Abstract
Recently, high levels of fluorine have been detected in mine water, posing a potential risk to human health in light of the comprehensive utilization of mine water. Focusing on the Shendong area, this study aims to reveal the occurrence, spatial distribution, main sources, and health risks associated with fluorine in mine water using Piper diagrams, statistical analysis, SEM–EDS of rocks, and health risk assessment models. A total of 101 samples, including 68 water samples and 32 rock samples, were collected and analyzed. Results show that the average concentration of fluorine in the study area is mostly greater than 1 mg/L, belonging to high-fluorine mine water. Mine water with elevated levels of fluorine is with an abundance of Na+ and a scarcity of Ca2+. The distribution of fluorine content in mine water of Shendong mine varies significantly with the depth of mining depth, resulting in lower fluoride levels in 1# and 2# mine water and higher levels in 3# mine water. The primary cause of elevated fluorine in 3# mine water is attributed to 3# roof groundwater related to the prolonged water–rock process between groundwater and rock formations. Health risk assessments demonstrate that mine waters from Shigetai, Daliuta, and Huojitu pose no health risks to infants, children, males, or females, whereas waters from Halagou, Buertai, Cuncaota, Bulianta, Shangwan, and Wulanmulun present potential hazards. Health risks associated with mine water are closely correlated with its fluorine concentration. To ensure compliance with drinking and living standards, a proposed solution involves coagulation precipitation followed by adsorption to remove fluorine from mine water. This study provides valuable insights into the distribution, sources, risk assessment, and mitigation of fluorine in mine water and groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is crucial for the optimal functioning of the human body as it serves as an essential micronutrient. However, Prolonged and excessive intake of fluoride can lead to the development of dental or skeletal fluorosis, as well as potentially more severe consequences such as genetic harm, cognitive impairment, or cancer (Xiong, et al. 2007; Jha et al. 2013; Yadav et al. 2019). The element fluorine, belonging to the halogen group in the periodic table, is plentiful in Earth’s crust (Podgorski and Berg 2022). Meanwhile, it exhibits exceptional reactivity and electronegativity. Hence, fluoride is widespread in the environment and can result in F− accumulation in groundwater (Amini et al. 2008). The worldwide occurrence of geogenic fluorine groundwater is a global health concern affecting more than 260 million people (Ayoob and Gupta 2006; Kumar, et al. 2016; Yadav et al. 2017). Hence, the World Health Organization (WHO) suggests that the recommended guideline for the national standard of drinking water is 1.5 mg/L (WHO, 2004). In China, the government has established a permissible limit of F− in drinking water at 1.0 mg/L for water quality safety.
The origin of geogenic fluorine in groundwater can be attributed to the dissolution of fluoride-containing minerals such as fluorite, hornblende, mica, fluorapatite, and others (Li et al. 2015). Extensive research has been carried out on the hydrochemical processes governing high-fluoride groundwater (Zabalat et al. 2016; Wang et al. 2009; Li et al. 2017). Elevated concentrations of fluoride are commonly observed in aquifers distinguished by high pH, alkalinity, reduced calcium levels (Wang et al. 28,29,, b), and elevated temperature conditions (Wang et al. 2023). High pH levels with a higher concentration of OH− can replace F− in minerals containing fluorine, while dissolved calcium can bind with fluoride and prevent from dissolution of fluorite. Additionally, elevated temperatures can accelerate chemical weathering processes and promote the solubilization of fluorine (Saxena and Ahmed 2003; He et al. 2020).
Previous studies mainly focused on fluorine’s source and hydrochemical processes in groundwater. However, few scholars have been devoted to studying the occurrence, and main source of fluorine in mine water. Unlike high-fluorine groundwater, the occurrence of elevated fluorine in mine water is impacted by principal geological deposits and mining disturbance. Thus, multiple methods were used to study the hydrogeochemical characteristics and formation mechanism of high-fluoride mine water (Wang, et al. 2022). However, the health risks assessment of fluorine in mine water has not been studied. The distribution of fluorine provides the basis for health risk assessment and health risk assessment of mine water is closely related to occurrence of fluorine. In addition, sources of fluorine can provide important opinions for mine water treatment.Thus, studying occurrence, main source and health risks of fluorine in mine water, integrally is indispensable and meaningful. It is reported that the distribution of high-fluorine mine water is primarily concentrated in the arid and semi-arid regions of northwest China. (Zhang et al. 2021). However, the northwestern mining areas are characterized by ecologically fragile and water-scarce (Xu et al. 2022). Mine water will serve as a water source for industrial production, living, and ecological utilization in the mining region.
Shendong Mine is located in Northwest China. The annual production of mine water in the Shendong mining area is estimated to be around 106 million tons, primarily attributed to extensive underground coal mining activities. Thus, it was chosen as a research area. And the aims of this study are (1) to analyze characteristics of fluorine distribution of mine water in shendong mine (2) to identify main stratum source and mineral source of fluorine (3) to conduct a health risk assessment and put forward correlative treatment measures of high-fluorine mine water.
Study Area
Location and Climate
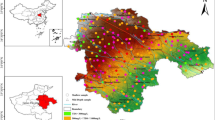
The study area is located at the border of Shaanxi Province and Inner Mongolia Autonomous Region, with an altitude of 1015 ~ 1190 m. It is the transformation zone from the Mu Us Desert to the Loess Plateau. The landform is mainly hilly, without obvious mountains, with terrain relatively flat. The mining area is about 3 356.11 km2, including 9 mine fields named Buertai(BET), Cuncaota(CCT), Ulan Moron(UM), Bulianta(BLT), Shangwan(SW), Halagou(HLG), Shig-etai(SGT), Daliuta(DLT), and Huojitu(HJT) (Fig. 1). The region has a temperate continental semi-arid climate characterized by an annual temperature of 11 °C, annual rainfall of 360 mm, and annual evaporation capacity of 2 500 mm. And evaporation is almost ten times greater than rainfall.
The groundwater mainly comes from the infiltration and recharge of precipitation, so the groundwater in the area is relatively poor. The surface water system is Groye River, a tributary of the middle reaches of the Yellow River, and its tributary Wulan Mulan River flows through the whole mining area.
Geology and Hydrogeology
The region is located on the slope of northern Shaanxi from the eastern flank of the Erdos Platform syncline in North China Platform, it is a part of the early and Middle Jurassic coal-bearing basin of Erdos, and a successor basin formed based on the platform. Since the Mesozoic era, the tectonic movement in Earth’s history has been mainly vertical. Therefore, there is no magmatic activity, few faults, and a simple structure. Most of the area is covered by Quaternary loose sediments, with bedrock exposed only along the Ulan Mulun River.
The mining area’s geological layers consist of Jurassic, Neogene, and Quaternary, from old to young (Fig. 1). Among them, Yan’an Formation is a coal-bearing formation, and at present, mining activities primarily focus on extracting 1#, 2#, and 3#coal seams. The major aquifers include a porous aquifer of the Quaternary Salawusu Formation (Q4), a fissured aquifer within the Zhi-luo Formation (J2z), a fissured aquifer within the Yan’an Formation (J1-2y).
The dominant lithology within the Quaternary comprises sand gravel and sand layers, with an average thickness of 436 m. The unit water inflow ranges from 0.065 to 0.901 L/s·m, while the permeability coefficient varies between 0.87 and 11.345 m/d. The lithology of Zhiluo Formation consists of medium, fine-grained sandstone and coarse sandstone, with argillaceous cement, unit water inflow of 0.00681L/s·m, permeability coefficient of 0.0367 m/d, and water richness is weak to very weak. The Zhiluo Formation also includes three sections: the water-bearing rock section from bottom to 1# coal roof, the water-bearing rock section from bottom to 2# coal roof and the water-bearing rock section from 2# coal floors to 3# coal roof. The lithological composition of J1-2y is medium-coarse sandstone, and the fractures are poorly developed overall. The specific water yield of the borehole of this aquifer varies from 0.00001 to 0.0011 L/(s⋅m).
Materials and Methods
Water Samples Collection
Water samples were gathered from nine minefields to study the distribution characteristics of fluorine-containing mine water and the main source of fluorine. A total of 68 samples were collected including 42 groups of mine water, 6 groups of surface water, and 16 groups of groundwater. In detail, this includes 7 groups of 1# coal mine water, 12 groups of 2# coal mine water, 23 groups of 3# coal mine water. The Quaternary groundwater, Zhiluo Formation groundwater, 1# coal roof groundwater, and 2# coal roof groundwater is 3 groups, respectively, while 3# Coal roof groundwater is 8 groups. Mine water samples were obtained from the underground coal face, goaf, mining roadway, and central sump. Additionally, surface water samples were collected from the Wulanmulun River near the study area. The groundwater sample wells were drained for 5–10 min until the temperature and pH reached a state of relative stability. Then, the samples were collected using high-density Polyethylene bottles. Samples for cation and fluorine analysis were acidified to a pH of 2 by adding pure nitric acid, thereby preventing the formation of precipitates in the water sample. Additionally, drilling operations were conducted to extract 32 rock samples collected from various layers.
Water Samples Analysis
The measurement of pH and TDS was conducted using a water quality analyzer equipped with multiple parameters (HACH). The water samples were subjected to analysis for Na+, Ca2+, Mg2+, and K+ using inductively coupled plasma-optical emission spectrometry (ICP-OES, Optima 7000 DV). And, F–, Cl–, NO3–, and SO42− were determined by employing an ion chromatograph (ICP-AES, iCAP6300). The precision of this analysis anion and cation are both within ± 1%. Besides, the HCO3− concentration was measured using the chemical method, with a precision level falling into a range of ± 5%. To guarantee the precision of the data, the charge balance error (%CBE) was calculated for each water sample. The %CBE values of at least 95% of the water sample’s accuracy level should be within a range of ± 5%, indicating that the water quality data were reliable. Fluorine-bearing rocks were extracted through the utilization of an acidic reagent and subsequently analyzed using ion chromatography for fluorine content. The mineral composition was determined by X-ray diffraction (XRD, X’PERT3), while the microstructure of fluorinated minerals were examined using a scanning electron microscope (SEM, SIGAM).
Health Risk Assessment (HRA)
HRA is a quantitative approach that establishes a connection between water environment contamination and its impact on human health, aiming to depict the potential risks posed to individuals when exposed to polluted surroundings. (Chaudhry and Sachdeva 2020; Omonona and Okogbue 2021). It utilizes the internationally recognized procedure and standards established by the US Environmental Protection Agency (EPA) (USEPA 1989).
The procedure comprises four steps, specifically the identification of hazards, analysis of the dose–effect relationship, evaluation of exposure levels, and characterization of risk (Liu et al. 16,17,18,, b, c; Vaiphei and Kurakalva 2021). Fluorine in groundwater or mine water is toxic but not carcinogenic. Typically, the evaluation of the potential risks associated with a toxic substance relies on the reference dose. the non-carcinogen risk assessment model was used as following
In Eq. (1).
\(HI\) is the non-carcinogenic risk index.
\(CDI\) is the average daily exposure dose of a non-carcinogenic substance (mg⋅kg−1⋅d−1).
\(Rf{D}_{i}\) is the reference dose of the noncarcinogenic substance(mg⋅kg−1⋅d−1).
In Eq. (2).
\({C}_{i}\) represents the measured concentration of fluorine in mine water.
IR represents the drinking rate (L⋅d−1).
EF represents the exposure frequency (d⋅a−1).
ED represents the exposure duration (a).
ABW represents average body weight of residents.
AET represents life expectancy (d).
Table 1 shows the parameters of the mine water fluorine health risk assessment model.
Results and Discussion
Hydrochemistry of Mine Water
Table 1 provides 42 groups of the main chemical components of mine water from study area. pH and TDS are crucial parameters reflecting the quality of mine water. As illustrated, the pH levels of water samples obtained from 9 mines range between 7.0 and 10.1, suggesting predominantly slightly alkaline to alkaline conditions. The TDS of the mine water from 9 mining areas is greater than 500 mg/L. Especially, TDS of the three mining areas from BET, SW and HLG is greater than 1000 mg/L, exceeding the quality standards of groundwater and drinking water, and belong to high salinity mine water. The fluorine average concentrations of mine water from BRT, WLML,CCT,BLT, SW and HLG is 8.45, 2.34, 4.75, 4.5, 4.03, 6.36 mg/L respectively, exceeding 1 mg/L, and can be categorized as high -fluorine mine water. The coexistence of high-fluoride and salinity is consistent with reports by Jia, especially in arid or semi-arid inland basins (Jia et al. 2018). Meanwhile, the mean concentration of fluorine of mine water from SGT, DLT and HJT is 0.38, 0.79 and 0.89 mg/L, respectively, that were less than 1 mg/L, belonging to low-fluorine mine water (Table 2).
The piper diagram (Fig. 2a) drawn based on Table 1 is used to describe the overall hydrochemical facies of mine water. The mine water has relatively high proportions of Ca2+ and Na+ for cations, while HCO3−,Cl−and SO42− for anions. Thus, the main water chemical types of mine water include HCO3-Na type, Cl-Na type and SO42−-Ca type. It has been reported that a distinct correlation exists between the chemical types and concentration of fluorine in groundwater (Chen, et al., 2021). Obviously, the mine water of BRT, WLML, CCT, BLG, SW and HLG is high-fluorine mine water, hydrochemical phase is basically HCO3-Na type and Cl-Na type, and the cation is dominated by Na+. And, the mine water in SGT, DLT and HJT is low-fluorine mine water, hydrochemical phase is SO42− -Ca type, and the anion is dominated by Ca2+. It is evident from Fig. 2a that most of high-F mine water is characterized by high-concentration of Na+ and low concentration of Ca2+.
The relationship between Na+/Ca2+ and F− concentration (Fig. 2b) was drawn to study how F− concentration changes as changes in the concentration ratio of Na+ vs Ca2+. It can be seen that the ratio of Na+/Ca2+ was positively correlated with the concentration of F−. The fitted correlation coefficient is 0.63, suggesting that the correlation is well. The low levels of Ca2+ and high levels of Na+ in mine water with high levels of fluorine can be attributed to the formation of CaF2 precipitate through the easy combination of Ca2+ with F−.However, an increase in the sodium ion concentration and a decrease in the calcium concentration of water is conducive to the enrichment of fluorine. This aligns with the preceding result that the concentration of fluoride in groundwater grows with a higher proportion of sodium and calcium (Wang et al. 2023). Meanwhile, many researchers on high-fluorine groundwater mentioned low concentrations of Ca2+ and high concentrations of Na+ are conducive to fluorine enrichment (Guo et al. 2007; Wang et al. 2021a, b).
Characteristics of Fluorine Distribution in Mine Water
The concentration of fluorine in mine water varies significantly both vertically and horizontally. And contour map of fluorine concentration in the study area (Fig. 3a) and a fluorine concentration box diagram of different mines and different coal seams (Fig. 3b) were drawn. Horizontally, the distribution difference in fluorine concentration is obvious. To be specific, The fluorine concentration in mine water of DLT and HJT, in the southeast direction, is lower than 1 mg/L whereas that of BET CCT and WLML range from 3 to 5 mg/L. Besides, the fluorine concentration of SGT mine water is also lower than 1 mg/L. From Fig. 3b, the mean concentration of fluorine in mine water of DLT and HJT were 0.79 and 0.89 mg/L, respectively. Mine water samples from the above two mining areas were produced by 2# coal seams mining. Additionally, the mean fluorine concentration of mine water from BET, WLML, CCT, BLT, and SW was 8.45, 2.34, 4.75, 4.5, 4.03 mg/L, respectively. Mine water samples from the above five mining areas were produced by 3# coal seams mining.
Meanwhile, the distribution of fluorine in mine water also changes significantly in the vertical direction. The fluoride content of 1# coal mine water is 0.7–0.93 mg/L, with a mean of 0.85 mg/L. While the fluoride content of 2# coal mine water varied between 0.22 and 0.68 mg/L, with a mean concentration of 0.38 mg/L. And, the fluoride content of 1#, 2# coal mine water is all less than 1.0 mg/L. Contrarily, the mean fluorine concentration of 3# coal mine water is 10.78 times and 4.82 times as that of 1# and 2# mine water, respectively. To summarize, as the mining depth increases, the fluorine concentration of mine water also increases. In other words, the concentration of fluorine in the mine water of 3# coal mine is higher than that of 2# coal and greater than that of 1# coal. Therefore, the primary source of fluorine in 3# coal mine water will be discussed as follows.
The Main Source of Fluorine in Mine Water
Stratum Source of Fluorine
To find stratum sources of fluorine, surface water and groundwater samples from the main supply sources of mine water were collected, and violin maps (Fig. 4a) were drawn to characterize fluorine concentrations in different types of water samples. As shown, the level of fluoride in surface water and Quaternary groundwater is lower than 1 mg/L, indicating that it does not exceed the standard. The fluorine concentration of 1# coal roof groundwater and 1# mine water, 2# coal roof groundwater, and 2# mine water is also less than 1 mg/L. However, the fluorine concentration of 3# coal roof water and 3# coal mine water exceeds 1 mg/L. This proves that the direct water filling source of mine water is the related coal roof groundwater. Hence, the elevated level of fluoride of 3# coal mine water is strongly associated with groundwater from its roof aquifer.
Li reported that primary source of fluoride in groundwater is derived from fluorinated minerals in sediments (Li, et al. 2020). During prolonged water–rock interactions, fluoride dissolved from rocks is released, thereby increasing fluoride concentrations in groundwater. (Adimalla and Qian 2020). To further confirm the reason for the high-level fluorine of 3# coal mine water, the curves of fluorine content in the rocks of Quaternary, Zhiluo Formation, 1# coal roof, 2# coal roof, and 3# coal roof with depth were drawn. The fluorine content of the Quaternary and Zhiluo formations is 200 ~ 500 mg/kg, and the mean values are 334 and 361 mg/kg, respectively. The fluorine content of 1# coal roof rocks varies from 260 to 307 mg/kg, having a mean content of 284 mg/kg. And, the fluorine content of 2# coal roof rocks ranges from 200 to 350 mg/kg, having a mean content of 313 mg/kg, that of 3# coal roof rocks ranges between 400 and 900 mg/kg, having a mean content of 688 mg/kg. 3# coal roof rock fluorine level is much higher than that in other strata, this result is consistent with the previous one that the contents of water-soluble F− in the shallow sediments were lower than those in deep sediments (Wang, et al. 28,29,, b). High concentration fluorine 3# coal roof rock leads to the root cause of high-fluorine content of 3# coal roof groundwater and 3# coal mine water. The findings confirm that the primary source of fluorine in 3# coal mine water is predominantly derived from 3# coal roof rock. Further discussion is necessary to identify the main minerals containing fluorine.
Mineral Source of Fluorine
Minerals are the basic building blocks of rocks. Thus, we further focused on the fluorine-enriched mineral using X-ray diffraction to analyze mineral composition and using SEM–EDS to study micro-morphology and main element proportion of the main fluorine-containing minerals. The results show that the rock types of 3# coal roof are mainly sandstone, siltstone and mudstone. The main fluorinated minerals in the rocks include fluorapatite, hornblende, illite and kaolinite. As shown in Fig. 5, Fluorapatite is hexagonal columnar while hornblende appeared as a fibrous aggregate, under the scanning electron microscope. Fluorapatite and hornblende belong to silicate minerals and fluorine mainly occurs in the mineral lattice in an insoluble state (Fig. 5a). Besides, the surface of illite and kaolinite exhibited fluorine attachment. The main form of fluorine in clay minerals is an exchangeable state (Fig. 5b).
The increased fluoride concentration in groundwater is primarily attributed to the direct impact of dissolving and releasing fluorine-bearing minerals. The chemical processes implicated in the interaction between water and rocks can be represented by the following equation:
It can be seen from the analysis results in 4.1 that the groundwater environment is weakly alkaline to alkalinity.
Therefore, hydroxide ions can replace fluorine ions in silicate, leading to fluorine enrichment in groundwater. In addition, the presence of OH− can displace fluoride in clay minerals (illite and kaolinite), leading to an increased concentration of fluorine in groundwater.
Health Risk Assessment of Mine Water
The primary route of human exposure to fluorine in mine water is through drinking water potentially affects human health directly (Lacson et al. 2021). Long-term consumption of drinking water with a high-fluorine content can result in dental or skeletal fluorosis (Yadav et al. 2017; Hossain and Patra 2019) Hence, The risk to human health from fluorine in the mine water was assessed using the model suggested by USEPA. As the non-carcinogenic risk threshold HI is set at 1, a HI < 1 signifies that the human health risk associated with fluorine remains within an acceptable range. Conversely, a HI > 1 indicates that fluorine poses an unacceptable level of risk to human health. and there exists a positive correlation between the value of HI and the extent of non-carcinogenic health risks (Malakootian et al. 2020). Since infants and children are more vulnerable than males and females, the exposure parameters of infants, children, males, and females are different in the health risk assessment model. Therefore, the health risks of infants, children, males, and females from mine water intake in the study area are calculated, respectively, according to Eq. (3) and Eq. (4). HI value box chart for infants (Fig. 6a), children (Fig. 6c), males (Fig. 6c), and females (Fig. 6d) were plotted separately.
The calculated HI ranged from 0.2077 to 8.169, 0.2077 to 8.169, 0.2077 to 8.169, 0.2077 to 8.169 for infants, children, males, and females, respectively, with average values of 2.6723, 1.5115, 1.5115, 1.5115, respectively. They all exceed the permissible limit (HI = 1), and fluoride intake presents an intolerable risk to human well-being including infants, children, males, and females. When the fluorine intake object is the same, the HI value of mine water from different mining areas is also different. Take infants for example, the ranking of infants’ average HI value is as follows: HLG > BRT > CCT > BLT > SW > WLML > HGT > DLT > SGT. Among them, drinking mine water of SGT, DLT, and HJT does not pose health risks to infants, while drinking mine water of HLG, BRT, CCT, BLT, SW, and WLM will present a potential hazard to infants. The ranking of children, males, and females is the same as infants.
The evaluation results show that intake of mine water from HLG, BRT, CCT, BLT, SW, WLML, and HJT are more risker than intake of mine water from SGT, DLT, and HJT, due to fluorine concentration of A being higher than that of B.
To conduct a more comprehensive analysis of the health hazards associated with mine water in the Shendong mining area, the health risk zoning map of the mining area is developed based on HI value. The spatial distribution of the HI for different groups can be observed in Fig. 7. Concerning previous research results (Liu et al. 2021a, b), we defined a HI index of less than 3 as a low-risk area, a HI index of 3–5 as a medium-risk area, and a HI index greater than 5 as a high-risk area. Comparing Fig. 7a and b, it is found that the risk of mine water to infants is greater than that to children, which is consistent with the above analysis results. In addition, the high-risk areas for infants, children, males and females are the same, mainly in BRT and CCT mines. The middle-risk areas are mainly distributed in BLT, SW, WLML, and HLG. The low-risk areas are primarily found in SGT, HJT, and DLT. Compared with the fluorine concentration diagram in Fig. 3, it can be seen that there exists a positive correlation between the level of fluorine in mine water and the HI index. In other words, the health risk of mine water to the human body is positively correlated with the level of fluorine in mine water, and the higher the concentration of fluorine, the higher the induced health risk. This is consistent with the research conclusion of Liu, and he put forward that pollutant concentration is the main controlling factor that threatens human health (Liu et al. 2021c) (Fig. 7).
Based on the calculation results of HI of mine water, it can be seen that the health risk induced by mine water in the northwestern minefield of the study area is large. For residents, if mine water is not treated, it will cause great harm no matter whether it is discharged or directly used. Therefore, the mine water must be defluorinated to reduce the health risk to the local population.
Based on the calculation results of HI of mine water, it can be seen that health risk induced by mine water in the northwestern mine of the study area is high. If mine water is not treated, it will cause great harm to local residents whether it is discharged or directly used.
Fluorine Removal in Mine Water
According to the above conclusions, mine water fluorine treatment should be strengthened. And the effective removal of fluorine from mine water is essential to optimize the sustainable utilization of mine water resources. At present, the predominant treatment techniques for water contaminated with fluorine encompass precipitation, adsorption, and membrane separation technologies (Tang and Zhang 2015). The precipitation method encompasses chemical precipitation and coagulation precipitation. In the chemical precipitation method, limestone or calcium chloride is added to facilitate the reaction between calcium and fluoride ions, resulting in CaF2 precipitation. This process can reduce the residual fluorine content to approximately 1% of its original mass concentration. However, the continuous addition of lime water may decrease the sediment formation rate and maintain a minimum level of 1% fluorine content in water. Therefore, this method is primarily employed for treating high-concentration industrial wastewater (with a fluoride concentration greater than 12 mg/L). The coagulation precipitation method utilizes coagulants to generate positively charged colloidal particles that adsorb fluoride ions from water. These colloidal particles then aggregate into larger flocculent precipitates for effective fluorine removal. This approach suits fluctuation-free fluorine-containing water but cannot directly reduce fluoride levels to 1 mg/L using a single technique. The adsorption method relies on physical and chemical adsorbents to achieve a final fluoride concentration below 1 mg/L. It is particularly applicable when dealing with low-fluoride-concentration water or wastewater that still exhibits a higher-than-desired fluoride concentration after undergoing precipitation treatment while maintaining low treatment costs. Considering the advantages and disadvantages of various fluoride removal processes mentioned above along with large volumes of stable-fluoride-concentration mine water, we propose employing a combined approach involving coagulation precipitation followed by adsorption to remove fluorine. Simultaneously, this treatment method can be applied to remove fluoride from groundwater or mine water in other regions where concentrations range from 1 mg/L to 10 mg/L.
Conclusions
The efficient utilization of mine water is crucial for the mining area in Northwestern China. This research studied the hydrochemistry and primary sources of high-fluorine mine water, as well as the potential health risks associated with fluorine contamination.
The average concentration of fluorine in the study area is mostly greater than 1 mg/L, belonging to high-fluorine mine water, The hydrochemical phase of high-fluorine mine water is HCO3-Na and Cl-Na type, with elevated levels of Na+ and reduced levels of Ca2+. Fluoride concentrations of mine water continuously increase along the depth of coal mining. Therefore, 1# and 2# mine water have low fluoride levels, while 3# mine water has high-fluoride levels. Closed hydrogeological conditions and long-term water–rock interactions in confined aquifers favor F– accumulation in 3# coal roof groundwater, resulting in F– enrichment of 3# coal mine water.
Health risk assessment results show that if mine water from SGT, DLT and HJT will not pose health risks to infants, children, males, and females, while mine water from HLG, BRT, CCT, BLT, SW, WLM will present a potential hazard to infants, children, males, and females. The health risks of mine water posed to the human body are strongly associated with fluorine concentration of mine water. These results suggest the necessity for addressing the treatment of mine water quality to satisfy living and drinking water quality standards and a health risk assessment system suitable for residents. Considering the characteristics of various fluoride removal processes and fluorine concentration of mine water, we propose employing a combined approach involving coagulation precipitation followed by adsorption to remove fluorine. This fluorine treatment technology has implications for similar concentrations of mine water and groundwater elsewhere in the world.
Data Availability
This manuscript contain associated data.
References
Adimalla N, Qian H (2020) Spatial distribution and health risk assessment of fluoride contamination in groundwater of Telangana: a state-of-the-art. Geochemistry 80(125):548. https://doi.org/10.1016/j.chemer.2019.125548
Amini M, Mueller K, Abbaspour KC et al (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42:3662–3668. https://doi.org/10.1021/es071958y
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487. https://doi.org/10.1080/10643380600678112
Chaudhry AK, Sachdeva P (2020) Groundwater quality and non-carcinogenic health risk assessment of nitrate in the semi-arid region of Punjab, India. J Water Health 18:1073–1083. https://doi.org/10.2166/wh.2020.121
Chen J, Gao Y, Qian H et al (2021) Hydrogeochemical evidence for fluoride behavior in groundwater and the associated risk to human health for a large irrigation plain in the Yellow River Basin. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.149428
Guo QH, Wang YX, Ma T et al (2007) Geochemical processes controlling the elevated fluoride concentration in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor 93(1):1–12
He XD, Li PY, Ji YJ et al (2020) Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: occurrence, distribution and management. Exposure Health 12:355–368. https://doi.org/10.1007/s12403-020-00347-8
Hossain M, Patra PK (2019) Hydrogeochemical characterization and health hazards of fluoride enriched groundwater in diverse aquifer types. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113646
Jha SK, Singh RK, Damodaran T et al (2013) Fluoride in groundwater: toxicological exposure and remedies. J Toxicol Environ Health B Crit Rev 16:52–66. https://doi.org/10.1080/10937404.2013.769420
Jia Y, Xi B, Jiang Y (2018) Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: a review. Sci Total Environ 643:967–993
Kumar M, Das N, Goswami R et al (2016) Coupling fractionation and batch desorption to understand arsenic and fluoride co-contamination in the aquifer system. Chemosphere 164:657–667. https://doi.org/10.1016/j.chemosphere.2016.08.075
Lacson CFZ, Lu MC, Huang YH (2021) Fluoride-containing water: a global perspective and a pursuit to sustainable water de-fluoridation management an overview. J Clean Prod 280:124236. https://doi.org/10.1016/j.jclepro.2020.124236
Li CC, Gao XB, Wang YX (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165. https://doi.org/10.1016/j.scitotenv.2014.11.045
Li JX, Zhou HL, Qian K et al (2017) Fluoride and iodine enrichment in groundwater of North China Plain: evidences from speciation analysis and geochemical modeling. Sci Total Environ 598:239–248. https://doi.org/10.1016/j.scitotenv.2017.04.158
Li JX, Wang YT, Zhu CJ et al (2020) Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci Total Environ 730:138877
Liu JT, Peng YM, Li CS et al (2021a) Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.115947
Liu JT, Gao ZJ, Zhang Y et al (2021b) Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.129164
Liu J, Peng Y, Li C et al (2021c) An investigation into the hydrochemistry, quality, and risk to human health of groundwater in the central region of Shandong Province, North China. J Clean Prod 282:125416
Malakootian M, Mohammadi A, Faraji M (2020) Investigation of physicochemical parameters in drinking water resources and health risk assessment: a case study in NW Iran. Environ Earth Sci 79(9):195
Omonona OV, Okogbue CO (2021) Hydrochemical evolution, geospatial groundwater quality and potential health risks associated with intake of nitrate via drinking water: case of Gboko agricultural district, central Nigeria. Environ Earth Sci. https://doi.org/10.1007/s12665-021-09448-2
Podgorski J, Berg M (2022) Global analysis and prediction of fluoride in groundwater. Nat Commun. https://doi.org/10.1038/s41467-022-31940-x
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736. https://doi.org/10.1007/s00254-002-0672-2
Tang D, Zhang G (2015) Efficient removal of fluoride by hierarchical ce–fe bimetal oxides adsorbent: thermodynamics, kinetics, and mechanism. Chem Eng J 283:721–729
USEPA (1989) Risk assessment guidance for superfund volume I: human health evaluation manual (part A). Office of Emergency and Remedial Response, Washington
Vaiphei SP, Kurakalva RM (2021) Hydrochemical characteristics and nitrate health risk assessment of groundwater through seasonal variations from an intensive agricultural region of upper Krishna River basin, Telangana, India. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2021.112073
Wang YX, Shvartsev SL, So CL et al (2009) Genesis of arsenic/fluoride-enriched soda water: a case study at Datong, northern China. Appl Geochem 24:641–649. https://doi.org/10.1016/j.apgeochem.2008.12.015
Wang YX, Li JX, Ma T et al (2021a) Genesis of geogenic contaminated groundwater: As, F and I. Crit Rev Environ Sci Technol 51:2895–2933. https://doi.org/10.1080/10643389.2020.1807452
Wang Z, Guo HM, Xing S et al (2021b) Hydrogeochemical and geothermal controls on the formation of high fluoride groundwater. J Hydrol 598(1–2):126372
Wang TT, Xue JK, Shang HB et al (2022) Fluorine pollution characteristics and formation mechanism of mine water in Shaanxi and Inner Mongolia Contiguous Area. J China Coal Soc 47(11):1743–1749 (in Chinese)
Wang TT, Jin DW, Xue JK et al (2023) Source and release mechanism of fluorine in coal mine goaf water-rock system, Shaanxi and Inner Mongolia contiguous area. Coal Geol Explor 51(2):1–11. https://doi.org/10.12363/issn.1001-1986.22.11.0855. (in Chinese)
WHO (2004) Fluoride in drinking-water: background document for development of WHO guidelines for drinking-water quality, Geneva
Xiong XZ, Liu JL, He WH (2007) Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res 103:112–116. https://doi.org/10.1016/j.envres.2006.05.008
Xu F, Li P, Du Q et al (2022) Seasonal hydrochemical characteristics, geochemical evolution, and pollution sources of lake sha in an arid and semiarid region of Northwest China. Exposure Health 15(1):231–244. https://doi.org/10.1007/s12403-022-00488-y
Yadav KK, Singh JK, Gupta N et al (2017) A review of nano bioremediation technologies for environmental cleanup: a novel biological approach. J Mater Sci Technol 8:740–757
Yadav KK, Kumar S, Pham QB et al (2019) Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2019.06.045
Zabala ME, Manzano M, Vives L (2016) Assessment of processes controlling the regional distribution of fluoride and arsenic in groundwater of the Pampeano Aquifer in the Del Azul Creek basin (Argentina). J Hydrol 541:1067–1087. https://doi.org/10.1016/j.jhydrol.2016.08.023
Zhang Z, Li GQ, Su XB et al (2021) Geochemical controls on the enrichment of fluoride in the mine water of the Shendong mining area, China. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.131388
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No.42307113, 52204262) the Natural Science Foundation of Shaanxi province (2023-JC-QN-0291) and National Key Research and Development Program of China (2023YFC3700067).
Author information
Authors and Affiliations
Contributions
Tiantian Wang: Analyziing data,writing original manuscript. Wei Zhao, Zihan Wang, and Zhenfang Zhou: Conducting simulation experiment, analyzing data, editing. Jian Yang: Reviewing, editing. Feng Xu, Jiankun Xue and Guoqing Li: Water sampling and testing.
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that there is no conflict of interest present.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Zhao, W., Wang, Z. et al. Occurrence, Main Source and Health Risks of Fluorine in Mine Water. Expo Health (2024). https://doi.org/10.1007/s12403-024-00660-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12403-024-00660-6