Coll Cardiol Img. 2010;3:893–5.
Goldstein JA, Grines C, Fischell T, et al. Coronary embolization following balloon dilation of lipid-core plaques. J Am Coll Cardiol Img. 2009;2:1420–4.
Saeed B, Banerjee S, Brilakis ES. Slow flow after stenting of a coronary lesion with a large lipid core plaque detected by near-infrared spectroscopy. EuroIntervention. 2010;6:545.
Schultz CJ, Serruys PW, van der Ent M, et al. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound: a potential key to predict distal embolization and no-reflow? J Am Coll Cardiol. 2010;56:314.
Goldstein JA, Maini B, Dixon SR, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4:429–37.
Raghunathan D, Abdel-Karim AR, Papayannis AC, et al. Relation between the presence and extent of coronary lipid core plaques detected by near-infrared spectroscopy with postpercutaneous coronary intervention myocardial infarction. Am J Cardiol. 2011;107:1613–8.
Brilakis ES, Abdel-Karim AR, Papayannis AC, et al. Embolic protection device utilization during stenting of native coronary artery lesions with large lipid core plaques as detected by near-infrared spectroscopy. Catheter Cardiovasc Interv. 2012;80:1157–62.
Maini A, Buyantseva L, Maini B. In vivo lipid core plaque modification with percutaneous coronary revascularization: a near-infrared spectroscopy study. J Invasive Cardiol. 2013;25:293–5.
Erlinge D, Harnek J, Goncalves I, Gotberg M, Muller JE, Madder RD. Coronary liposuction during percutaneous coronary intervention: evidence by near-infrared spectroscopy that aspiration reduces culprit lesion lipid content prior to stent placement. Eur Heart J Cardiovasc Imaging. 2015;16:316–24. This pilot study demonstrated that aspiration thrombectomy of lesions causing STEMI resulted in 28% reduction in culprit lesion lipid content.
Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–97.
Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–98.
Kini AS, Baber U, Kovacic JC, et al. Changes in Plaque Lipid Content After Short-Term Intensive Versus Standard Statin Therapy: The YELLOW Trial (Reduction in Yellow Plaque by Aggressive Lipid-Lowering Therapy). J Am Coll Cardiol. 2013;62:21–9. YELLOW was the first study that used NIRS to determine longitudinal changes on intracoronary plaque lipid content. High dose rosuvastatin for 7 weeks was associated with significant reduction in maxLCBI 4mm .
Takano M, Mizuno K, Yokoyama S, et al. Changes in coronary plaque color and morphology by lipid-lowering therapy with atorvastatin: serial evaluation by coronary angioscopy. J Am Coll Cardiol. 2003;42:680–6.
Takarada S, Imanishi T, Kubo T, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009;202:491–7.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9.
Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–43.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–55.
Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–7.
Oemrawsingh RM, Cheng JM, Garcia-Garcia HM, et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64:2510–8. First prospective cohort study to demonstrate that NIRS can assist in predicting the risk of future adverse cardiovascular events.
Madder RD, Husaini M, Davis AT et al. TCT-398 identification of vulnerable patients by intracoronary near-infrared spectroscopy. J Am Coll Cardiol. 2014: 64(11_S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Barbara A. Danek and Aris Karatasakis declare that they have no conflicts of interest. Ryan D. Madder reports research support and speaker honoraria from InfraRedx. James E. Muller reports consulting with InfraRedx. Sean Madden reports employment by InfraRedx. Subhash Banerjee reports research grants from Gilead and the Medicines Company, consultant/speaker honoraria from Covidien and Medtronic, ownership in MDCARE Global (spouse), and intellectual property in HygeiaTel. Emmanouil S. Brilakis reports consulting/speaker honoraria from Abbott Vascular, Asahi, Boston Scientific, Elsevier, Somahlution, St Jude Medical, and Terumo; research support from Guerbet and InfraRedx; and spouse’s employment by Medtronic.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Danek, B.A., Karatasakis, A., Madder, R.D. et al. Experience with the Multimodality Near-Infrared Spectroscopy/Intravascular Ultrasound Coronary Imaging System: Principles, Clinical Experience, and Ongoing Studies. Curr Cardiovasc Imaging Rep 9, 7 (2016). https://doi.org/10.1007/s12410-015-9369-2
Published:
DOI: https://doi.org/10.1007/s12410-015-9369-2