Abstract
Shahu Lake is a well-known tourist lake in Ningxia, northwest China. This study analyzed the general hydrochemical characteristics of the lake water to determine the spatiotemporal variability of contaminants and to assess the potential health risk associated with the surface water. Lake water was monitored at three stations each month throughout 2013. Samples were analyzed for pH, total dissolved solids, temperature, EC, major ions (Na+, K+, Ca2+, Mg2+, SO4 2−, Cl−, HCO3 −, and CO3 2−), ammonia nitrogen (NH4-N), fluoride (F−), phenol, and heavy metals (As, Hg, and Cr6+). The results suggest that Na+ is the dominant cation in the lake water, and Cl− and SO4 2− are the dominant anions, resulting in a primary water type of Cl·SO4–Na. Contaminant levels (NH4-N, F−, As, Hg, Cr6+, and phenol) vary through time and space (spatiotemporal variations). As and F− are high in the summer season; Cr6+ and Hg do not significantly change over the year; and the NH4-N and phenol concentrations also remain relatively stable. Concentrations of all these substances are within the acceptable limits for recreational water use (1.5, 1.5, 0.1, 0.001, 0.05, and 0.01 mg/L for NH4-N, F−, As, Hg, Cr6+, and phenol, respectively). An assessment model recommended by the Ministry of Environmental Protection of the P.R. China was used in the study to conduct the health risk assessment. While workers do experience higher non-carcinogenic and carcinogenic risks than tourists, the risks are low and negligible. As and Cr6+ contribute the most to total carcinogenic and non-carcinogenic risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a worldwide environmental problem, limiting sustainable socio-economic development and affecting the health of people who use contaminated water for domestic uses (Wu and Sun 2016; Li et al. 2015). Lake water is usually used for recreation and fishing, and its quality is deteriorating in many places because of climate change and human pollution (Juma et al. 2014). Many scholars have conducted lake water quality studies around the world. Chapra et al. (2009) analyzed chloride trends in Great Lakes (United States), and found that the chloride concentrations in these lakes were increasing. Yu et al. (2010) assessed eutrophication using a synthesized trophic state index model in Gucheng Lake, China. Jirsa et al. (2013) studied major and trace element geochemistry of two athalassic endorheic lakes in Kenya during an extreme drought. Saeed and Hashmi (2014) evaluated effects of human activity on water quality and bacterial diversity in Rawal Lake, Islamabad. All these lake water quality studies benefit lake water quality protection and lake water resources management.
Shahu Lake (also known as Sand Lake) is the largest lake in Ningxia, northwest China. Operated by Shahu Lake Tourism Company, it is a national 5A-class tourist location, attracting hundreds of thousands of domestic and foreign tourists each year. It is famous for its scenery and abundant fishery resources. The lake, however, is experiencing water quality deterioration because of tourism, industrial, and agricultural development around the lake. The lake water deterioration may ultimately endanger ecosystem sustainability and biodiversity; therefore, the area has attracted significant attention from local governmental officials and researchers. Lu et al. (2001) analyzed the ecological and environmental problems in Shahu Lake, proposing several remedial measures. Qu (2007) analyzed tourism impacts on lake water quality by assessing DO, suspended matter, BOD, NH4 +, TP, and F−. Luo and Qu (2011) assessed water quality variability trends at Shahu Lake using principal component analysis and a fuzzy comprehensive evaluation method. Wang et al. (2008) studied TN, TP, and organic matter distribution, and Ren et al. (2008) assessed heavy metal pollution in the surface sediments of Shahu. Chen and Qian (2016) engaged in numerical modeling to characterize replenishment water, lake water, and groundwater interactions in this area. These local research studies help protect the lake water ecosystem.
However, these studies did not focus on the negative health impacts of polluted water on human beings. When tourists play and workers work on and around the lake, their bodies may contact the water. As a result, lake water contaminants, such as ammonia nitrogen, fluoride, and heavy metals, may pose non-carcinogenic and carcinogenic risks to tourists and the workers. Previous studies have not determined whether health risks posed by the lake water through the dermal contact exposure pathway are at acceptable levels. Therefore, the main objectives of this study were (1) to characterize the major ion geochemistry of lake water, (2) to analyze the spatiotemporal variability of contaminants, including ammonia nitrogen, fluoride, phenol, and selected heavy metals (As, Hg, and Cr6+) in Shahu Lake, and (3) to evaluate health risks to tourists and workers who are exposed to lake water through the dermal contact pathway. This study provides information for local lake administrators to support water quality management. It also provides international researchers with new reference information for similar studies.
Materials and Methods
Study Area
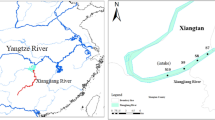
Shahu Lake is the largest lake in Ningxia, in northwest China (Fig. 1). It is located in the north part of Ningxia, at longitude 106°18′E, and latitude 38°45′N, covering 8.2 km2 (Zhao et al. 2010). The average lake water depth is 2.2 m (Chen and Qian 2016), with a maximum depth of 6 m. Located in a semiarid area, there is little precipitation but intense evaporation. The average annual precipitation in the area is 172.5 mm, with 66.6% concentrated in July to September. The average evaporation rate is 1755.1 mm per year, which is ten times the annual precipitation. Intense evaporation is the primary factor causing lake water salinization. The average annual temperature is 9.74 °C (Chen and Qian 2016).
South of the lake is a sand dune surrounded by a canal linking the lake’s inlet and outlet. The sand dune covers 12.74 km2 (Fig. 1). The lake and the dune are important elements of the tourist attraction. Tourists are transported to the dune by hovercrafts and motorboats. The lake is primarily recharged by the Yellow River water through irrigation canals during May to September and from October to November (Chen and Qian 2016). Because of intense evaporation, the lake water is rarely discharged at the outlet, keeping the water level stable. As such, the lake is actually a closed water body, putting it at risk of becoming a salt lake, with deteriorating lake water quality. People in the area rely on groundwater for domestic use; Shahu Lake water is primarily used for recreation and aquaculture.
Geographically, the area is a part of the alluvial–lacustrine plain (Qian et al. 2014), fine sand and clay rich in carbonates and silicates have been deposited in the area. In most of the area, groundwater level is high and the depth to groundwater level is usually less than 3 m, favorable for the evaporation of shallow groundwater (Qian et al. 2014). Because the lake contains stagnated water, there is a chance of increasing inorganic substances due to the interactions between the water and the rocks. In addition to impacts from human activities, the lake water quality may undergo deterioration due to intense evaporation in the area and water–rock interactions.
Data Collection and Analysis
Data used in this study were collected from the Shizuishan Environmental Monitoring Station, which regularly monitors lake water quality. Samples were collected each month throughout 2013, except in January and February when the lake water was frozen. Major ions were also not analyzed in March, due to exclusion from regular monitoring parameters before April 2013. Samples were collected from three monitoring sites (Fig. 1). Station 1 is located near the outlet of the lake; Station 2 belongs to an aquaculture zone where the inlet of the lake is situated; and Station 3 is the wharf serving as a tourist attraction at the middle section of the lake. In total, 30 lake water samples were collected.
Contaminants monitored for this study included ammonia nitrogen (NH4-N), fluoride (F−), phenol, and heavy metals (As, Hg, and Cr6+). In addition, pH, total dissolved solids (TDS), temperature, electric conductivity (EC), and major ions in the lake water were also analyzed to delineate general characteristics of the lake water. Temperature, pH, and EC were measured in situ using a portable multi-parameter water quality monitoring device (HACH HYDROLab). The other parameters were analyzed in the Laboratory of Shizuishan Environmental Monitoring Station. The study applied standard analytical procedures recommended by the American Public Health Association (2012) (Table 1).
Health Risk Assessment
The main exposure pathway for contaminated lake water was determined to be dermal contact, given that the lake water is usually used for recreational purposes. It is also used for fishing; however, there are no data on the contaminants found in the fish consumed by tourists and residents. Therefore, this study only assessed human health risks through dermal contact. The assessment models recommended by the Ministry of Environmental Protection of the P.R. China (2014) were adopted. Contaminants considered for non-carcinogenic risk assessment included ammonia nitrogen, fluoride, volatile phenols, and heavy metals (As, Hg, and Cr6+); As and Cr6+ were also included in the carcinogenic assessment.
The chronic daily intake (CDI) dose through dermal contact can be calculated using Eqs. (1) and (2).
In these expressions, C represents the contaminant concentrations in lake water (mg/L); SA is the skin surface area exposed to water (cm2); K is the coefficient of skin permeability, estimated at 0.002 for Cr6+ and 0.001 cm/h for other contaminants (Yang et al. 2015; USEPA 2004); and t is the duration of contact (h). ED, EF, EV, BW, AT, H, and ABSd denote, respectively, the exposure duration (a), exposure frequency (d/a), daily exposure frequency of dermal contact, average body weight (kg), average time of duration of the health effects (days), average height (cm), and the absorption ratio of dermal contact. CF is a conversion factor which equals 0.001 in this study. SER signifies skin exposure ratio. This study adopted values recommended by the Ministry of Environmental Protection of the P.R. China (2014) for BW (56.8 kg), AT (21,600 days for carcinogenic effect and 7200 days for non-carcinogenic effect), H (156.3 cm), and SER (0.32). For ED, 20 years and 1 year were assigned to workers and tourists, respectively. This signified that tourists would visit this lake only once in their lives, while workers work there for 20 years. EF values of 1 and 220 were assigned to tourists and workers, respectively. EV is 2 and 10 for tourists and workers, respectively, and t is 0.3 h for the duration of each exposure event. ABSd was also derived from Ministry of Environmental Protection of the P.R. China guidelines (2014) and is 0.03 for As and 0.001 for other contaminants.
Potential non-carcinogenic risks are usually represented using the hazard quotient (HQ, Yang et al. 2015), computed using Eq. (3). The hazard index (HI), representing the total hazard from multiple contaminants, is calculated by adding the HQs of all contaminants (Eq. 4) as follows:
In this expression, RfDdermal is the reference dosage for non-carcinogenic pollutants through the dermal contact exposure pathway [mg/(kg d)]. It is computed by multiplying RfDo and ABSgi. RfDo represents the reference dosage for non-carcinogenic pollutants through the oral intake exposure pathway, and ABSgi is the gastrointestinal absorption factor. The value of ABSgi is 1 for all the studied contaminants considered in this study, except Cr6+ and Hg, which have ABSgi values of 0.025 and 0.07, respectively (USEPA 2015). The RfDo values for NH4-N, F−, volatile phenols, As, Hg, and Cr6+ are 0.97, 0.04, 0.3, 0.0003, 0.0003, and 0.003, respectively. The parameter n signifies the total number of non-carcinogenic contaminants considered. If HQ and HI are less than 1, then the non-carcinogenic risk is acceptable, otherwise it is unacceptable (Wu and Sun 2016; Li et al. 2016a; Yang et al. 2015; Yu et al. 2010).
For carcinogenic risks, when CDI × SF < 0.01, Eq. (5) applies. Otherwise, Eq. (6) is used instead (Li and Qian 2011; Li et al. 2014; Yang et al. 2015). Equation (7) calculates the total carcinogenic risk from multiple carcinogenic contaminants.
In this expression, CR represents the carcinogenic risk; SF is the slope factor of each toxic contaminant; and n signifies the number of carcinogenic contaminants.
Results and Discussion
General Hydrochemical Characteristics of the Lake Water
Table 2 presents the annual minimum, maximum, and mean analytical values for major parameters at each sampling station. The pH value expresses the acidity or alkalinity of water (Ravikumar et al. 2013). Water with low pH is corrosive and harms skin and eyes (Rao and Rao 2010). The pH values of water at the stations ranged from 8.23 to 8.93 (Station 1), 8.35 to 8.94 (Station 2), and 8.36 to 9.77 (Station 3), with average values of 8.65, 8.70, and 8.74, respectively. The highest pH and the highest mean pH was seen in samples from Station 3. This may be due to the more intensified human activity at this station. A pH value between 6 and 8.5 indicates a productive water body (Garg et al. 2010); however, the mean pH of water at the three stations in this study were within the permissible limit of 6–9 (Bureau of Quality and Technical Supervision of China 2002).
COD and BOD are important parameters indicating the lake water quality. In this study, COD ranges from 19.2 to 20.1 mg/L with the highest mean value observed at Station 1 and lowest at Station 2. This is indicative of the greater seriousness of organic pollution of water at Station 1 than other stations. Similarly, the highest mean COD was detected from Station 1 and lowest from Station 2, convincing the statement that organic pollution is more serious at Station 1.
TDS is an indicator of water salinity and represents dissolved salts in water, including bicarbonates, chlorides, calcium, and magnesium. The average TDS values in lake water at Stations 1, 2, and 3 were 4188, 4156, and 4194 mg/L, respectively. This indicates that the water salinity in Shahu Lake is high, likely because of a high degree of evaporation, intensified human activities, and inadequate lake water outflow. The water EC also indicates water salinity (Wu and Sun 2016). The EC values of lake water at different stations were 5320–7600, 5550–7590, and 5460–7590 µS/cm, respectively; these values indicate that the lake water belongs to very high salinity class (EC > 2250, Wu and Sun 2016). The highest average EC value was recorded at Station 3, likely due to intense evaporation and human activities such as waste disposal.
In Shahu Lake, the mean concentration of Ca2+ ranges from 44.9 to 47.0 mg/L. Lake water collected at Station 1 contained slightly higher Ca2+ levels than lake water collected at Stations 2 and 3. However, Ca2+ levels at all stations were generally low, indicating weak impacts from water–rock interactions in the lake. The concentrations of Mg2+ in lake water varied between 187.0–366.0 mg/L at Station 1, 196.0–286.0 mg/L at Station 2, and 204.0–289.0 mg/L at Station 3. These levels are low enough for the water to be considered acceptable for irrigation and fishery. The low concentration of Ca2+ and Mg2+ leads to lake water being classified as medium hard water (total hardness within 75–150 mg/L as CaCO3, WHO 2004).
Na+ concentrations in lake water were high compared to Ca2+ and Mg2+, ranging from 1027.0 to 1545.0, 996.0 to 1280.0, and 1024.0 to 1514.0 mg/L at Stations 1, 2, and 3, respectively. Na+ averages at all three stations were higher than 1100 mg/L, which is five times higher than the permissible limit (200 mg/L) for multiple purposes. The concentrations of K+ in lake water were the lowest of all the major cations, ranging on average from 21.1 to 21.5 mg/L; the levels of this cation are usually controlled by rock weathering.
For anions, SO4 2− concentrations at Stations 1, 2, and 3 varied from 1086.0 to 1443.0, 1094.0 to 1281.0, and 1009.0 to 1267.0 mg/L, respectively, with the highest level observed at Station 1. The SO4 2− concentration exceeds the acceptable limit for drinking and irrigation (these limits are 250 mg/L for drinking purpose and 350 mg/L for irrigation). The mean Cl− concentration was also very high in lake water collected for this study. Its mean concentrations were 1031.9, 988.2, and 968.7 mg/L for lake water at Stations 1, 2, and 3, respectively. The high SO4 2− and Cl− concentration may be due to intense evaporation and extensive agricultural activities in this area. Recharge water entering the lake contains high concentration of SO4 2− and Cl−; this water originates from agricultural return water and municipal wastewater. Compared with SO4 2− and Cl−, the HCO3 − concentration is lower. Its concentration varied from 500.2 to 595.4, 480.1 to 617.9, and 492.3 to 618.5 mg/L, respectively, for Stations 1, 2, and 3, with the highest detected at Station 3. High concentrations of carbonate and bicarbonate ions can harm soils and plants. This is because high concentrations of them induce sodium hazards, by enhancing the tendency of calcium and magnesium to precipitate as carbonates. This results in an increased proportion of sodium (Li et al. 2016b; Ravikumar et al. 2013; Wu et al. 2015).
The concentration of Na+ is higher than Mg2+, which in turn exceeds Ca2+ + K+. This makes Na+ the dominant cation. There were more Cl− anions than SO4 2−, which in turn exceeded HCO3 − + CO3 2− (Fig. 2). Major ion concentrations at different stations were close to each other, suggesting that the lake water at different stations is influenced by similar factors. The high abundance of Na+, Cl−, and SO4 2− results in the lake water being mainly a Cl·SO4–Na type. All water samples were plotted in similar regions of the Piper diagram (Fig. 3).
The Cl− and SO4 2− type water is mainly the result of progressive salinization (Li et al. 2016b), due to rock weathering and evaporation. However, aerial deposition and human activities can also introduce Cl− and SO4 2− into water bodies (Li et al. 2016c). “Gibbs diagrams” help analyze the natural mechanisms that drive water formation (Gibbs 1970). They divide water formation mechanisms into three types: rock dominance, evaporation dominance, and precipitation dominance. Figure 4 shows that all water samples align with the evaporation dominance zone of the diagrams. This suggests that evaporation plays a dominant role in the formation lake water geochemistry. The potential evaporation rate in the study area is very high (1755.1 mm per year), resulting in intense lake water evaporation, elevating major ion concentrations, and deteriorating water quality.
Spatiotemporal Variability of the Contaminants
Lake water contaminants may change differently over time due to diverse factors. Figure 5 illustrates the temporal variations of F−, NH4 +, Cr6+, phenol, As, and Hg; contaminants differ over time. F− increases during the summer season and declines during the winter season. A similar trend can be observed for the As concentration. These trends may be due to the recharge water entering during the summer season. This recharge water includes municipal and industrial wastewater and agricultural return water, with diverse contaminants such as As and F−. The high temperature and intense evaporation may also lead to elevated As and F− during the summer season. The high temperature may favor dissolution reactions of As and F− containing minerals and compounds.
Cr6+ and Hg do not experience significant increasing or decreasing trends over the course of a year. Their concentrations do, however, vary in different months. Cr6+ concentrations are high in March, June, and August, and Hg concentrations are high in March, June, and October. The significant variations of Cr6+ and Hg in different months may be due to the combined effects of different factors, such as natural release from sediments, addition from recharge water, contamination by human activities, and concentration due to evaporation.
In contrast to other contaminants, NH4-N and phenol concentrations remain relatively stable during the year. NH4-N and phenol are both chemically active contaminants. As such, they can be easily transformed into other forms or states, depending on the redox conditions and environmental conditions. For example, phenol easily volatilizes at a low temperature. NH4-N can be transformed into nitrogen gas or nitrous oxides such as NO, NO2 −, and NO3 − under oxidizing and/or acidic conditions (Dou et al. 2016). Possible NH4-N transformation reactions are as follows:
Lake water contaminant levels at different stations show spatial variability. Table 3 shows that NH4-N ranges from 0.376 to 0.781, 0.325 to 0.803, and 0.380 to 0.795 mg/L at Stations 1, 2 and 3, respectively. Average levels are lowest at Station 1, higher at Station 2, and the highest at Station 3. This indicates more significant contamination at Station 3. Similarly, mean F− concentration was lowest at Station 1, higher at Station 2, and highest at Station 3, with ranges of 0.661–1.270, 0.662–1.230, and 0.665–1.280 mg/L, respectively, at Stations 1, 2 and 3. The NH4-N and F− concentrations are below acceptable limits for recreational purpose (1.5 mg/L for both NH4-N and F−, Bureau of Quality and Technical Supervision of China 2002).
Both As and Hg are toxic elements in the environment (Cott et al. 2016; Rebelo and Caldas 2016), and were present at trace levels in the lake water. The allowable limits of As and Hg in water for recreation are 0.1 and 0.001 mg/L, respectively (Bureau of Quality and Technical Supervision of China 2002). As concentrations ranged from 2.40 to 8.70, 2.20 to 9.20, and 2.40 to 8.50 μg/L, with means of 4.99, 4.87, and 4.96 μg/L for samples from Stations 1, 2, and 3, respectively. The Hg concentration was even lower than As. Its average values are 0.026 (Station 1), 0.025 (Station 2), and 0.027 (Station 3). Station 1 samples had the highest As mean, while Station 3 samples had the highest Hg means. As discussed above, the As concentration is primarily influenced by water recharge and natural climate conditions, such as high temperature and intense evaporation. Hg concentrations, however, are impacted by diverse factors such as industrial and agricultural pollution.
Cr6+ is also a highly toxic element (Kiran et al. 2016) due to its ability to penetrate cell membranes (Wang et al. 2016). The acceptable limit of Cr6+ in water for recreation is 0.05 mg/L (Bureau of Quality and Technical Supervision of China 2002). In this study, the level ranged from 0.004 to 0.010, 0.004 to 0.008, and 0.004 to 0.012 mg/L at Stations 1, 2, and 3, respectively. These levels are well below the acceptable limit. Station 3 had the highest average value of Cr6+ and Station 2 had the lowest.
The spatial variability of phenol was weaker than the other contaminants, with the same mean at the three stations. The specific concentration ranges at Stations 1, 2, and 3 were 0.0013–0.0024, 0.0011–0.0028, and 0.0013–0.0030 mg/L, respectively. These phenol concentrations were below the acceptable limit for recreation (0.01 mg/L, Bureau of Quality and Technical Supervision of China 2002).
Health Risk Assessment
All contaminants discussed above are at levels within acceptable limits for recreational use. However, tourists and workers may be exposed to health risks if they frequently contact water containing carcinogenic and non-carcinogenic contaminants. Table 4 lists the carcinogenic and non-carcinogenic health risks for tourists and workers when exposed to F−, NH4-N, Cr6+, phenol, As, and Hg. Table 5 and Fig. 6 provide the contribution of each contaminant to the non-carcinogenic quotients and carcinogenic risks, respectively, for comparison.
As Table 4 shows, HItourist ranges from 4.78 × 10−9 to 5.00 × 10−9; the non-carcinogenic risk at Station 1 is slightly higher than that at the other stations. However, the values of HItourist are far lower than the threshold of 1. This suggests that the non-carcinogenic risks to tourists are quite low and negligible, and the lake water is safe for tourists in terms of non-carcinogenic risk. The HI values for workers are 4–5 magnitudes higher than for tourists, because the workers there usually do such work as catching fish and operating boats for tourists, and they have higher daily exposure rates through dermal contact (EV) and a higher annual contact frequency (EF) than tourists. This means that workers have a greater chance of being exposed to the lake water, and if exposed may be exposed for longer periods. However, the non-carcinogenic risk for workers is also significantly lower than the threshold, indicating that the lake water is also safe for workers in terms of non-carcinogenic risk.
Workers experience higher carcinogenic risk than tourists. Table 4 shows that the carcinogenic risk for workers ranges from 1.19 × 10−8 to 1.22 × 10−8 with the highest mean CR value at Station 1 and the lowest at Station 2. In contrast, the carcinogenic risk for tourists ranges from 5.41 × 10−13 to 5.55 × 10−13. The carcinogenic risks for tourists and workers are well below the acceptable level (1 × 10−6) recommended by the Ministry of Environmental Protection of the P.R. China (2014). This suggests that the lake water is a safe environment for tourists and workers.
Of all contaminants considered in this study, As contributes the most to the total hazard quotients due to its high toxicity, followed by Cr6+ and F− (Table 5). As and Cr6+ have small reference dosages. Hg is also highly toxic and is assigned a very low reference dosage; however, it contributes little to the total hazard quotients in this study, because of its low concentration in the lake water. NH4-N and phenol contribute least to the total quotients, because they can be easily transformed and/or removed from the water, and they are not as toxic as the other contaminants. In terms of carcinogenic risk, As contributes more to the total carcinogenic risk than Cr6+ does at all three stations (Fig. 6). Therefore, any preventive measures should consider methods to reduce As exposure or risk.
Conclusions
Tourist lake water is rarely studied in terms of its hydrochemical characteristics and its health impacts on tourists and workers. This study investigated the major ion chemistry and contamination status of several contaminants (ammonia nitrogen, fluoride, phenol, and heavy metals) in lake water at the popular Shahu Lake. Health risks to tourists and workers from the selected contaminants were assessed to verify the lake water’s suitability for recreation. The following conclusions can be drawn.
-
1.
Na+ was the dominant cation, followed sequentially by Mg2+, Ca2+, and K+. Cl− and SO4 2− were the dominant anions, followed by HCO3 − + CO3 2−. This resulted in the formation of Cl·SO4–Na type water. There are only slight differences in the water at different stations in terms of the concentrations of major parameters. Evaporation is the primary influencing factor on the major ion chemistry of the lake water.
-
2.
As and F− concentrations are high in summer season due to water recharge, high temperature, and intense evaporation. Cr6+ and Hg, influenced by the combined effects of diverse factors, showed no obvious increasing and decreasing trend over the year. The NH4-N and phenol concentrations remain relatively stable during the year, because they are easily transformed to other forms or states. These contaminant concentrations also show slight spatial variability, because of different degrees of human activities and diverse environmental conditions. Mean concentrations of NH4-N, F−, Hg, and Cr6+ were higher at Station 3 than at the other stations; the concentration of As was highest at Station 1; and the phenol concentration was the same at the three stations. All contaminant levels in the lake water were well below the acceptable limits for recreational purposes.
-
3.
The mean carcinogenic risk and the mean non-carcinogenic risk for workers range from 1.19 × 10−8 to 1.22 × 10−8 and 1.05 × 10−4 to 1.10 × 10−4, respectively. The carcinogenic risk and the non-carcinogenic risk for tourists are in the range of 5.41 × 10−13 to 5.55 × 10−13 and 4.78 × 10−9 to 5.00 × 10−9, respectively. Workers experience higher non-carcinogenic and carcinogenic risks than tourists. However, the risks are within the acceptable limits or thresholds, indicating that the lake water quality is safe for tourists and workers. As and Cr6+ contribute the most to the total carcinogenic and the total non-carcinogenic risks; as such, any future preventive measures should focus on avoiding exposure or minimizing risk associated with these metals.
This study may stimulate further lake water research. The origin of the pollutants in the lake water should be determined, and the factors influencing their migration and transport should also be well studied. Besides, as the Shahu Lake is also used for fishery, the study on the impacts of contaminants on fish and in turn on consumers is also an important subject deserving attention. In addition, the health risk assessment model can also be improved by sensitivity analysis. This is a critical research topic in all health risk assessment studies.
References
American Public Health Association (2012) Standard methods for the examination of water & wastewater, 22nd end. American Public Health Association, Washington
Bureau of Quality and Technical Supervision of China (2002) Environmental quality standards for surface water, (GB 3838-2002). China Environmental Science Press, Beijing (in Chinese)
Chapra SC, Dove A, Rockwell DC (2009) Great Lakes chloride trends: long-term mass balance and loading analysis. J Great Lakes Res 35:272–284. doi:10.1016/j.jglr.2008.11.013
Chen J, Qian H (2016) Characterizing replenishment water, lake water and groundwater interactions by numerical modelling in arid regions: a case study of Shahu Lake. Hydrol Sci J. doi:10.1080/02626667.2015.1133910
Cott PA, Zajdlik BA, Palmer MJ, McPherson MD (2016) Arsenic and mercury in lake whitefish and burbot near the abandoned Giant Mine on Great Slave Lake. J Great Lakes Res 42(2):223–232. doi:10.1016/j.jglr.2015.11.004
Dou Y, Howard KWF, Qian H (2016) Transport characteristics of nitrite in a shallow sedimentary aquifer in Northwest China as determined by a 12-day soil column experiment. Expo Health 8(3):381–387. doi:10.1007/s12403-016-0206-x
Garg RK, Rao RJ, Uchchariya D, Shukla G, Saksena DN (2010) Seasonal variations in water quality and major threats to Ramsagar reservoir, India. Afr Environ Sci Technol 4(2):61–76
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090. doi:10.1126/science.170.3962.1088
Jirsa F, Gruber M, Stojanovic A, Omondi SO, Mader D, Körner W, Schager M (2013) Major and trace element geochemistry of Lake Bogoria and Lake Nakuru, Kenya, during extreme drought. Chem Erde 73:275–282. doi:10.1016/j.chemer.2012.09.001
Juma DW, Wang H, Li F (2014) Impacts of population growth and economic development on water quality of a lake: case study of Lake Victoria Kenya water. Environ Sci Pollut Res 21(8):5737–5746. doi:10.1007/s11356-014-2524-5
Kiran B, Rani N, Kaushik A (2016) Environmental toxicity: exposure and impact of chromium on cyanobacterial species. J Environ Chem Eng 4:4137–4142. doi:10.1016/j.jece.2016.09.021
Li P, Qian H (2011) Human health risk assessment for chemical pollutants in drinking water source in Shizuishan city, Northwest China. Iran J Environ Health Sci Eng 8(1):41–48
Li P, Qian H, Wu J (2014) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, Northwest China. Environ Geochem Health 36(4):693–712. doi:10.1007/s10653-013-9590-3
Li P, Qian H, Howard KWF, Wu J (2015) Building a new and sustainable “Silk Road economic belt”. Environ Earth Sci 74(10):7267–7270. doi:10.1007/s12665-015-4739-2
Li P, Li X, Meng X, Li M, Zhang Y (2016a) Appraising groundwater quality and health risks from contamination in a semiarid region of Northwest China. Expo Health 8(3):361–379. doi:10.1007/s12403-016-0205-y
Li P, Wu J, Qian H (2016b) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua county, China. Arab J Geosci 9(1):15. doi:10.1007/s12517-015-2059-1
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016c) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8(3):331–348. doi:10.1007/s12403-016-0193-y
Lu XZ, Liu MS, Hu HB, Wang HJ, Deng CH (2001) Problems of ecological environment and their remedial measures in Sand Lake scenic area Ningxia. J Nanjing For Univ 25(3):89–92 (in Chinese)
Luo YZ, Qu XN (2011) Variation trend analysis of water quality in Sand Lake of Ningxia Hui Autonomous Region. Bull Soil Water Conserv 31(5):246–249 (in Chinese)
Ministry of Environmental Protection of the P.R. China (2014) Technical guidelines for risk assessment of contaminated sites, (HJ 25.3–2014). China Environmental Science Press, Beijing (in Chinese)
Qian H, Wu J, Zhou Y, Li P (2014) Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan Plain. Hydrol Process 28:3554–3562. doi:10.1002/hyp.9915
Qu X (2007) Effect of tourism development on water environment of Sand Lake in Ningxia. J Arid Land Resour Environ 21(3):105–108 (in Chinese)
Rao GS, Rao GN (2010) Study of groundwater quality in greater Visakhapatnam city, Andhra Pradesh (India). J Environ Sci Eng 52(2):137–146
Ravikumar P, Mehmood MA, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261. doi:10.1007/s13201-013-0077-2
Rebelo FM, Caldas ED (2016) Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res 151:671–688. doi:10.1016/j.envres.2016.08.027
Ren JD, Ren XR, Jin Y, Ma CM, Luo T (2008) Characteristics of contaminated sediments of heavy metal in Shahu Lake. Ningxia Eng Technol 7(3):224–227 (in Chinese)
Saeed A, Hashmi I (2014) Evaluation of anthropogenic effects on water quality and bacterial diversity in Rawal Lake, Islamabad. Environ Monit Assess 186(5):2785–2793. doi:10.1007/s10661-013-3579-3
USEPA (2004) Risk assessment guidance for superfund, volume I: human health evaluation manual (Part E, supplemental guidance for dermal risk assessment). EPA/540/R/99/005.Washington: office of emergency and remedial response
USEPA (2015) Regional screening levels (RSLs)—generic tables. https://www.epa.gov/risk/regional-screening-levels-rsls-generictables-November-2015. Accessed 15 Sept 2016
Wang P, Ren XR, Jin Y, Ma CM (2008) Characteristics of contaminated sediments in Shahu Lake. Ningxia Eng Technol 7(1):5–8 (in Chinese)
Wang D, He S, Shan C, Ye Y, Ma H, Zhang X, Zhang W, Pan B (2016) Chromium speciation in tannery effluent after alkaline precipitation: isolation and characterization. J Hazard Mater 316:169–177. doi:10.1016/j.jhazmat.2016.05.021
WHO (2004) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva, p 515
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329. doi:10.1007/s12403-015-0170-x
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588. doi:10.1007/s12665-015-4018-2
Yang X, Duan J, Wang L, Li W, Guan J, Beecham S, Mulcahy D (2015) Heavy metal pollution and health risk assessment in the Wei River in China. Environ Monit Assess 187:111. doi:10.1007/s10661-014-4202-y
Yu FC, Fang GH, Ru XW (2010) Eutrophication, health risk assessment and spatial analysis of water quality in Gucheng Lake, China. Environ Earth Sci 59:1741–1748. doi:10.1007/s12665-009-0156-8
Zhao HX, Qiu XC, Yang YM, Li GD (2010) Evaluation and analysis on trophic level in Shahu Lake. Hubei Agr Sci 49(10):2414–2417 (in Chinese)
Acknowledgements
We are grateful to the support from the National Natural Science Foundation of China (41502234 and 41602238), the Foundation of Outstanding Young Scholar of Chang’an University (310829153509), the Research Funds for Young Stars in Science and Technology of Shaanxi Province (2016KJXX-29), the General Financial Grant from the China Postdoctoral Science Foundation (2015M580804 and 2016M590911), the Special Financial Grant from the China Postdoctoral Science Foundation (2016T090878), the Special Financial Grant from the Shaanxi Postdoctoral Science Foundation, the Innovation Training Program for Undergraduate Students of Chang’an University (201510710072 and 201610710073), the Joint Foundation of Key Laboratory of Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences (KF201601), and the Fundamental Research Funds for the Central Universities (310829151072 and 310829161014). Anonymous reviewers and the Editor are sincerely thanked for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, P., Feng, W., Xue, C. et al. Spatiotemporal Variability of Contaminants in Lake Water and Their Risks to Human Health: A Case Study of the Shahu Lake Tourist Area, Northwest China. Expo Health 9, 213–225 (2017). https://doi.org/10.1007/s12403-016-0237-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0237-3