Abstract
Haridwar once regarded as the holiest city of India has fast assumed the garb of an industrial destination after the establishment of the integrated industrial estate (IIE) Haridwar in the year 2000. IIE Haridwar is flanked by the Rajaji National Park and rural/agricultural areas in addition to urban residential, commercial and other industrial areas. Five heavy metals Cobalt (Co), Chromium (Cr), Iron (Fe), Nickel (Ni) and Zinc (Zn) were monitored monthly at 18 groundwater locations for a year in and around IIE Haridwar. Co was detected in 94 %, Fe in 99 % and Cr in 98 % samples; and Ni in 90 % and Zn in 99 % of the 216 samples. Co, Cr, Fe and Ni were found to exceed standard guideline limits in 196/216, 199/216, 71/216 and 147/216 samples, respectively. Two-way ANOVA showed main effects of season on concentrations of Fe and Zn. Significant correlations were identified between metal pairs Co–Cr and Fe–Zn. PCA identified two principal components, the anthropogenic pollution factor with loadings on Co and Cr and geogenic factor with loadings on Zn and Fe. HCA supported the findings of PCA and formed three clusters. Health risk assessment showed non-carcinogenic risk at all 17/18 locations due to Cr indicating adverse impact of industrial activity on human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater conventionally considered a safe reserve of good quality water worldwide is now found to be contaminated with heavy metals in excess of natural background loads due to increasing urbanization and industrialization. Groundwater contamination may occur naturally from normal geological phenomena such as ore formation, weathering of rocks and leaching or due to increased population, urbanization, industrial activities, agricultural practices, exploration and exploitation of natural resources (Akinmosin et al. 2009). Anthropogenic activities, like industrial production and unsafe disposal of industrial wastes agricultural wastes and domestic sewage, release heavy metals into the environment (Sirajudeen et al. 2012). The percolating wastewater picks up a large number of heavy metals and reaches the aquifer system and contaminates ground water. These heavy metal-bearing wastewaters are of considerable concern because they are highly toxic, non-biodegradable and probably carcinogenic in nature (Dermentzis et al. 2011). Metals are the most persistent contaminants in the aquatic environment (Chai et al. 2010; Li et al. 2014). Although the industrial sector accounts for only three per cent of the annual water withdrawals in India, its contribution to water pollution, particularly in urban areas, is considerable.
While some metals, such as Fe, Cr and Cu, act as micronutrients to maintain human and animal biological health, they can become toxic after exceeding acceptable levels. High concentration exposure is not necessary to produce a state of toxicity in the body, as heavy metal accumulation occurs in body tissues gradually, and over time, it can reach toxic concentration levels, much beyond acceptable limits. Human health risk assessment through drinking water consumption has thus become the prime focus of environmental researchers globally (Khan et al. 2014). Mining and processing activities, production of alloys and chemicals, sewage effluents, urban run-off, and agricultural run-off are major anthropogenic contributors of Co to the aquatic environment. Oral exposure to Co in humans results in adverse effects on respiratory, cardiovascular, gastrointestinal, haematological, hepatic, renal, endocrine, dermal, ocular, hypothermic, and body weight (ATSDR 2004). Cr and its salts are used in the leather tanning industry; the manufacture of catalysts, pigments and paints; fungicides; the ceramic and glass industry; photography; for chrome alloy and metal production; chrome plating; and corrosion control. As a result, Cr has become a major factory run-off pollutant that is beginning to become a global trend (Hu 2002). A number of epidemiological studies of workers in Cr-production facilities have demonstrated an association between inhalation of Cr(VI) and upper respiratory irritation and atrophy, lower respiratory effects, and renal effects (USEPA 1998). The toxicity of Cr stems from its tendency to be corrosive and to cause allergic reactions (Howard 2002). Inhalation and retention of materials containing Cr(VI) can cause perforation of the nasal septum, asthma, bronchitis, pneumonitis, inflammation of the larynx and liver, and increased occurence of bronchogenic carcinoma. Skin contact of Cr(VI) compounds can induce skin allergies, dermatitis, dermal necrosis and dermal corrosion (Bielicka et al. 2005). In general, the chromium content of surface waters reflects the extent of industrial activity (WHO 2003a). Ni, a hard, silvery-white metal, which combines with other metals to form alloys, is used mainly in the production of stainless steels, non-ferrous alloys and super alloys. Other uses of Ni and Ni salts are in electroplating, as catalysts, in Ni–Cadmium batteries, in coins, in welding products, and in certain pigments and electronic products (IARC 1990). Allergic contact dermatitis is the most prevalent effect of Ni in the general population (WHO 2007). Fe which is the second-most abundant metal in the earth’s crust is a common constituent in soil and ground water. Iron oxides are used as pigments in paints and plastics, and as coagulants in water treatment. Anaerobic groundwaters may contain iron (II) at concentrations up to several milligrams per litre without discoloration or turbidity in the water when directly pumped from a well. Taste is not usually noticeable at iron concentrations below 0.3 mg/L, although turbidity and colour may develop in piped systems at levels above 0.05–0.1 mg/L. No health-based guideline value for iron is proposed (WHO 2003a, b, c).The primary anthropogenic sources of zinc in the environment are from metal smelters and mining activities The production and use of zinc in brass, bronze, die castings metal, alloys, rubbers, and paints may also lead to its release to the environment through various waste streams (EPA 2005). Waste streams from Zn- and other metal-manufacturing industries, domestic wastewater and run-off can discharge Zn into waterways (ATSDR 2005). Oral exposure to high levels of Zn in humans can result in several systemic effects, the most sensitive of which are related to diminished copper status. Acute toxicity arises from the ingestion of excessive amounts of Zn salts, either accidentally or deliberately as an emetic or dietary supplement. Vomiting usually occurs after the consumption of more than 500 mL of Zinc Sulfate (WHO 2003c). Nickel may be present in some groundwaters as a consequence of dissolution from nickel ore-bearing rocks. Nickel is used mainly in the production of stainless steels, non-ferrous alloys, and super alloys. Other uses of nickel and nickel salts are in electroplating, as catalysts, in nickel–cadmium batteries, in coins, in welding products, and in certain pigments and electronic products. Allergic contact dermatitis is the most prevalent effect of Ni in the general population. With reference to carcinogenicity, it was concluded that occupational exposure to Sulfidic and Oxidic Nickel at high concentrations causes lung and nasal cancer (WHO 2007; Sharma et al. 2011).
One of the main objectives behind the creation of the separate state of Uttarakhand was to ensure rapid economic development of the area. Following its new industrial policy of 2003, Uttarakhand achieved an impressive industrial growth rate of 18.18 % in 2006, just three years after inception, compared to the national growth rate of 10.1 %. The State Industrial Infrastructural Development Corporation of Uttaranchal (SIDCUL) established four industrial areas in the state of which integrated industrial estate (IIE) Haridwar is one. The district is home to more than 38 private/government industrial areas. Haridwar known worldwide for its religious significance is fast assuming the garb of an industrial destination after the establishment of IIE Haridwar in the year 2000. The heavy engineering industry—Bharat Heavy Electricals Limited (BHEL) and Bahadrabad old industrial area are located within 1–2 km range of IIE Haridwar. All these industrial areas are sustained by ground water, and it is likely that ground water contamination is compounded by high concentration of industries over a small area. The main objectives of the study were (1) to assess concentrations and distribution of heavy metal in the study area, (2) to identify the sources of pollution and (3) to assess the Human Health Risk due to ingestion of ground water.
Study Area
Haridwar district is located in southwestern part of Uttarakhand State between latitudes 290 35′ and 300 40′ North and longitudes 770 43′ to 780 22′ East and can be located in Survey of India Degree Sheet Nos. 53 J, F, G and K. The district experiences moderate subtropical-to-humid climate with three distinct seasons, viz. summer followed by rainy and winter seasons. The topography of the district is undulating in the northern part and more or less plain towards south. The altitude ranges from 869 to 232 m. Hydrogeolological investigations reveal that the ground water flows in the southwest direction. The ground water conditions in alluvial parts of Hardwar district are considerably influenced by the varying lithology of the subsurface formations. The fluvial deposits of Indogangetic Plains exhibit significant variations, both laterally and vertically. The main source of water, which sustains groundwater in the district, is rainfall. The other sources of groundwater replenishment are infiltration from canals and irrigation return flow. The common ground water abstraction structures in Hardwar district are shallow and deep tubewells. Dug wells are used for drinking and other domestic purposes up to a limited extent. Hydrogeological surveys carried out in Hardwar district show that water levels range from 0.78 to 50.20 m bgl in pre-monsoon period and from 0.64 to 48.56 m bgl during post-monsoon period, respectively. The stage of ground water development is 96.40 %, and the district is categorized as critical. Ground water in Hardwar district occurs under unconfined, confined and semi-confined conditions. The aquifers are separated with thick clay with considerable thickness, which act as confining layers. The water-level data suggest the presence of multilayer aquifer system. The first one is unconfined, and the others are semi-confined or confined. The depth of the first unconfined aquifer ranges from 4 to 8 m bgl, and the those of the others range from 18 to 25 m bgl, 40 to 60 m bgl and 90 to 120 m bgl. The northern and northeastern parts of the district comprise boulders, pebbles, gravels, sand and clay, which form a good recharge zone. Alluvium is the main water-bearing formation in the area, which consists of coarse sand, fine sand and silt. Geomorphologically, Hardwar district can be divided into four geomorphic units. These are flood plain, lower piedmont plain, upper piedmont plain and structural hills. The higher areas of Siwalik and Bhabar are situated in the northern and northeastern parts of the Hardwar district (CGWB 2009).
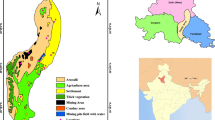
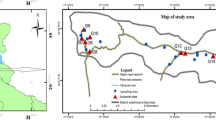
Eighteen sampling locations (L1, L2, L3,…, L18) spread over five land-use areas were identified in the study area ( Fig. 1). Three sampling locations were identified in each land-use area. IIE Haridwar (L1, L2, L3) has more than 585 industrial units spread over 8.23 km2. Industries in IIE Haridwar include a mix of pharmaceutical, plastics and allied, electrical and electronics, metal and fabrication, food and agro, textiles, paper and packaging, chemicals, and general manufacturing industries. Aneki rural area (L3, L4, L5) is a rural/agricultural landuse area characterized by low-density unplanned housing with no piped water supply and sewerage facilities. Aneki Rural area is characterized by shallow-to-intermediate aquifer (Singhal et al. 2010) and is geologically part of the upper piedmont plain which occurs all along the south of Siwalik hills in variable lateral and areal extents: formed at the foothills by the coalescence of several alluvial fans comprising boulders, gravel and clay. Shivalik Nagar (L7, L8, L9) which is one of the prime residential areas of Haridwar with low-density housing has the Bahadrabad old industrial area (L10, L11, L12) in close proximity. Bahadrabad Old industrial area also comprises mixed nature of industries such as electroplating, metal and fabrication, packaging, electrical, electronics, etc. Bahadrabad Old Industrial area and Shivalik Nagar are parts of lower piedmont plain characterized by flat-to-undulating plain with gradient towards southwest having micro relief; sediments vary from fine clastic-to-coarse clastic with variable run-off and filtration. Rajaji National Park (RNP) (L13, L14, L15) is a protected area created in 1983 by amalgamation of three wildlife sanctuaries. The park spreads over 820 sq.km covering three districts of Uttarakhand: Haridwar, Dehradun and Pauri Garhwal, and represent the Shivalik eco-system. The Shivalik trail is 10 million years old and very rich in fossils. RNP is home to 23 species of mammals, 315 species of birds and the Asian elephant. The park forms the northern periphery of IIE Haridwar. RNP is part of the structural hills characterized by high relief and deep incised drainage with steep and sharp hill slope and well-defined crest line. This terrain shows rugged topography and homogenous lithology and is densely forested indicating the presence of loose alluvial material. The Railway Station arterial road (L16, L17, L18) is the commercial hub of the city and houses the Railroad Station and State level Bus Terminus. It is characterized by commercial establishments like hotels and shops and heavy vehicular traffic.
Materials and Method
Sampling and Analytical Methodology
Heavy metals (Cr, Co, Ni, Fe and Zn) were monitored monthly at 18 locations over a year from January 2012 to December 2012. Three locations were sampled monthly from each land-use, accounting for a total of 216 (3 samples × 6 locations × 12 months) samples. Sampling was done by grab sampling method in early morning. Groundwater was sampled in 1-L plastic containers after flushing out initial discharge of water from handpumps/tubewells for a few minutes. 250 mL of sample was oven dried at 105 °C overnight until the sample evaporated. 25 mL of 5 % of Nitric Acid solution was poured into the beaker after which the sides of the beakers were cleaned with a glass rod to ensure that the residues from evaporated sample were dissolved in the Nitric Acid. The sample was then filtered through a Whatman filter paper into a 25-mL volumetric flask and made up to volume of the flask by adding 5 % of HNO3 solution. Sample blank was prepared in the same manner as was done for the sample using Double Distilled Water. Heavy Metals were analysed by aspirating samples in Atomic Absorption Spectrophotometer (AAS) at respective wavelengths of metals to be identified. Calibration curves produced using quality-control standards were used to evaluate data from each set of samples. Reagents, procedural blanks, and samples were measured three times, and the average of three values was used.
Methodology for Assessing Distribution of Metals
Descriptive Statistics were used to assess distribution of metals in the study area. The dataset was first examined for the presence of non-detect data. As the total amount of non-detect data was less than 15 % of the total data, non-detect data was substituted with half of the limit of detection (LOD) of the respective heavy metal. Two-way ANOVA was conducted to examine spatial and seasonal variations of heavy metals in the study area. Residual analysis was performed to test for the assumptions of the two-way ANOVA. Outliers were assessed by inspection of a boxplot, and normality was assessed using Shapiro–Wilk test for each group combination of independent variables. Homogeneity of variances was assessed by Levene’s test. In case of detection of outlier, the outlier was removed and substituted with value of nearest highest variable. Data which were not normalised or which had significant Levene’s test scores were transformed before the analysis.
Pollution Source Apportionment Methodology
Coefficient of Correlation was used to find correlation between heavy metals. The main factors of pollution were identified with principal component analysis (PCA) while hierarchial cluster analysis (HCA) was applied to identify homogenous clusters of sampling locations. Correlation is a method used to evaluate the degree of interrelation and association between two variables (Nair et al. 2005). Bivariate correlation is useful for determining the strength and direction of the association between two variables. A correlation of 1 indicates a perfect positive relationship between two variables. A correlation of −1 indicates that one variable changes inversely with relation to the other. A strong correlation is indicated by 0.8 > r < 0.1, moderate correlation by 0.6 > r < 0.8 and low correlation by r < 0.6. A correlation of zero indicates that there is no relationship between the two variables (Kapil et al. 2009). Pairs of heavy metals with strong correlations were likely to have similar pollution sources. PCA and HCA are common multivariate statistical methods used in environmental studies (Han et al. 2006) to simplify datasets by reducing components or grouping variables into homogenous clusters. PCA was applied to determine the sources or main factors of pollution. According to the combination of criteria for factor selection, eigenvalues higher than 1.0 were extracted. Hierarchical Cluster analysis (HCA) was performed first to check the results of the PCA by clustering the metals and then to identify homogenous locations based on distribution of heavy metals.
Health Risk Assessment Methodology
Non-carcinogenic health risk assessment was carried out according to usual reliable exposure pathways of contaminants recommended by USEPA (1989). The average daily dose (ADD) is the dose rate averaged over a pathway-specific period of exposure expressed as a daily dose on a per-unit-body-weight basis. The ADD is used for exposure to chemicals in unfiltered groundwater. For direct ingestion (ADDi), the equation used was
For dermal contact with chemicals in groundwater, dermally absorbed average daily dose (ADDd) can be estimated by
where ADD(i)/ADD(d) is the average daily dose (mg/L-day); C is the metal concentration in mg/L; IR is the ingestion rate (2.5 L/d); EF is the Resident Exposure frequency (350 days/year); ED is the exposure duration (26 years); BW is the Resident Body Weight (80 kg); AT is the Averaging time-resident (365 days/year); SA is the skin surface area available for contact (20,900 cm2), and ET is the Resident Water Exposure Time during bathing and shower (0.71 h/event). Standard exposure factors were sourced from guidance update of USEPA (2014). Kp is the dermal permeability coefficient (2 × 10−3 cm/h for Cr: 4 × 10−4 cm/h for Co: 2 × 10−4 cm/h for Ni: 6.0 × 10−4 cm/h for Zn: and 1 × 10−3 for all other inorganic metals (USEPA 2004).
A hazard quotient (HQ) is the ratio of an exposure level of a single toxic substance to the reference dose (RfD) for that substance. Because RfDs are generally exposure pathway-specific (e.g. oral or dermal RfD), the HQ is a single-substance/single-exposure pathway ratio. HQ is the health risk likely to be without non-carcinogenic health effects during a specified duration of exposure for an individual metal and is computed using the equation:
where Exposure represents both ADD(i) and ADD(d) values for each metal in mg/kg/day, and RfD is the oral RfD in mg/kg/day. The RfD is “an estimate of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious (non-cancer) effects during a lifetime”. By definition, exposures below the RfD are unlikely to produce an adverse effect; above this value, an exposed individual may be at risk for the effect. RfD values for Cr, Ni and Zn were taken from USEPA (2011). As RfD values for Fe have not been estimated by the U.S. EPA, health risk assessment (HRA) was not done for Fe. RfD values for Cr, Co, Ni and Zn were based on EPA evaluations (USEPA 2009, 2011). For non-carcinogenic risk, a HQ > 1 signifies adverse non-carcinogenic effects of concern, while HQ < 1 can be interpreted as an acceptable level. A Hazard Index (HI) is the sum of two or more HQs. A HI is usually a single-substance/multiple-exposure pathway ratio, a multiple-substance/single-exposure pathway ratio, or a multiple-substance/multiple-exposure pathway ratio. For non-carcinogenic effects, a concentration is calculated that corresponds to an HI of 1, which is the level of exposure to a chemical from all significant exposure pathways in a given medium below which it is unlikely for even sensitive populations to experience adverse health effects. A HI > 1 suggests that ingestion and dermal contact with the water could have adverse impacts on the residents’ health. HI was not calculated for iron as RfD values for iron have not been estimated by the U.S. EPA.
Statistical Analysis
Ground water data were analysed statistically using SPSS 21, while Surfer 11 was used for mapping and graphical data.
Results and Discussion
Distribution of Heavy Metals in Study Area
Groundwater in the study area was found to be free of odour. pH of all samples ranged between 6.8 and 7.3. Co was detected in 94 %, Fe in 99 %, Cr in 98 % samples, Ni in 90 % and Zn in 99 % of the samples. Table 1 and Fig. 2 depict distribution of heavy metals in the study area. Maximum concentrations of heavy metals in the study area were in the order: Zn (0.66 mg/L) > Fe (0.6 mg/L) > Co (0.36 mg/L) > Ni (0.29 mg/L) > Cr (0.26 mg/L). Co levels exceeded health reference level (HRL) of 0.07 mg/L (USEPA 2009) at 196/216 locations. Gaur et al. (2011) reported Co levels within permissible limits in Haridwar region. The maximum concentration (0.39 mg/L) of Co in the study area was found at L17 (commercial area) in summer followed by (0.32 mg/L) at L18 (commercial) in summer. Co levels exceeded HRL at all locations in the rural area. Quazi et al. (2014) similarly reported high Co levels in groundwater of villages near diversified industries in a study of other industrial area. Annual average Co concentration by landuse was maximum (0.15 mg/L) in RNP (L13/L14/L15) and minimum (0.11 mg/L) at IIE Haridwar (L1/L2/L3)). Co concentration in study area was more in summer compared to other seasons. Cr concentration exceeded guideline limit of 0.05 mg/L (WHO 2011; BIS 2012) in 199/216 samples. Cr level in study area was maximum at L4 (0.26 mg/L) rural location in winter. Annual average Cr level by landuse was maximum (0.15 mg/L) at Bahadrabad old industrial area (L10/l11/L12) followed by 0.13 mg/L at commercial landuse locations (L16/L17/L18). Cr concentration was maximal in winter and minimal in summer. Highest concentration of Fe in study area was 0.56 mg/L at L17 (commercial land use) in summer. This exceeded the guideline limit of 0.3 mg/L (WHO 2011). Fe concentration exceeded WHO guideline limit in 71/216 samples. Average concentration of Fe for the study area was the greatest in monsoon followed by those in winter and summer. This was likely the result of increased rusting of pipe castings of handpumps/tubewell and increased metal dissolution in monsoon and post-monsoon. Groundwater at locations L6 (rural) and L11, L12 (old industrial) was found to be of pale yellow colour—a likely result of rusting in the cast iron pipeline of the hand pumps. Elinge et al. (2011) reported that the presence of Fe was responsible for the brownish-red colour of the water when allowed to stay for some minutes. Fe was found to exceed guideline limit in 11/30 samples in IIE Haridwar. High levels of Fe have been reported in industrial areas by several researchers (Bharti et al. 2013; Ravichandran and Jayaprakash 2011; Thomas et al. 2011). Ni concentrations exceeded guideline limit of 0.07 mg/L (WHO 2011) in 147/216 samples. Ni concentration was maximum (0.3 mg/L) at L2 (IIE Haridwar) in winter. Annual average Ni level by landuse was maximum (0.9 mg/L) at IIE (L1/L2/L3) followed by 0.08 mg/L at rural area (L4/L5/L6). Similar findings were reported in a separate study where parametric tests showed no statistical difference in high nickel levels in ground water of an industrial estate and nearby hamlets (Etim and Onianwa 2013). High levels of Ni due to a mix of anthropogenic activities and wastes from automobiles, repair shops, electroplating unit and sewage run-off (Sirajudeen et al. 2012) and pollution by motor parts waste, sewage waste and domestic waste (Virha et al. 2010) have been reported. Average Nickel levels for the study area were high in monsoon season. The higher availability of Ni in monsoon compared to other seasons was likely to be a consequence of dissolution from nickel ore-bearing rocks. Zn levels in study area were less than guideline limit of 5 mg/L (WHO 2011) at all locations and seasons. Weak adsorptive nature of Zn in soil could be the likely cause for its low infiltration rate in groundwater. Annual average zinc level by landuse was maximum at 0.3 mg/L for urban residential landuse followed by 0.29 mg/L at commercial landuse. Zn concentrations were higher in monsoon compared to other seasons. The Zn levels below acceptable limits in groundwater of industrial areas and their environs have similarly been reported by several researchers (Ullah et al. 2009; Thomas et al. 2011; Alshikh 2011; Shivasharanappa and Huggi 2012; Zamani et al. 2012; Bharti et al. 2013; Musa et al. 2013; Ramola and Singh 2013). The maximum concentration of Zn was 0.66 mg/L at L9 in summer. Two-way ANOVA was applied to examine the main and interaction effects of two independent variables, season and location, on metal concentration. Statistically significant main effect of season: F(2216) = 3.751, p = .026, partial n2 = .044 was found on “Fe concentration” score. For winter and summer, “Fe concentration” score was .104 (95 % CI, from .012 to .197) points higher for winter than summer. For Zn, statistically significant main effect of season on “Zn concentration” score, F(2196) = 3.273, p = .04, partial n2 = .039, was observed.
Pollution Source Apportionment
Firstly, correlation coefficients were worked out between metals for all locations in the study area to find the strength and direction of metal correlations. Secondly, correlations were found between metals for respective landuse areas to identify correlations between metals that were characteristic of the landuse they originated from. For the overall study area, moderate positive correlation was found between Co and Cr (+.33) and Fe-Zn (+.57). Correlation between metals for respective landuses (Table 2) showed that Cr-Co correlation ranged from moderate to strong for all landuses except RNP. All the locations are characterized by urban/industrial/commercial activities, whereas the RNP is a protected area. Thus, it could be inferred that the source of Co-Cr contamination was anthropogenic in nature. Strong Cr-Ni correlation (>+.9) was observed in IIE Haridwar and adjacent rural area of Aneki. Industrial effluents from electroplating industries contain high amounts of heavy metal ions such as Co, Cr, Ni, Cu, Cadmium (Cd) and Zn. Only 30–40 % of all metals used in plating processes are effectively utilized, i.e. plated on the articles. The rest of the metals contaminate the rinse waters during the plating process, when the plated objects are rinsed upon removal from the plating bath (Konstantinos et al. 2011). Galvanizing iron, welding, electroplating, etc. activities in IIE Haridwar were likely to contribute to Co, Cr, Ni and Zn contamination of groundwater in adjacent areas. Zhang and Li (1987) reported effects of environmental contamination of well water in villages adjacent to a chromium alloy plant. Strong correlation between Co and Fe was seen in urban residential area (+.84), RNP (+.93) and commercial area (+.98). Bahadrabad old industrial area showed strong positive correlation between Fe and Ni (+.94), while urban residential area of Shivalik Nagar showed moderate Fe–Ni correlation (+.68). Ni–Zn correlations were strong in urban residential area (+.99), rural area (+1.0) and RNP (+.85). Fe-Zn correlations were found to be strong at RNP (+.7) and commercial area (+1.0). Zn and Ni are most common metals emitted by vehicular traffic. Often, Ni easily undergoes activation and combines quickly with iron. A considerable part of Ni finds its way into the environment as a result of the burning of diesel oil containing Ni (Barałkiewicz and Siepak 1999). Correlations between different metals indicated that likely sources of Cr, Co and Ni were anthropogenic sources such as industrial activity, vehicular pollution, sewage infiltration and urban run-off etc.
PCA was then applied to the normalized datasets of five heavy metals. PCA revealed two components that had eigenvalues greater than 1 and which explained 38.703 and 33.19 % of the total variances, respectively. Visual inspection of the scree plot indicated that two components (Fig. 3) explaining 71.89 % of the total variance should be retained. A Varimax orthogonal rotation was employed to aid interpretability, and pollution sources were identified through the representation of the factor scores in factor analysis. (Reisenhofer et al. 1998; Kowalkowski et al. 2006; Kannel et al. 2008). The higher the factor scores, the higher the factor’s influence (Felipe-Sotelo et al. 2007). Factor 1 accounting for 38.703 % of the total variance was found to have high loading on metals Zn and Fe. As Zn was found to be below permissible standard limits at all locations and Fe is one of the metals having abundant crustal presence, it was concluded that this factor was geogenic in nature. Enrichment Factor Analysis supported the conclusion as Fe and Zn showed insignificant enrichment. The second factor had high loading on metals Co and Cr amounting to a variance of 33.19 % indicating anthropogenic sources of contamination such as urban and industrial activities. This component was named the anthropogenic pollution load factor. The findings of PCA clearly supported the findings of Correlation Analysis.
Lastly, HCA was performed using Wards method and squared Euclidean distance to examine the dataset for interrelationships and to reduce the number of variables into small homogenous groups or clusters with similar characteristics of metallic concentration. HCA classified the heavy metals into two groups based on spatial similarities and dissimilarities. HCA for variable “Metals” showed two clusters of metals Fe and Zn in one cluster and metals Cr, Co and Ni in another cluster also indicating similar source of pollution for Fe and Zn and another source of pollution for Cr, Co and Ni. The result of HCA was consistent with finding of PCA and Correlation Analysis. HCA was then used to identify the number of homogenous clusters resulting from the set of 18 locations (Fig. 3). The HCA identified 3 clusters based on evaluation of agglomeration schedule and dendrogram. Results of Cluster Analysis and the Dendrogram (Fig. 3) showed that locations (L1 and L16) formed Cluster 1; locations (L2, L3, L4, L7, L9, L12, L17 and L18) formed Cluster 2; and Locations (L5, L6, L8, L10, L11, L13, L14 and L15) formed Cluster 3. One-way ANOVA was applied to determine those classifying variables (metals) which were significantly different between the clusters. Results of one-way Anova showed that between groups, means are significant for Co; F(2,17) = 6.405, p = 0.010, Fe; F(2,17) = 7.268, p = 0.006 and Zn; F(2,17) = 15.203, p = 0.000, indicating these three variables reliably distinguished among the three clusters. Tukey post hoc test was used to establish differences between clusters. For the metal Co, result of the Tukey post hoc test showed that the cluster means were different for Clusters 1 and 3 (p = .009). For Fe, cluster means were different for Clusters 2 and 3, while for Zn, means were different between Cluster 1 and Cluster 2 (p = 0.000), between Cluster 1 and Cluster 3 (p = 0.21) and between Cluster 2 and Cluster 3 (p = 0.011). Result of cluster analysis along with interpretation of activities at the study locations showed Cluster 1 was characterized by low Co and Zn contents. Cluster 2 was characterized by high Co, Fe and Zn contents, while Cluster 3 was characterized by average Fe and Zn contents.
Health Risk Assessment
Non-carcinogenic risk to Human Health of adults was assessed by working out Oral and Dermal Exposures to groundwater. Table 3 lists the HQ and HI by metal and location. HQ for Cr exceeded 1 at all locations except L14 and L18. HQ for Cr was maximum at 1.95 at L10 in winter followed by 1.81 at L11 in monsoon. Both these locations are part of the Bahadrabad Old Industrial area. HQ for Cr was minimal at L18 in summer. In general, high Cr concentrations are representative of industrial activity. In a study on heavy metal contamination in the industrial area of Kattedan, the range of Cr in ground water was found to exceed the permissible guideline limits, and the human exposure assessment revealed that the concentration of Cr was much higher than the permissible levels in people residing in the study area (Sekhar et al. 2006). HQ for all other metals was below 1 indicating no non-carcinogenic hazard due to Oral or Dermal exposure. HI was found to be greater than 1 at all locations except L18. In winter season, HI was maximum at L7 (1.95), urban residential area. HI in RNP ranged between 1.7 and 1.85. This clearly indicates that the protected area is being impacted by anthropogenic activity happening in its surroundings. HI at all locations except L18 indicated the presence of non-carcinogenic hazard from oral ingestion of the ground water due to cumulative effect of contaminants. One-way ANOVA for main effect of location on HI was statistically significant, F(17, 54) = 6.661, p = .000. Tukey post hoc Test was significant (p < .05) for L18 paired with all other locations. Two-way ANOVA showed that HI was independent of seasonal groundwater table fluctuation. The HI values by season for all groundwater locations were used to construct Health Hazard Maps (Fig. 4). Hazard Maps for heavy metals were created using Surfer program by averaging and smoothing the data; the contour lines were constructed based on the distance and interpolation gridding method.
Conclusions
Co was detected in 94 %, Fe in 99 %, Cr in 98 % samples; and Ni in 90 % and Zn in 99 % of the 216 samples. Co, Cr, Fe and Ni were found to exceed standard guideline limits in 196/216,199/216, 71/216 and 147/216 samples, respectively. Zn levels were within WHO’s acceptable standard limits at all locations and seasons. Fe, Ni and Zn metals were available more in monsoon compared to other seasons, while Co and Cr concentrations in the study area were maximum in summer and winter, respectively. Correlation coefficients showed moderate positive correlation between metals Co and Cr and Fe and Zn for the study area. Two-way ANOVA results were not significant for main and interaction effects of location and season on Co, Cr and Ni. Two-way ANOVA result was statistically significant for seasonal effects on Fe and Zn with concentrations in winter being more than those in other seasons indicating the possibility of metal dissolution in groundwater after monsoon. PCA identified the anthropogenic (industrial/urban) pollution load factor with loadings on Co and Cr and geogenic pollution load factor with loadings on Zn and Fe. Factors identified by PCA were in consonance with Correlation Analysis. Results of HCA along with interpretation of activities at the study locations showed Cluster 1 was characterized by low Co and Zn contents. Cluster 2 was characterized by high Co, Fe and Zn contents, while Cluster 3 was characterized by the moderate Fe and Zn contents. HQ for Cr exceeded 1 in all locations but one, while HI showed Non-carcinogenic Hazard in all locations except in L18. HQ > 1 for Cr and its emergence as pollution load factor in PCA clearly show contamination due to industrial/anthropogenic activities in the study area. This study shows that the protected area of RNP is also impacted due to Cr contamination from its surrounding land uses. This study also suggests that groundwater could be ingested only after taking due precautions.
References
Akinmosin A, Osinowo OO, Oladunjoye MA (2009) Radiogenic components of the Nigeria Tars and Deposits. Earth Sci Res J 13(1):64–73
Alshikh A (2011) Analysis of heavy metals and organic pollutants of ground water samples of South Saudi. Life Sci J 8(4):438–441
ATSDR (2004) Toxicological profile for Cobalt. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry
ATSDR (2005) Toxicological review for Zinc. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, June 2005
Barałkiewicz D, Siepak J (1999) Chromium, nickel and cobalt in environmental samples and existing legal norms. Pol J Environ Stud 8(4):201–208
Bharti PK, Pawan KP, Singh V (2013) Impact of industrial effluents on ground water and soil quality in the vicinity of industrial area of Panipat city, India. J Appl Nat Sci 5(1):132–136
Bielicka A, Bojanowska I, Wiśniewski A (2005) Two faces of chromium—pollutant and bioelement. Pol J Environ Stud 14(1):5–10
BIS 10500 (2012) Drinking water-specification (second revision), Gr 6. Bureau of Indian Standards, New Delhi
CGWB (2009) Groundwater brochure of Haridwar District. Central Ground Water Board Government of India Ministry of Water Resources, Uttaranchal
Chai LY, Wang ZY, Wang YY et al (2010) Ingestion risks of metals in groundwater based on TIN model and dose-response assessment—a case study in the Xiangjiang watershed, central-south China. Sci Total Environ 408:3118–3124
Dermentzis K, Christoforidis A, Valsamidou E (2011) Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. Int J Environ Sci 1(5):2011
Elinge CM, Itodo AU, Peni IJ, Birnin-Yauri UA, Mbongo AN (2011) Assessment of heavy metals concentrations in bore-hole waters in Aliero community of Kebbi State. Adv Appl Sci Res 2(4):279–282
Etim EU, Onianwa PC (2013) Impact of effluent of an industrial estate on Oruku River in Southwestern Nigeria. World Appl Sci J 21(7):1075–1083
Felipe-Sotelo M, Andrade JM, Carlosena A, Tauler R (2007) Temporal characterisation of river waters in urban and semi-urban areas using physico-chemical parameters and chemometric methods. Anal Chim Acta 583:128–137
Gaur S, Joshi MC, Saxena SK, Dutt HK (2011) Analytical study of water safety parameters in ground water samples of Uttarakhand in India. Journal of Applied Pharmaceutical Science 01(09):166–169
Han YM, Du Cao PX, Posmentier JJ (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355:176–186
Howard H (2002) Human health and heavy metals exposure. In: McCally M (ed) Life support: the environment and human health. MIT Press, Cambridge
Hu H (2002) Human health and heavy metals exposure. Chapter 4. In: McCally M (ed) Life support: the environment and human health. MIT Press, Cambridge
IARC (1990) Nickel and nickel compounds. In: Chromium, nickel and welding. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 49. International Agency for Research on Cancer, Lyon, pp 257–445
Kannel PR, Lee S, Lee YS (2008) Assessment of spatial–temporal patterns of surface and ground water qualities and factors influencing management strategy of groundwater system in an urban river corridor of Nepal. J Environ Manage 86:595–604
Kapil DM, Mamta K, Sharma DK (2009) Hydrochemical analysis of drinking water quality of Alwar District Rajasthan. Nat Sci 7(2):30–39
Khan S, Shah IA, Muhammad S, Malik RN (2014) Arsenic and heavy metal concentrations in drinking water in Pakistan and risk assessment; a case study. Hum Ecol Risk Assess. doi:10.1080/10807039.2014.950925
Konstantinos D, Achilleas C, Evgenia V (2011) Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. Int J Environ Sci 1(5):697–710
Kowalkowski T, Zbytniewski R, Szpejna J, Buszewski B (2006) Application of chemometrics in river water classification. Water Res 40:744–752
Li J, Li FD, Liu Q et al (2014) Impacts of Yellow River irrigation practice on trace metals in surface water: a case study of the Henan-Liaocheng Irrigation Area, China. Hum Ecol Risk Assess 20:1042–1057
Musa OK, Shaibu MM, Kudamnya EA (2013) Heavy metal concentration in groundwater around Obajana and its environs, Kogi State, North Central Nigeria. Am Int J Contemp Res 3(8):170–177
Nair GA, Mohamed AI, Premkumar K (2005) Physico chemical parameters and correlation coefficients of ground waters of North-East Libya. Pollut Res 24(1):1–6
Quazi MA, Khattak AA, Khan MSA, Chaudhry MN, Mahmood K, Akhter B, Iqbal N, Ilyas S, Ali UA (2014) Spatial distribution of heavy metals in ground water of Sheikhupura district Punjab, Pakistan. J Agric Res 52(1):99–110
Ramola B, Singh A (2013) Heavy metal concentrations in pharmaceutical effluents of industrial area of Dehradun (Uttarakhand). India. Int J Environ Sci Res 2(2):140–145
Ravichandran K, Jayaprakash M (2011) Seasonal variation on physico-chemical parameters and trace metals in groundwater of an industrial area of north Chennai, India. Indian J Sci Technol 4(6):646–649. ISSN: 0974–6846
Reisenhofer E, Adami G, Barbieri P (1998) Using chemical and physical parameters to define the quality of karstic freshwaters (Timavo River, North-eastern Italy): a chemometric approach. Water Res 32:1193–1203
Sekhar KC, Chary NS, Kamala CT, Vairamani M, Anjaneyulu Y, Balaram V, Sorlie JE (2006) Environmental risk assessment studies of heavy metal contamination in the industrial Area of Kattedan, India—a case study. Hum Ecol Risk Assess 12(2):408–422. doi:10.1080/10807030500531513
Sharma V, Verma SM, Sakhuja N, Arora D (2011) Impact of heavy metals (Chromium and Nickel) on the health of residents of Jagadhri city due to intake of contaminated underground water. Arch Appl Sci Res 3(5):207–212
Shivasharanappa SP, Huggi MS (2012) Study on the physico-chemical characteristics of ground water of Bidar city and its industrial area. Int J Appl Biol Pharm Technol 3(1):359–367
Singhal DC, Israil M, Sharma VK, Kumar B (2010) Evaluation of groundwater resource and estimation of its potential in Pathri Rao watershed, district Haridwar (Uttarakhand). Curr Sci 98(2):162–170
Sirajudeen J, Manikandan SA, Naveen J (2012) Seasonal variation of heavy metal contamination of ground water in and around Uyyakondan channel Tiruchirappalli district, Tamil Nadu. Der Chemica Sinica 3(5):1113–1119
Thomas DR, Sunil B, Latha C (2011) Physico-chemical analysis of well water at Eloor industrial area-seasonal study. Curr World Environ 6(2):259–264
Ullah R, Malik RN, Qadir A (2009) Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. Afr J Environ Sci Technol 3(12):429–446
USEPA (1989) Risk assessment guidance for superfund volume I human health evaluation manual. United States Environmental Protection Agency EPA/540/l-89/002, December 1989
USEPA (1998) Toxicological review of Hexavalent chromium. In: Support of summary information on the integrated risk information system (IRIS). United States Environmental Protection Agency August 1998. CAS No. 18540-29-9
USEPA (2004) Risk assessment guidance for superfund volume I; Human health evaluation manual (part E, supplemental guidance for dermal risk assessment). United States Environmental Protection Agency. EPA/540/R/99/005. OSWER 9285.7-02EP. PB99-963312
USEPA (2005) Toxicological review of zinc and compounds. In: Support of summary information on the integrated risk information system (IRIS). U.S. Environmental Protection Agency Washington D.C. EPA/635/R-05/002. (CAS No. 7440-66-6)
USEPA (2009) Contaminant information sheets for the final CCL 3 Chemicals. Office of Water (4607M). United States Environmental Protection Agency. EPA 815-R-09-012
USEPA (2011) 2011 edition of the drinking water standards and health advisories. United States Environmental Protection Agency. EPA 820-R-11-002
USEPA (2014) Human health evaluation manual, supplemental guidance: update of standard default exposure factors. United States Environmental Protection Agency OSWER Directive 9200.1-120
Virha R, Biswas AK, Kakaria VK, Qureshi TA, Borana K, Malik N (2010) Seasonal variation in physicochemical parameters and heavy metals in water of Upper Lake of Bhopal. Bull Environ Contam Toxicol. doi:10.1007/s00128-010-0172-0
WHO (2003a) Chromium in drinking water, background document for development of WHO guidelines for drinking-water quality. World Health Organisation. WHO/SDE/WSH/03.04/04
WHO (2003b) Iron in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization. WHO/SDE/WSH/03.04/08
WHO (2003c) Zinc in drinking water, background document for development of WHO guidelines for drinking-water quality. World Health Organization. WHO/SDE/WSH/07.08/55
WHO (2007) Nickel in drinking water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization.WHO/SDE/WSH/07.08/55
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization. ISBN 978 92 4 154815 1
Zamani AA, Mohammad RYMR, Parizanganeh A (2012) Multivariate statistical assessment of heavy metal pollution sources of groundwater around a lead and zinc plant. Iran J Environ Health Sci Eng 9:29. http://www.ijehse.com/content/9/1/29
Zhang J, Li X (1987) Chromium pollution of soil and water in Jinzhou. J Chin Prev Med 21:262–264
Acknowledgments
This research work of Author Dipali Bhaskar Kulkarni was supported by the University Grants Commission (UGC) under grant No. F.7-70/2007(BSR)/UGC-BSR Research Fellowships in Sciences, Bio-Sciences, Agricultural Sciences, Medical Sciences and Engineering Sciences. The authors wish to thank the Department of Environmental Sciences, Pantnagar Agricultural University, Pantnagar, Uttarakhand, India for providing instrumentation and facilities for the analysis of heavy metals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest arising from the direct application of this research work.
Rights and permissions
About this article
Cite this article
Bhutiani, R., Kulkarni, D.B., Khanna, D.R. et al. Water Quality, Pollution Source Apportionment and Health Risk Assessment of Heavy Metals in Groundwater of an Industrial Area in North India. Expo Health 8, 3–18 (2016). https://doi.org/10.1007/s12403-015-0178-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0178-2