Abstract
Humans are widely exposed to multiple metals, some of which are suspected to be hepatotoxic, and diabetes mellitus (DM) is a risk factor for chronic liver disease. This cross-sectional study aimed to explore the association of multi-metal exposure and liver function in elderly with diabetes mellitus. We enrolled 1663 subjects aged ≥ 60 years and measured the levels of 22 plasma metals and liver function parameters (total bilirubin (TBIL), alanine transaminase (ALT), and aspartate transaminase (AST). Generalized linear regression model (GLM) and Bayesian kernel machine regression (BKMR) were used to investigate the correlations of multi-metal combined exposure with liver function parameters in the elderly. Multivariate GLM results shown that plasma Fe was positively related with TBIL and plasma Ti and Sr were negatively associated with TBIL in DM elderly. Positive associations were found between Ti-AST and Sn-ALT. In BKMR model, multi-metal combined exposure showed a “U” sharp association with TBIL in overall elderly and an inverse association in DM elderly. Further analysis of the BKMR model revealed that Fe, Ti, and Sr combination exposure had interaction effect with TBIL in the overall and non-DM groups; however, no interaction effect was found in the DM elderly group. Our results suggested that combined exposure of plasma Fe, Ti, and Sr was inversely associated with TBIL in elderly with DM. Potential mechanisms of the complex metal effects on liver function in DM elderly deserve further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal exposure in the environment was increasing with the development of industrialization and urbanization (Caylak and Tokar 2012; Yang et al. 2017). Metals are easily absorbed by humans and accumulated in the body, causing serious and irreversible hazards in human health (Astuti et al. 2022; Mallongi et al. 2022); for example, lead and cadmium induce adverse health effects on blood and kidneys (Caylak and Halifeoglu 2007; Li et al. 2022). The elderly are exposed to multiple metals via different modes, and liver function is important for digestive, immunologic, and hematopoietic processes. The proportion of the elderly population increases worldwide and their health problems, especially liver function, should not be ignored (Shen et al. 2021).
The ability of the liver to regenerate decreases with increasing age; in particular, the elderly are more susceptible to chronic liver diseases, such as chronic liver fibrosis and non-alcoholic fatty liver disease (Hoare et al. 2010; Kim et al. 2015), which could reduce their immune response capacity. Total bilirubin (TBIL), alanine transaminase (ALT), and aspartate transaminase (AST) are common proteins and enzyme biomarkers in the blood that are used to evaluate hepatic function (McGill. 2016; Gholizadeh et al. 2020). Serum TBil is an important indicator of hepatobiliary and hemolytic diseases (Leem et al. 2018). Abnormal TBIL level can induce hepatocellular injury (Hamoud et al. 2018). The level of AST and ALT suggest the integrity of hepatocytes. Serum levels of AST and ALT increase when hepatocellular injury or death (Andria et al. 2013; Tang et al. 2016). In this regard, factors associated with liver function should be explored, such as lifestyle, exercise, nutrition, and environment (Yang et al. 2020a; Zhang et al. 2020).
Increasing lines of evidence indicate that environmental metal exposures were associated with hepatic function and liver toxicity (Cave et al. 2010; Chung et al. 2020). Many heavy metals accumulate in water and soil, and then enter the human body through drinking water, diet, and other ways, causing health damage (Anwar et al. 2020; Astuti et al. 2021a). Epidemiological studies revealed that increasing serum bilirubin level was positively associated with plasma Fe but negatively associated with plasma Mo in manganese-exposed workers (Ge et al. 2020). Previous study also reported that blood Cd exposure had significant positive statistical correlations with serum ALT in 12,099 non-professional Korean adults (Park et al. 2021). Meanwhile, increasing urine Hg was positively associated with serum AST in 4582 US adults (Cave et al. 2010). Animal studies also found acute exposure to Cd and Pb led to higher levels of bilirubin and cell damage (Andjelkovic et al. 2019). Acute Cd exposure also increased the serum AST and ALT levels in mice (Vicas et al. 2021).
Humans exposed to many metals (Astuti et al. 2021b), but previous studies of hepatotoxicity mostly focused on single metals and young people. In elderly, metal exposure could reduce gluconeogenesis and induce the accumulation of liver fat, resulting in hepatic steatosis (Allaire and Gilgenkrantz 2020). Diabetes mellitus (DM) is a chronic multi-risk metabolic disease characterized by hyperglycemia, with an incidence of 8.8% all over the world (Koye et al. 2018; Carlsson. 2019). Liver function injury induced by DM is a serious health problem; as one of the main mechanisms involved, oxidative stress causes the disorder of glucose and lipid metabolism and leads to hepatocyte apoptosis and necrosis (Xu et al. 2018; Yang et al. 2020a, b). Researches on the effects of multi-metal combined exposure on liver function in elderly patients with DM are relatively limited. Hence, the correlation between multi-metal exposure and liver function parameters, especially in elderly with DM, should be investigated.
This study based on Boshan elderly cohort study (BSECS) aimed to explore the associations of multi-metal exposure with liver function in elderly, especially those with DM. We evaluated the mixture of metals that might affect liver function, determined their dose–effect relationship, and identified the metals that mainly affected the liver function.
Materials and Methods
Study Design and Populations
This cross-sectional study based on the ongoing BSECS, which is a prospective cohort in Boshan established in August 2020. Boshan, located in the east of China, the center of Shandong province, is an industrial city with an aging population. All the study participants were elderly aged ≥ 60 years. A total of 1708 participants were recruited, and those with integrated information on baseline characteristics, medical examinations, and lifestyles such as smoking habits, drinking habits and exercise were considered. After excluding participants those with missing basic information, biometric sample, and a history of medication for liver disease within 2 weeks before the physical examination, a diagnosis of hepatitis B, and non-type 2 DM, 1663 elderly individuals were finally obtained for the present study.
Peripheral venous blood (5 mL) was obtained one times from the participants after overnight fasting when elderly was taking physical examination. Plasma samples, which were separated from blood, stored in a refrigerator at − 80 °C until metals were measured. This study was approved by the medical ethics committee of Shandong First Medical University (ID: R202103030030). Each participant signed an informed consent form prior to the interview and biometric sample collection.
Liver Function Testing and Diagnosis of DM
The liver function of the elderly was assessed by serum TBIL, ALT, and AST, which were measured by an automatic biochemistry analyzer (NT1000, NEUSOFT, Nan Jing, China). The reference intervals of these liver function indices in adults are as follows: TBIL = 3.4–17.1 mmol/L, AST = 8–40 U/L, and ALT = 5–40 U/L. All the diabetic patients included in our study had type 2 DM. The diagnostic criteria of DM are as follows: (1) 8 h fasting plasma glucose (FPG) ≥ 7.0 mmol/L; HbA1c1evel ≥ 6.5%; and/or 2 h plasma glucose level ≥ 11.1 mmol/L during a 75-g oral glucose tolerance test (OGTT) (Yang et al. 2020b); (2) medical diagnosis of DM; and (3) intake of anti-diabetic medications.
Plasma Metal Determination
The plasma metals were measured by inductively coupled plasma mass spectrometry (ICP-MS, TQ; Thermo): magnesium (Mg), aluminum (Al), calcium (Ca), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), selenium (Se), rubidium (Rb), strontium (Sr), molybdenum (Mo), cadmium (Cd), titanium (Ti), antimony (Sb), barium (Ba), stannum (Sn), and lead (Pb). The plasma sample was diluted by acidic solution containing 1% nitric acid (Fisher), 5% n-butyl alcohol (Across, Denmark), and 0.01% Triton (Triton X-100, Inc.). Metal detection was carried out according to the protocol we previously reported (Hou et al. 2019). Standard reference materials, namely, certified reference materials (ClinChek® human plasma controls for trace elements Level 1 and Level 2, no.8885; Recipe Chemicals), were selected for quality control. These standard reference materials were tested for each of the 25 samples to ensure the stability of the results. The concentration of 22 plasma metals lower than the limit of detection (LOD) was filled with LOD/\(\sqrt{2}\).
Assessments of Covariates
Data on socio-demographics and lifestyle were collected by experienced interviewers using face-to-face interviews. The baseline information of the participants included age, sex, height, weight, and body mass index (BMI, weight in kilograms divided by height in meters square). Lifestyle habits included smoking status (smoking at least once each week for more than 6 months), drinking status (drinking at least one time per week for more than 6 months), and exercise frequency (never, ≥ 1 times/week and 1 times/day). Total cholesterol (TC), Triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), which affects liver function parameters, were determined by an automatic biochemistry analyzer (NT1000, NEUSOFT, Nan Jing, China).
Statistical Analysis
In the descriptive statistical analysis of baseline and clinical characteristics, continuous variables were described by mean ± standard deviation for normally distributed data or median (25th, 75th) for non-normally distributed data. Categorical variables were described by frequency (percent). Wilcoxon rank-sum test and Chi-square test were used to calculate differences in baseline characteristics. The concentrations of plasma metals were presented as median (25th, 75th) and were normalized because they were skewed distributions. Considering that DM could affect liver function parameters (Ballatori 1991; Tien Tai et al. 2022), we divided the participants into two groups, namely DM and non-DM.
Spearman’s analysis was used to explore the correlations among 22 plasma metals. The univariate generalized linear regression model (GLM) was used to explore the linear associations between single-metal exposure and liver function parameters. False discovery rate (FDR) was used to correct all results. Data were considered statistically significant at 5% FDR-adjusted p-value. Least absolute shrinkage and selection operator (LASSO) was used to select plasma metals that were independently associated with TBIL, AST, and ALT (Vriens et al. 2017). In the LASSO regression model, lambda (λ) with the minimum mean square error predicted from tenfold cross-validation was chosen as the optimal penalty parameter. We used multivariate GLM to explore the association between plasma metals (selected by both LASSO model and univariate GLM) and liver function indicators. The results were shown as Beta and its 95% confidence interval (CI). We also divided the plasma metal concentrations into tertiles and estimated the associations between liver function parameters and the plasma metals concentrations, and the first tertiles was referred as reference. The median tertiles concentration was treated as a continuous variable to calculate the linear trend. Previous studies have shown that age, sex, BMI, smoking, drinking, TC, TG, HDL-C, and LDL-C are all factors affecting liver function (Yang et al. 2020a; Zhang et al. 2020), so they were included as adjustment factors in our study model. A novel environmental pollution statistical approach, namely, Bayesian Kernel machine regression (BKMR) (Bobb et al. 2018), was used to explore the joint effect of 22 plasma metals on TBIL, AST, and ALT. The plasma metals correlated with liver function parameters as determined by LASSO and univariate GLM were included in the BKMR model and adjusted with the same factors as in the GLM model. In BKMR model, the importance of plasma metals was assessed by posterior inclusion probabilities (PIP). When the other plasma metal concentrations were fixed at the median, the univariate exposure–response curve of plasma metals (95% credible intervals) with liver function parameters was presented. We evaluated the combined effect and potential interactions among the plasma metals by calculating the expected changes in the liver function parameters in relation to particular quartiles in all the plasma metal mixtures from their median levels.
All statistical analyses were performed by R software (version 4.1.0. R Foundation for Statistical Computing). Associations were considered statistically significant as two-tailed P < 0.05.
Results
Baseline Characteristics and Plasma Metal Levels
The baseline and clinical characteristics of the 1,663 elderlies (male: 44.3%, female: 55.7%) were described in Table 1. The average age of the elderly was 69.33 ± 7.93 years old, and 40.8% of them exercised ≥ 1 times /week. Most of them self-reported that they were not currently smoking (83.0%) and drinking (75.8%). The morbidity of DM in our study population was 21.9%. The average baseline levels of TBIL, AST, and ALT were 14.57 ± 5.28 (μmol/L), 29.55 ± 10.72 (U/L), and 24.05 ± 12.42 (U/L), respectively (Table 1). The details of other clinical characteristics such as BMI, TG, HDL-C, and LDL-C are shown in Table 1. The detection rates of 22 plasma metals were all above 95.3% (Table S1). The Spearman’s rank-order correction analysis of 22 plasma metal concentrations is shown in Fig. S1. Results showed that some plasma metals were correlated significantly with one another (− 0.487 ≤ r ≤ 0.736 in overall, − 0.493 ≤ r ≤ 0.747 in non-DM group, and − 0.329 ≤ r ≤ 0.733 in DM group).
Associations of Single Metals with TBIL, ALT, and AST
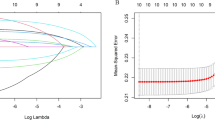
In the univariate linear models, TBIL was negatively associated with plasma Ti, V, Zn, and Sr and positively associated with plasma Cr, Fe, Ni, Rb, and Sb; AST had negative correlations with plasma Cr, Fe, Ni, Co, Cu, and Sb and positive correlations with plasma Ti, V, Zn, Sr, and Cd; ALT was negatively associated with plasma Zn and positively associated with plasma Sn (Fig. 1, Tables S2, S3). However, plasma Fe, Co, Cu, Zn, and Cd had no statistical association with AST at the 5% FDR (Fig. 1). Based on the categorical analysis results, the trends for the three liver function parameters across increasing tertiles of plasma metals were consistent with the univariate linear models (Tables S4–S9). All the models were adjusted by age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C. The LASSO penalized regression analysis selected independent metals associated with TBIL, ALT, and AST (Fig. 2 and Table S10). Plasma Fe, Ti, and Sr were selected by LASSO regression as the significant stressors of TBIL (Fig. 2A); and plasma Ti and Sn were identified as the significant stressors of AST (Fig. 2B) and ALT (Fig. 2C), respectively.
The univariate linear associations of plasma metals and liver function indexes in overall and subgroups. Model A overall group; Model B non-diabetes mellitus group; Model C diabetes mellitus group. All the models: single metal was incorporated in the GLM separately adjusting for age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C. Except for themselves, each group was adjusted by the other covariates. Red marks replaced positive association, blue marks replaced negative association, and black marks replaced no statistical significance. FDR false discovery rate-adjusted p-values
The prediction error of the LASSO regression model as a function of the penalty parameter (log λ) (the figure at right) and the LASSO solution path with metal coefficient profiles based on the penalty parameter (log λ) (the figure at left). For each metal, the vertical red line represents the cross-validated optimum of λ (minimum mean-squared error), and forced variables and several metals were selected at the λ indicated by the vertical red line. As indicated by the vertical red line, A Fe, Ti, and Sr were selected at the lambda (λ = 0.496) for the analysis of TBIL; B Ti was selected at the lambda (λ = 3.161) for the analysis of AST; C Sn was selected at the lambda (λ = 1.033) for the analysis of ALT
RCS analysis was used in the overall, DM, and non-DM groups to further explore the dose–effect relationship of single plasma metals and liver function indicators. Plasma Ti, V, Cr, Fe, Ni, Zn, and Rb (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05) had non-linearity dose–effect relationship, and plasma Sr and Sb (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) had a linearity dose–effect relationship with TBIL in the overall group. The curves showed that plasma Ti, V, Cr, Ni, Cd, and Sb (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05) had non-linearity relationship to AST and a linearity relationship was found between plasma Zn and Sn (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) and ALT in the overall group (Fig. S2A). In the DM group, plasma Ti, V, Fe, Ni, Zn ,and Rb (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05) had non-linearity dose–effect relationship and plasma Cr, Sr, and Sb (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) had linearity dose–effect relationship to TBIL (Fig. S2C). The curves showed that plasma Ti, V, Ni, and Sb (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05) had non-linearity relationship and Cr and Cd had linearity relationship to AST. A linearity relationship was also found between plasma Zn and Sn (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) and ALT (Fig. S2C). In the non-DM group, plasma V, Ni, Zn, Cr, and Sr had non-linear relationships (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05) and plasma Ti, Fe, Rb, and Sb had a linear relationship to TBIL (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) (Fig. S2B). Plasma V, Ti, Ni Cr, Cd, and Sb had a non-linear relationship to AST (all of Poverall association < 0.001, all of Pnonlinearity association < 0.05), and plasma Zn and Sn had significant linear relationship to ALT (all of Poverall association < 0.001, all of Pnonlinearity association > 0.05) (Fig. S2B).
Associations of Multi-metal Combined Exposure and TBIL
Given that the LASSO regression model only selected one metal associated with AST and ALT, we only performed multi-linear regression analysis for TBIL. The plasma metals, which selected by LASSO regression and univariate linear models, were incorporated into the multivariate GLM regression model to explore associations with TBIL (Table 2). Each group contained unadjusted and adjusted models. The results of the adjusted models showed that plasma Ti (β = − 0.15, 95%CI − 0.21, − 0.10) and Sr (β = − 0.07, 95%CI − 0.12, − 0.03) were negatively associated with TBIL and plasma Fe was positively associated with (β = 0.28, 95%CI 0.23, 0.33) TBIL (Table 2). The results of the unadjusted model were consistent with those of the adjusted model (Table 2) and after FDR correction, the results were also had statistical significance. Moreover, we investigated the association between plasma metals and liver function parameters in non-DM and DM groups. In both groups, plasma Ti (non-DM: β = − 0.16, 95%CI − 0.23, − 0.09; DM: β = − 0.13, 95%CI − 0.24, − 0.02) was negatively related with TBIL and plasma Fe was positively correlated with TBIL (non-DM: β = 0.29, 95%CI 0.24, 0.35; DM: β = 0.26, 95%CI 0.16, 0.36). Meanwhile, only plasma Sr (β = − 0.15, 95%CI − 0.25, − 0.06) had negative association with TBIL in the DM groups. The categorical analysis results indicated the significant dose-dependent increasing trends for TBIL across increasing tertiles of plasma Fe, while decreasing trends were observed for TBIL across increasing tertiles of plasma Ti and Sr (P-trend values < 0.05) (Table 3). After FDR correction, the results were also had statistical significance (Table 3). All the models were adjusted by age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C. Each group was adjusted by the other covariates except itself. The adjusted factors were consistent with the overall groups.
The BKMR model was used to explore the combined effects of multi-metal exposure and TBIL. The posterior inclusion probability (PIP) levels for the BKMR analyses were 1.000 (Ti), 1.000 (Fe) and 0.1648 (Sr) in the overall group (Table S11). The BKMR results showed the overall effect of cumulative metal mixture on TBIL. When all of the metals were set with their 70th value as reference, the relationship between the three metals on TBIL showed an “U” shape association and the overall effect was lowest at 0.4 quantile in the overall and non-DM groups (Fig. 3); by contrast, a negative relationship was found between the three metals and TBIL in the DM group (Fig. 3). The multi-metal combination exposure (Ti, Fe, and Sr) had interaction effect in the overall and non-DM groups. However, no interaction was found between the metals (Ti, Fe and Sr) combined exposure and serum TBIL in the DM group (Fig. 4). The bivariate exposure–response function showed that plasma Ti and Sr had interaction effect on TBIL in the overall and non-DM groups, but not in the DM group (Fig. 5). The univariate metal exposure–response revealed that plasma Ti and Sr had negative dose–effect relationship to TBIL in each group. However, plasma Fe had positive dose–effect relationship to TBIL in the overall and non-DM groups and. showed a reverse U-sharp association with TBIL in the DM group (Fig. 6). Interestingly, all of the univariate metal exposure–response results were consistent with the single-metal dose–response relationships in the RCS model (Fig. S2).
The overall effect of cumulative mixture with TBIL. The lines were overall effect of three metals mixtures (estimates and 95% credible intervals) on TBIL level, when all of exposures were at a particular quantile as compared to when all of them were at their 70th value. Model A overall group; Model B Non-diabetes mellitus group; Model C diabetes mellitus group. Adjusted factors: age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C in elderly. Except for themselves, each group was adjusted by the other covariates
A comparison of the risks associated with a single exposure based on all of the other exposures' 75th percentiles versus their 25th percentiles. Model A overall group; Model B Non-diabetes mellitus group; Model C Diabetes mellitus group. Adjusted factors: age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C in elderly. Except for themselves, each group was adjusted by the other covariates
Bivariate exposure–response function for pairwise metals: the correlation between plasma metals and TBIL when plasma metal fixed at 25th (red), 50th (green) and 75th (blue) percentile, and the remained plasma metals were held at the median. Model A overall group; Model B Non-diabetes mellitus group; Model C Diabetes mellitus group. Adjusted factors: age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C in elderly. Except for themselves, each group was adjusted by the other covariates
Univariate exposure–response functions for each plasma metal (95% confidence interval) and serum TBIL with the other two plasma metals fixed at the median value. Blue line acted as estimates and gray band acted as 95%CI. A overall group; B Non- diabetes mellitus group; C Diabetes mellitus group. Adjusted factors: age, sex, BMI, smoking, drinking, TC, TG, LDL-C, and HDL-C in elderly
Discussion
For the first time, this study reported that the combined exposure of plasma Ti, Fe, and Sr showed an “U” sharp association with TBIL in the overall and non-DM elderly and had an inverse association with TBIL in the DM elderly. For the univariate exposure–response functions in the overall group, TBIL was positively correlated with plasma Fe and negatively associated with plasma Ti and Sr. Moreover, positive associations were found between Ti-AST and Sn-ALT. In the DM elderly, except for plasma Fe, Ti, and Sr showed an inverse “U” sharp association with TBIL; the other findings were in accordance with those in the overall and non-DM elderly.
We used different models to identify the most significant metals statistically associated with liver function parameters. Table S12 summarizes the results of the LASSO, GLM, and BKMR regression analyses. Based on the LASSO and GLM regression, Fe, Ti, and Sr were associated with TBIL; Ti was associated with AST; and Sn was associated with ALT. The BKMR model identified plasma Fe, Ti, and Sr as important contributors to TBIL. The association between the three metals (Fe, Ti, and Sr) and TBIL was U-shaped in the overall and non-DM groups and negative in the DM group. Moreover, multi-metal combination exposure (Ti, Fe and Sr) had interaction effect in the overall and non-DM groups. The bivariate exposure–response function showed that plasma Ti and Sr had interaction effect on TBIL in the overall and non-DM groups. In the DM group, plasma metals had no interaction effects with TBIL upon multi-metal combination exposure and bivariate exposure–response function. Moreover, the dose–effect relationship of single-metal exposure and liver function parameters was in accordance with the univariate metal exposure–response in the multi-metal exposure model. The BKMR model is a multi-pollutant approach that brings together highly correlated pollutants and allows estimate non-linear, nonadditive exposure–response functions, as well as joint and interactive correlations between pollutants and health (Bobb et al. 2018). In general, the results of single-metal dose–effect relationship and BKMR regression were relatively consistent.
Our study firstly found that plasma Ti had a negative correlation with serum TBIL but had positive association with serum AST. Compared with previous studies, the serum levels of TBIL, ALT, and AST in the present study were relatively higher than the normal median level (Wang et al. 2017; Edvardsson et al. 2018). The concentration of plasma Ti (median = 40.39 μg/L) was also higher than in other works (median = 11.8 and 12.07 μg/L) (Hou et al. 2019; Ge et al. 2020). Hydrogen peroxide (H2O2) and other oxidative stress markers (such as superoxide dismutase) in the liver significantly increased in rats exposed to titanium dioxide nanoparticles (TiO2 NPs) by intraperitoneal injection (Duan et al. 2010; Wani et al. 2021). Previous studies revealed that TiO2 NPs increased the expression of oxidation products such as H2O2 and decreased the antioxidant activity of glutathione (GSH) (An et al. 2020). Therefore, we could speculate that elevated plasma Ti levels aggravate the oxidative stress capacity of the liver, leading to liver function damage and metabolic imbalance of liver function parameters. Bilirubin is an endogenous antioxidant and a lipophilic hydrogen peroxide scavenger that can be oxidized to biliverdin, which is mediated into circulation by biliverdin reductase (BVR) to amplify the antioxidant effect (Shum et al. 2021; Stocker et al. 1987). Therefore, we supposed that the negative association between plasma Ti and TBIL was correlated with the ability of bilirubin to remove hydrogen peroxide (Shum et al. 2021). Moreover, Yang et al. revealed that TBIL in mouse had a positive dose–dependent relationship to exposure to TiO2 NPs (Yang et al. 2017); that is, exposure to high doses of TiO2 NPs caused complicated diseases and increased the activities of AST and ALT. Hence, AST and ALT are the special markers of serious inflammation or liver injury. Wang et al. (2007) revealed that the accumulation of TiO2 NPs in mouse liver led to severe histopathological changes, such as hydropic degeneration and liver damage, thereby increasing the levels of serum AST and ALT. The results of our present epidemiology study were consistent with those of previous animal studies, although the exposure dose of Ti in mice was higher than in human (Brand et al. 2020). Therefore, follow-up studies should be conducted to explore the possible mechanism.
In this study, plasma Fe had a positive dose–effect relationship to serum TBIL in elderly. Bilirubin is the main end-product of heme catabolism and heme synthesis is one of the major pathways of iron utilization; as such, elevated plasma Fe levels could promote the conversion of heme to bilirubin (Kronenberg. 2010; Evstatiev and Gasche. 2012). Ge et al. reported the positive correlation between plasma Fe and serum bilirubin level in a population-based study, which was consistent with our present study (Ge et al. 2020). Moreover, the univariate metal exposure–response revealed that plasma Fe had a reverse U-shape association in elderly with DM. Previous studies also reported that hyperglycemia promotes the formation of advanced glycation end-products (AGEs), resulting in higher levels of reactive oxygen species (ROS) in DM (Vasan et al. 2003; Abo El-Nasr et al. 2020). Excess iron can also increase the release of harmful ROS (Mehta et al. 2019). Hence, we could assume that excess iron accelerates liver damage in DM. However, the underlying mechanism remains unclear and needs to be confirmed in future studies.
We also first found negative dose–response relationship between plasma Sr and serum TBIL in the elderly, especially those with DM. Plasma Sr, an essential trace element, has considerable influences on human health. Jiang et al. (2018) reported that Sr could prevent hepatic lipogenesis by alleviating endoplasmic reticulum stress (ERS), which is the key mechanism of bilirubin toxicity Schiavon et al. (2018). ERS can participate in the activation of hepatic stellate cells and accelerate hepatocyte fibrosis (Zuo et al. 2019; Pan et al. 2021). Cellular stress is one of the characteristics of DM (Tirosh et al. 2021), and hepatic ERS plays an important role in insulin resistance (Hotamisligil. 2010). Hyperglycemic environments could exacerbate ERS and induce apoptosis by excessive ERS (Sari et al. 2010). Therefore, the negative relation between plasma Sr and serum TBIL may be associated with the fact that Sr could alleviate ERS, especially in patients with DM.
We also observed that the concentration of plasma Sn was positively related with ALT level. Animal studies showed that Sn mainly accumulated in the kidney, liver, and other organs (Skaug et al. 2018) and caused further liver function injury. Tang et al. reported that Sn exposure induced lipid peroxidation and oxidative stress, leading to hepatocyte damage (Tang et al. 2021). ALT was considered as a special indicator of hepatocyte damage and death (Andria et al. 2013; Tang et al. 2016). One of the possible reasons for the positive association between plasma Sn and serum ALT was linked to the ability of Sn induces lipid peroxidation and oxidative stress. DM was characterized by metabolic disorders and high oxidative stress levels (Lovic et al. 2020), which were more susceptible as tin exposure. However, few researchers have investigated the association between plasma Sn and liver function parameters or liver function injury, and further research is needed to confirm the possible mechanism.
This study has several limitations. First, the elderly could not represent the entire elderly population in China. Second, this study adopted a cross-sectional design, which lacked the ability to verify the causal relationships between metal exposure and liver function indicators. In future, follow-up and prospective cohort studies should be conducted to verify the present results and determine the causality of metal exposure and liver function. Third, the metals in plasma could not completely represent the metal exposure level in the liver function. Finally, the potential mechanisms underlying the relationships between environmental metal exposure and liver function indicators remain unclear. Therefore, a variety of population and animal experiments are needed to verify the results in future study.
The strength of our study was that we were able to assess plasma metal levels in elderly with DM in relation to liver function. Various reports are available on the association of metals and liver function in occupational workers, but few studies investigated their association in urban elderly with DM. We examined the combined effects of 22 metals, as opposed to one single or several metals in previous studies. We also used different models to explore the metal exposure and liver function, including GLM and BKMR.
Conclusion
This study was firstly revealed that plasma Ti, Fe, and Sr combined exposure showed an inverse association with TBIL in elderly with DM. In single-metal models, plasma Fe showed an inverse “U” sharp association with TBIL, plasma Ti and Sr were negatively correlated with serum TBIL, and plasma Ti and Sn were positively correlated with serum AST and ALT. These associations should be validated in other population studies. Moreover, the underlying biological mechanisms should be clarified in future works.
Data Availability
All the data used for the study appear in the article.
Abbreviations
- DM:
-
Diabetes mellitus
- BSECS:
-
Boshan elderly cohort study
- ICP-MS:
-
Inductively coupled plasma source mass spectrometer
- TBIL:
-
Total bilirubin
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- LASSO:
-
The last absolute shrinkage and selection operator
- GLM:
-
Generalized linear model
- BKMR:
-
Bayesian kernel machine regression
- RCS:
-
Restricted cubic spline
- BMI:
-
Body mass index
- Mg:
-
Magnesium
- Al:
-
Aluminum
- Ca:
-
Calcium
- Ti:
-
Titanium
- V:
-
Vanadium
- Cr:
-
Chromium
- Mn:
-
Manganese
- Fe:
-
Iron
- Co:
-
Cobalt
- Ni:
-
Nickel
- Cu:
-
Copper
- Zn:
-
Zinc
- As:
-
Arsenic
- Se:
-
Selenium
- Rb:
-
Rubidium
- Sr:
-
Strontium
- Mo:
-
Molybdenum
- Cd:
-
Cadmium
- Sn:
-
Stannum
- Sb:
-
Antimony
- Ba:
-
Barium
- Pb:
-
Lead
- LOD:
-
The limit of detection
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- IQR:
-
Inter-quartile range
- HO-1:
-
Heme oxygenase-1
- ERS:
-
Endoplasmic reticulum stress
- AGEs:
-
Advanced glycation end-products
- ROS:
-
Reactive oxygen species
- NAFLD:
-
Non-alcoholic fatty liver disease
References
Abo El-Nasr NME, Saleh DO, Mahmoud SS, Nofal SM, Abdelsalam RM, Safar MM, El-Abhar HS (2020) Olmesartan attenuates type 2 diabetes-associated liver injury: cross-talk of AGE/RAGE/JNK, STAT3/SCOS3 and RAS signaling pathways. Eur J Pharmacol 874:173010. https://doi.org/10.1016/j.ejphar.2020.173010
Allaire M, Gilgenkrantz H (2020) The aged liver: beyond cellular senescence. Clin Res Hepatol Gastroenterol 44(1):6–11. https://doi.org/10.1016/j.clinre.2019.07.011
An H, Ling C, Xu M, Hu M, Wang H, Liu J, Song G, Liu J (2020) Oxidative damage induced by nano-titanium dioxide in rats and mice: a systematic review and meta-analysis. Biol Trace Elem Res 194(1):184–202. https://doi.org/10.1007/s12011-019-01761-z
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver and kidney. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16020274
Andria B, Bracco A, Attanasio C, Castaldo S, Cerrito MG, Cozzolino S, Di Napoli D, Giovannoni R, Mancini A, Musumeci A, Mezza E, Nasti M, Scuderi V, Staibano S, Lavitrano M, Otterbein LE, Calise F (2013) Biliverdin protects against liver ischemia reperfusion injury in swine. PLoS ONE 8(7):e69972. https://doi.org/10.1371/journal.pone.0069972
Anwar M, Bintara BA, Puji ARD, Muh S (2020) Effect of lead and cadmium to blood pressure on communities along coastal areas of Makassar Indonesia. Enfermerãa Clãnica 30:313–317. https://doi.org/10.1016/j.enfcli.2020.03.001
Astuti RDP, Mallongi A, Rauf AU (2021a) Natural enrichment of chromium and nickel in the soil surrounds the karst watershed. Global J Environ Sci Manag 7(3):1–18
Astuti RDP, Mallongi A, Rauf AU (2021b) Risk identification of Hg and Pb in soil: a case study from Pangkep Regency, Indonesia. Soil Sci Annu 72(1):1–15. https://doi.org/10.37501/soilsa/135394
Astuti RDP, Mallongi A, Choi K, Amiruddin R, Hatta M, Tantrakarnapa K, Rauf AU (2022) Health risks from multiroute exposure of potentially toxic elements in a coastal community: a probabilistic risk approach in Pangkep Regency, Indonesia. Geomat Nat Haz Risk 13(1):705–735. https://doi.org/10.1080/19475705.2022.2041110
Ballatori N (1991) Mechanisms of metal transport across liver cell plasma membranes. Drug Metab Rev 23(1–2):83–132. https://doi.org/10.3109/03602539109029757
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67. https://doi.org/10.1186/s12940-018-0413-y
Brand W, Peters RJB, Braakhuis HM, Maślankiewicz L, Oomen AG (2020) Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology 14(7):985–1007. https://doi.org/10.1080/17435390.2020.1778809
Carlsson S (2019) Etiology and pathogenesis of latent autoimmune diabetes in adults (LADA) compared to type 2 diabetes. Front Physiol 10:320. https://doi.org/10.3389/fphys.2019.00320
Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G (2010) Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect 118(12):1735–1742. https://doi.org/10.1289/ehp.1002720
Caylak E, Halifeoglu I (2007) Effects of sulfur-containing antioxidants on malondialdehyde and catalase levels of liver, kidney and brain in lead-exposed rats. Turk Klin J Med Sci 27(1):1–8
Caylak E, Tokar M (2012) Metallic and microbial contaminants in drinking water of Cankiri, Turkey. E-J Chem 9(2):608–614
Chung SM, Moon JS, Yoon JS, Won KC, Lee HW (2020) The sex-specific effects of blood lead, mercury, and cadmium levels on hepatic steatosis and fibrosis: Korean nationwide cross-sectional study. J Trace Elem Med Biol 62:126601. https://doi.org/10.1016/j.jtemb.2020.126601
Duan Y, Liu J, Ma L, Li N, Liu H, Wang J, Zheng L, Liu C, Wang X, Zhao X, Yan J, Wang S, Wang H, Zhang X, Hong F (2010) Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 31(5):894–899. https://doi.org/10.1016/j.biomaterials.2009.10.003
Edvardsson M, Sund-Levander M, Milberg A, Wressle E, Marcusson J, Grodzinsky E (2018) Differences in levels of albumin, ALT, AST, γ-GT and creatinine in frail, moderately healthy and healthy elderly individuals. Clin Chem Lab Med 56(3):471–478. https://doi.org/10.1515/cclm-2017-0311
Evstatiev R, Gasche C (2012) Iron sensing and signalling. Gut 61(6):933–952. https://doi.org/10.1136/gut.2010.214312
Ge X, Liu Z, Hou Q, Huang L, Zhou Y, Li D, Huang S, Luo X, Lv Y, Li L, Cheng H, Chen X, Zan G, Tan Y, Liu C, Zou Y, Yang X (2020) Plasma metals and serum bilirubin levels in workers from manganese-exposed workers healthy cohort (MEWHC). Environ Pollut 258:113683. https://doi.org/10.1016/j.envpol.2019.113683
Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, Esposito S, Khodadadi E, Yousefi M, Samadi Kafil H (2020) Alteration of liver biomarkers in patients with SARS-CoV-2 (COVID-19). J Inflamm Res 13:285–292. https://doi.org/10.2147/jir.s257078
Hamoud AR, Weaver L, Stec DE, Hinds TD Jr (2018) Bilirubin in the liver-gut signaling axis. Trends Endocrinol Metab 29(3):140–150. https://doi.org/10.1016/j.tem.2018.01.002
Hoare M, Das T, Alexander G (2010) Ageing, telomeres, senescence, and liver injury. J Hepatol 53(5):950–961. https://doi.org/10.1016/j.jhep.2010.06.009
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6):900–917. https://doi.org/10.1016/j.cell.2010.02.034
Hou Q, Huang L, Ge X, Yang A, Luo X, Huang S, Xiao Y, Jiang C, Li L, Pan Z, Teng T, Zhang H, Li M, Mo Z, Yang X (2019) Associations between multiple serum metal exposures and low birth weight infants in Chinese pregnant women: a nested case-control study. Chemosphere 231:225–232. https://doi.org/10.1016/j.chemosphere.2019.05.103
Jiang H, Guan Q, Xiao Y, Feng Z, Yu G, Pan Q (2018) Strontium alleviates endoplasmic reticulum stress in a nonalcoholic fatty liver disease model. J Med Food. https://doi.org/10.1089/jmf.2018.4186
Kim IH, Kisseleva T, Brenner DA (2015) Aging and liver disease. Curr Opin Gastroenterol 31(3):184–191. https://doi.org/10.1097/mog.0000000000000176
Koye DN, Magliano DJ, Nelson RG, Pavkov ME (2018) The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25(2):121–132. https://doi.org/10.1053/j.ackd.2017.10.011
Kronenberg F (2010) Association of bilirubin with cardiovascular outcomes: more hype than substance? Circ Cardiovasc Genet 3(4):308–310. https://doi.org/10.1161/circgenetics.110.957399
Leem AY, Kim HY, Kim YS, Park MS, Chang J, Jung JY (2018) Association of serum bilirubin level with lung function decline: a Korean community-based cohort study. Respir Res 19(1):99. https://doi.org/10.1186/s12931-018-0814-z
Li A, Mei Y, Zhao M, Xu J, Zhao J, Zhou Q, Ge X, Xu Q (2022) Do urinary metals associate with the homeostasis of inflammatory mediators? Results from the perspective of inflammatory signaling in middle-aged and older adults. Environ Int 163:107237. https://doi.org/10.1016/j.envint.2022.107237
Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A (2020) The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol 18(2):104–109. https://doi.org/10.2174/1570161117666190405165911
Mallongi A, Rauf AU, Daud A, Hatta M, Al-Madhoun W, Amiruddin R, Astuti RDP (2022) Health risk assessment of potentially toxic elements in Maros karst groundwater: a Monte Carlo simulation approach. Geomat Nat Hazards Risk 13(1):338–363. https://doi.org/10.1080/19475705.2022.2027528
McGill MR (2016) The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli j 15:817–828. https://doi.org/10.17179/excli2016-800
Mehta KJ, Farnaud SJ, Sharp PA (2019) Iron and liver fibrosis: mechanistic and clinical aspects. World J Gastroenterol 25(5):521–538. https://doi.org/10.3748/wjg.v25.i5.521
Pan X, Zhu Y, Wu X, Liu L, Ying R, Wang L, Du N, Zhang J, Jin J, Meng X, Dai F, Huang Y (2021) The interaction of ASIC1a and ERS mediates nerve cell apoptosis induced by insulin deficiency. Eur J Pharmacol 893:173816. https://doi.org/10.1016/j.ejphar.2020.173816
Park E, Kim J, Kim B, Park EY (2021) Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 266:128947. https://doi.org/10.1016/j.chemosphere.2020.128947
Sari FR, Watanabe K, Thandavarayan RA, Harima M, Zhang S, Muslin AJ, Kodama M, Aizawa Y (2010) 14–3–3 protein protects against cardiac endoplasmic reticulum stress (ERS) and ERS-initiated apoptosis in experimental diabetes. J Pharmacol Sci 113(4):325–334. https://doi.org/10.1254/jphs.10047fp
Schiavon E, Smalley JL, Newton S, Greig NH, Forsythe ID (2018) Neuroinflammation and ER-stress are key mechanisms of acute bilirubin toxicity and hearing loss in a mouse model. PLoS ONE 13(8):e0201022. https://doi.org/10.1371/journal.pone.0201022
Shen H, Zhang H, Gong W, Qian T, Cheng T, Jin L, Wang X, Luo D, Xu X (2021) Prevalence, causes, and factors associated with visual impairment in a Chinese elderly population: the Rugao longevity and aging study. Clin Interv Aging 16:985–996. https://doi.org/10.2147/cia.s304730
Shum M, Shintre CA, Althoff T et al (2021) ABCB10 exports mitochondrial biliverdin, driving metabolic maladaptation in obesity. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abd1869
Skaug V, Eilertsen E, Skogstad A, Levy FES, Berlinger B, Thomassen Y, Ellingsen DG (2018) Kinetics and tissue distribution of bismuth, tin and lead after implantation of miniature shotgun alloy pellets in rats. J Trace Elem Med Biol 48:224–232. https://doi.org/10.1016/j.jtemb.2018.04.002
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235(4792):1043–1046. https://doi.org/10.1126/science.3029864
Tang N, Zhang Y, Liu Z, Fu T, Liang Q, Ai X (2016) Correlation analysis between four serum biomarkers of liver fibrosis and liver function in infants with cholestasis. Biomed Rep 5(1):107–112. https://doi.org/10.3892/br.2016.681
Tang L, Zhang YH, Wang X, Zhang CC, Qin G, Lin Q (2021) Effects of chronic exposure to environmental levels of tributyltin on the lined seahorse (Hippocampus erectus) liver: analysis of bioaccumulation, antioxidant defense, and immune gene expression. Sci Total Environ 801:149646. https://doi.org/10.1016/j.scitotenv.2021.149646
Tien Tai T, Adachi Y, Taketani S (2022) A fluorescence-based quantitative analysis for total bilirubin in blood and urine. Lab Med 53(1):6–11. https://doi.org/10.1093/labmed/lmab043
Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, Yalcin A, Lee YG, Livne R, Ron S, Minsky N, Arruda AP, Hotamisligil GS (2021) Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab 33(2):319-333.e316. https://doi.org/10.1016/j.cmet.2020.11.009
Vasan S, Foiles P, Founds H (2003) Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys 419(1):89–96. https://doi.org/10.1016/j.abb.2003.08.016
Vicas SI, Laslo V, Timar AV et al (2021) Nano selenium-enriched probiotics as functional food products against cadmium liver toxicity. Materials (basel). https://doi.org/10.3390/ma14092257
Vriens A, Nawrot TS, Baeyens W, Den Hond E, Bruckers L, Covaci A, Croes K, De Craemer S, Govarts E, Lambrechts N, Loots I, Nelen V, Peusens M, De Henauw S, Schoeters G, Plusquin M (2017) Neonatal exposure to environmental pollutants and placental mitochondrial DNA content: a multi-pollutant approach. Environ Int 106:60–68. https://doi.org/10.1016/j.envint.2017.05.022
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2):176–185. https://doi.org/10.1016/j.toxlet.2006.12.001
Wang S, Zhang J, Zhu L, Song L, Meng Z, Jia Q, Li X, Liu N, Hu T, Zhou P, Zhang Q, Liu L, Song K, Jia Q (2017) Association between liver function and metabolic syndrome in Chinese men and women. Sci Rep 7:44844. https://doi.org/10.1038/srep44844
Wani MR, Maheshwari N, Shadab G (2021) Eugenol attenuates TiO(2) nanoparticles-induced oxidative damage, biochemical toxicity and DNA damage in Wistar rats: an in vivo study. Environ Sci Pollut Res Int 28(18):22664–22678. https://doi.org/10.1007/s11356-020-12139-3
Xu L, Li Y, Yin L, Qi Y, Sun H, Sun P, Xu M, Tang Z, Peng J (2018) miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics 8(20):5593–5609. https://doi.org/10.7150/thno.27425
Yang J, Luo M, Tan Z, Dai M, Xie M, Lin J, Hua H, Ma Q, Zhao J, Liu A (2017) Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ Toxicol Pharmacol 49:112–118. https://doi.org/10.1016/j.etap.2016.12.006
Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB (2020a) Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr 124(3):330–340. https://doi.org/10.1017/s0007114520001208
Yang X, Zhang P, Zhang F, Ke Z, Chen Q, Liu C (2020b) Protective effect of hypoglycemic granule against diabetes-induced liver injury by alleviating glycolipid metabolic disorder and oxidative stress. J Cell Biochem 121(5–6):3221–3234. https://doi.org/10.1002/jcb.29588
Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, Wang J, Li H, Liu G, Pan A (2020) Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer 122(7):1085–1093. https://doi.org/10.1038/s41416-020-0741-x
Zuo L, Zhu Y, Hu L, Liu Y, Wang Y, Hu Y, Wang H, Pan X, Li K, Du N, Huang Y (2019) PI3-kinase/Akt pathway-regulated membrane transportation of acid-sensing ion channel 1a/calcium ion influx/endoplasmic reticulum stress activation on PDGF-induced HSC activation. J Cell Mol Med 23(6):3940–3950. https://doi.org/10.1111/jcmm.14275
Acknowledgements
The author would like to thank all the patients included in our present study.
Funding
Shandong Province Medical and health Project (202102040656); Shandong First Medical University Science and Education Integration talents academic promotion plan funds (922/001003088002), Academic Promotion Program in the School of Public Health and Health Management (GW202212).
Author information
Authors and Affiliations
Contributions
JL contributed to conceptualization, methodology, formal analysis, investigation, data curation, and writing—original draft. JL contributed to methodology, resources, data curation, and writing. HY contributed to project administration, methodology, resources, data curation, and writing. JH contributed to methodology, resources, data curation, and writing. XX contributed to methodology, resources, and data curation. HZ contributed to project administration, supervision, and conceptualization. CH contributed to project administration, conceptualization, and methodology. XL contributed to project administration, and conceptualization. QH contributed to supervision, conceptualization, methodology, investigation, writing—original draft, writing—review & editing, visualization, and funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This study was approved by the Ethics Review Committees of Shandong First Medical University and performed in accordance with the Declaration of Helsinki in 1975. Written informed consents were obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Li, J., Yao, H. et al. Multi-metal Exposure and Liver Function in Elderly with Diabetes Mellitus: A Cross-Sectional Study in Eastern China. Expo Health 16, 897–910 (2024). https://doi.org/10.1007/s12403-023-00598-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-023-00598-1