Abstract
(Ultra) high-pressure homogenization ((U)HPH) is one of the emerging technologies being studied and developed for various applications in the food industry. (U)HPH was suggested as an effective tool for achieving microbial safety and extending the product shelf life of liquid foods in a continuous process while minimizing some negative attributes of thermal processing. The valve geometry, pressure level, inlet temperature, and the number of homogenization cycles are all factors affecting the level of microbial inactivation and the extent of the techno-functionalities of food biopolymers and matrices. Turbulence, high shear, cavitation, and temperature increase induced by (U)HPH treatments enhance emulsion stability, stabilize proteins in solutions, reduce particle size distributions, and increase the accessibility of health-promoting compounds. This review is a comprehensive and updated overview of the engineering aspects of the (U)HPH process, specifically focusing on (U)HPH modification of food components such as polysaccharides, proteins, and bioactive compounds. A detailed description of the potential applications in food products beyond microbial inactivation is also included.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the food industry is rapidly evolving and facing new consumers’ demands and global food trends. Nowadays, consumers are looking for healthier and safe food with minimal or no-added food preservatives and extended shelf life, together with the demand for more sustainable food resources (plant-based proteins, for example). Although conventional thermal processing methods are verified tools for ensuring microbiological food safety [18], the intense heat used in these methods can cause nutrient degradation (e.g., vitamins and volatile aroma compounds) and result in the formation of unwanted compounds and off-flavors, for example, acrylamide, chloropropanols, and furan [95, 127, 141]. Emerging technologies are often investigated as a replacement of conventional methods to minimize the effect of heat on food components while ensuring microbial safety and preserving nutrients as well as sensorial properties, and, in some cases, improve techno-functional properties. These technologies include high hydrostatic pressure (HHP), high-pressure homogenization (HPH), ohmic heating, pulsed electric fields (PEF), microwave heating, cold plasma, gamma irradiation, UV processing, and ultrasound [5, 40, 107, 134, 175, 183, 212].
In HHP processing, the pressure is applied uniformly and transmitted to the pre-packed product by the pressure-transmitting medium at ambient or subambient temperature for several minutes, without inducing a shearing effect. Although many products are commercially being treated with HHP for pasteurization (fruit and vegetable beverages, for example) [13, 130], this process is not continuous and thus only a relatively low processing volume is possible. On the other hand, HPH, also known as dynamic high-pressure homogenization or high-pressure valve homogenization, is an emerging continuous flow process technology enabling the homogenization and pasteurization, and in some cases sterilization, of fluids in one single step [122, 210], while the fluid is being subjected to high pressure for less than a second [166, 187].
Homogenization is a physical process in which a dispersed system, suspension or emulsion, is forced to flow at a high velocity through a narrow passage, a disruption valve, producing a smaller and narrow particle size distribution [12]. Conventional homogenization, usually up to 50 MPa [9], is widely utilized in the food industry to stabilize emulsions by preventing creaming and coalescence, to reduce particle size (dispersion) and to mix ingredients [12]. In contrast to (ultra) high-pressure homogenization ((U)HPH), conventional homogenization has no preservation effect on the treated fluid. A major difference between (U)HPH and conventional homogenization is the maximum pressure level reached, and it is dependent on the homogenizer design and characteristics such as gap size, seals, and valve geometry. UHPH reaches pressure levels up to 400 MPa [10, 210], while HPH reaches pressure levels between 50 and 200 MPa [63]. It should be noted that some authors differ on the cut-off point between HPH and UHPH [49, 166, 210]. High-pressure jet (HPJ) technology is a similar technology reported to reach up to 500–600 MPa by utilization of a nozzle (from diamond, sapphire, or ruby) restricting the flow and forcing the fluid to form a jet stream that hits the air around it and transforms the liquid into aerosols. Immediately after the nozzle, a heat exchanger is connected, allowing those aerosols to coalesce back to liquid by hitting the wall of the heat exchanger [72, 76, 77, 185].
(U)HPH has been demonstrated as a valuable tool with two main impacts, the first, mainly focuses on the physical changes of the fluid after being subjected to the treatment. Such changes have crucial importance in various applications, such as preparation and stabilization of emulsions and nanoemulsions, reduction in droplets, and particle size of emulsions and suspensions together with a narrower size distribution, changes in the techno-functional properties of proteins and polysaccharides, texture modification and changes, and improvement in rheological properties of fluids [9, 21, 70, 79, 122, 174, 210]. In the pharmaceutical, cosmetics, and biochemical industries, it can be a tool for handling solid lipid nanoparticles and crystalline solids, dispersions, emulsions with controlled droplet size, drug nanoparticles, and nanosuspension [24, 46, 88, 91, 131, 187, 188]. The effect of cell disruption induced by (U)HPH can also be used as a tool for improved extraction of intra-cellular compounds (e.g., proteins, enzymes, fatty acids) in bioengineering-related industries [11, 14, 90, 125]. The second major application is microbial inactivation. (U)HPH can induce a reduction of the microbial load of food products to the level of pasteurization and even sterilization [9, 42, 62, 100, 101, 134, 145, 210], depending on process parameters such as pressure level and the number of passes, process temperature (inlet and maximal temperature), high-pressure valve design, and the properties of the treated fluid itself [122, 187, 210].
(U)HPH applications for enzyme inactivation and improvement of techno-functional properties of food components have been also explored. More recently, an increased scientific focus has been given to the direct and indirect effects of (U)HPH on bioactive compounds. Currently, the majority of (U)HPH utilization in many aspects is still in a laboratory or pilot scale. The industrial usage of commercially available (U)HPH units is limited, due to operation, usually at the maximal achievable pressure levels with flow rates less than the industrial requirements and, in some cases, high energy consumption [49, 122, 166]. A comprehensive review of the main engineering aspects and the physics behind (U)HPH, along with opportunities in scaling up of the process to commercial scale, was recently published by Martínez-Monteagudo et al. [122]. The utilization of (U)HPH for pasteurization, sterilization, and enzyme inactivation, especially in the beverage industry, has been previously reviewed [9, 43, 46, 49, 63, 70, 122, 137, 154, 166, 187, 210], while others have reviewed the impact of (U)HPH on emulsion stability [16, 49, 52, 70, 122, 135, 174, 210]. In addition to the interest in the opportunities of (U)HPH in microbial inactivation, including patents on the topic [30, 117, 124], the technology was also studied regarding the influence on the techno-functional properties of proteins and polysaccharides and the manufacture of food products with improved functionality. The focus of this publication is an updated review of the engineering aspects of (U)HPH and the potential of this emerging technology for applications beyond microbial inactivation. Technological aspects, detailed descriptions, and working principles as related to applications in the development of novel food products are also discussed.
Principles of (Ultra) High-Pressure Homogenization
The design of high-pressure homogenizer usually consists of one or two stages restricting the fluid flow, depending on the desired application and the properties of the final product [70]. The recent developments of designs (intensifiers, different valve, and homogenization chamber geometries) and various high-pressure-resistant materials (e.g., ceramic, diamond, sapphire, seals) allow operating pressure levels of up to 400 MPa and process temperatures of up to 140–150 °C [10, 49, 166]. Commercially available units differ mostly in their high-pressure valve design affecting the pressure range and applicable flow rates at the laboratory, pilot, and industrial scale [49, 166]. Valve design and geometry were reported to influence vegetative microorganisms’ inactivation [45] and the formation of food nanoemulsions [47] even when the same pressure was used.
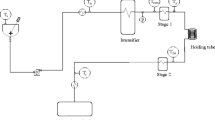
Two-stage (U)HPH systems are usually equipped with a positive displacement pump, intensifier, and heat exchangers before and/or after the high-pressure valve. The first stage is the high-pressure valve, and the second stage is the low-pressure valve. A schematic diagram of a two-stage (U)HPH system design is presented in Fig. 1. The fluid, depending on the processing objective (pasteurization, sterilization, emulsification), is pre-chilled or pre-heated to the desired inlet temperature by a heat exchanger. Then, it is pressurized by a pressure intensifier, to the required pressure, usually up to 400 MPa, and consequently, the fluid temperature rises due to hydrostatic compression. Afterward, the fluid is depressurized by passing through the high-pressure homogenization valve reaching after the first stage, a depressurization to 10–20 MPa. Due to the pressure drop, part of the kinetic energy is converted to heat, resulting in a temperature increase [49, 122, 210]. The second homogenization valve, the low-pressure valve, reduces the fluid pressure to atmospheric pressure and disrupts agglomerates that might have been formed during the first homogenization valve discharge. As the final product temperature can be high, immediate cooling by a heat exchanger is often employed after homogenization to minimize damage to thermolabile components.
A schematic flow diagram of a two-stage high-pressure homogenization processing system. Tr is the temperature of the fluid reservoir; Tin is the fluid inlet temperature before increasing pressure to the homogenization pressure p; Tp is the fluid temperature after hydrostatic compression; T1 is the fluid temperature after the high-pressure valve;T2 is the fluid temperature after the low-pressure valve; Tout is the final fluid temperature after cooling in the heat exchanger. Based on [46, 49, 70, 122, 210]
Valve Design and Temperature Increase
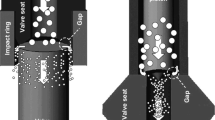
Valve geometry and design are crucial parameters affecting the process performance and characteristics of the final product. During (U)HPH, the fluid is forced to flow through the high-pressure valve passing a minute orifice (width of a few micrometers), increasing the fluid’s velocity. The disruption of the fluid particles (emulsion or suspensions) is mostly influenced by the valve geometry and the homogenization pressure level selected. The fluid itself is exposed to high pressure for a very short period, less than a second, and therefore hydrostatic effects are relatively small [166, 187]. Adjusting the initial fluid temperature, the pressure, the number of passes, and valve design may help to achieve the desired physical changes and process temperature [122]. Various valve geometries and designs are commercially available aiming for specific final applications [122]. Different commercial high-pressure homogenizers exist for laboratory and pilot-scale and a few ones fitting industrial requirements [122, 166, 210]. The three main types of valve geometries are counter jet, radial diffusers, and axial flow valves. While comprehensive descriptions of available homogenization valves are available [46, 122, 187, 210], Fig. 2 describes the most reported valve geometries [46, 70]. A nozzle-type geometry (Fig. 2a) was reported to be effective in the applications of droplet disruption [178], microbial inactivation, and cell rupture [152, 159]. The conical piston valve (Fig. 2b) was reported to be effective for breaking cell structures [20, 61, 103, 126], preparation of nanosuspensions [128] and nanoemulsions [47], solid lipid nanoparticles [177], and microbial inactivation [45, 57, 169]. The ceramic needle and seat (Fig. 2c) were reported to be effective for cell disruption [42, 57, 169, 193], preparation of nanosuspensions [59, 74, 186], protein denaturation [136], and microbial inactivation [51, 190]. Microfluidics (Fig. 2d) was reported to effectively disrupt cells, with relatively larger particles compared with the conical piston [61], heat denaturation of proteins [80], and formation of nanoparticles [99], and fine emulsion formation [24].
Velocity and Temperature Increase
In (U)HPH, the fluid is simultaneously subjected to homogenization and pressure effects. As the fluid depressurizes after the first homogenization valve and passes through a minute gap, the fluid velocity increases due to the tubing size reduction along with the corresponding drop in fluid pressure [49]. The patterns of fluid flow together with the velocity profile have been previously reviewed [122]. The increment in fluid velocity results in intense turbulence contributing to the disruption of the fluid dispersed phase and the homogenization effect. Forcing the fluid to pass through the small gap while the valve restricts its motion results in shearing between the fluid and the valve seat. Shear has a large effect on the droplet disruption. It was previously reported that the inertial and surface forces could be characterized by the Weber number (We in Eq. (1)) described before for disruption of a particle in a laminar flow [122, 195]. But for (U)HPH, the relation between the surface tension depends on the pressure and the temperature during homogenization (Eq. (2)) [122].
where ρ is the fluid density, v is the fluid velocity, l is the typical length, γ is the surface tension, p is the pressure of the system, V the total volume of the system, A total surface area, and T is the system temperature.
Mixing, emulsifying, and homogenization in the (U)HPH valves are considered to occur due to the turbulence created by the restricted fluid flow, leading to the increment in its velocity. The generated turbulence dissipates kinetic energy into heat, leading to an increase in fluid temperature [29, 78, 122]. The third phenomenon happening after the first (U)HPH valve is cavitation due to the sudden pressure drop [122]. Cavitation refers to the formation of air bubbles in the fluid and their subsequent collapse. Quantification and prediction of cavitation are possible using two dimensionless numbers: cavitation (Cv, Eq. (3)) and cavitation inception (Cin, Eq. (4)):
where p2 is the pressure after homogenization valve, p1 is the pressure before homogenization valve, pv is the liquid vapor pressure, ρ is the fluid density, and vmax is the fluid maximum velocity. The combination of three phenomena, turbulence, high shear, and cavitation, is mainly responsible for the effect of mixing, dispersion, emulsification, and reduction in particle size [29, 78, 122].
The total increment in temperature reflects two factors: heat compression and the combined effect of shearing turbulence and mixing (as a result of the instantaneous pressure drop). The rapid pressurization generated during the pressure build-up in the intensifier causes heat compression and an increase in temperature at a range of 2–3 °C per 100 MPa, depending on the fluid characteristics while this increment is reversible upon pressure release [46, 49, 122]. Due to the isotactic pressure and the work done during the pressure discharge through the homogenization valves, the kinetic energy of the fluid is lost in the form of heat and the fluid temperature increases irreversibly by 14–25 °C per 100 MPa, depending on the physicochemical properties of the fluid, the fluid inlet temperature, and the homogenizer design [46, 49, 122, 166, 187, 210]. For example, it was reported that the range of increasing temperature per 100 MPa for bovine milk was 16.6–19.5 °C when the inlet temperature ranged from 4 to 50 °C [73, 139, 142, 182]. For fruit juices and plant-based beverages, the increment was reported to be 15.0–22.6 °C per 100 MPa when inlet temperature ranged from 4 to 75 °C [143, 180, 189]. The increment in fluid temperature is mostly dependent on the homogenization pressure at a specific inlet temperature [210]. This increment was described to be a linear or polynomial function of pressure, based on empirical models reviewed in previous works [46, 49, 122, 181]. A general equation (Eq. (5)) for estimation of the fluid temperature during the process has been suggested [122]:
where Tp is the fluid temperature during the process, Tin is the fluid inlet temperature, δ is the heat compression of the fluid, ∆p the hydrostatic pressure, ξ homogenization heat, and heat loss that can be negligible if the system is well insulated (mostly relevant for laboratory-scale equipment) [122].
Effect on Bioactive Compounds and Properties of Food Matrices
(U)HPH has been suggested as a tool for improving the techno-functional properties of food and food components, investigated mainly in fruit juices. The utilization of (U)HPH in fruit juices processing is a promising example of a combination of the versatile applications of (U)HPH. The technology allows to achieve a reduction of microbial load, preservation of quality properties (such as the content of thermally sensitive compounds) of fresh products, and affect the physical properties, such as phase separation, in one single process. Each matrix reacts differently when subjected to (U)HPH processing and the effect on product properties cannot be easily predicted. Every fruit has a unique composition, and cell fragmentation exposes and releases internal and wall constituents (e.g., pectins and proteins), increasing the potential for particle-particle and particle-serum interactions. Also, the cell wall has its structure, and consequently different resistance to shear forces [104, 116]. Published data on fruit juices and juice concentrates showed that rheological properties were modified (e.g., decrease in apparent viscosity and thixotropic behavior changes) [104, 105, 173, 202, 213], decrease in sedimentation during storage [96, 156, 161, 173, 202], decrease in particle size with increasing homogenization pressure [7, 17, 22, 23, 96, 104, 105, 161, 173, 213], but reaching a critical size below which particles could not be further degraded regardless of the initial size [7, 161, 173]. While HPH was reported to induce a reduction in the thixotropic behavior of most fruit juices and juice concentrates [104, 213], an increase in this behavior was observed for tomato juices [96]. As further described, (U)HPH can reduce the consistency of many polysaccharide solutions, thus suggesting that such a technology could also be used to reduce the consistency of juice concentrates with low pulp content. From an engineering point of view, a lower consistency leads to lower friction during processing and distribution, thus minimizing energy requirements [104, 105]. Concentrated orange juice (COJ) is a common industrial raw material for the juice industry. As it is a concentrated product, its high consistency requires high amounts of energy for processing and handling. Leite et al. [104] evaluated the effect of HPH treatment on the rheological properties and flow behavior occurring in COJ. The apparent viscosity, the consistency index, and the mean particle diameter were reduced, while an increase in the flow behavior index was observed. Thus, the utilization of HPH presents an opportunity to minimize the energy required during COJ processing and distribution due to the reduction in the product’s consistency.
The effect of (U)HPH on the preservation of some of the health-promoting and bioactive compounds such as vitamins, polyphenols, compared with the conventional heat treatments was previously reviewed [122, 210]. Recently, Sharabi et al. [167] investigated the possible improvement of milk nutritional quality during shelf life by testing the stability of ascorbic acid and riboflavin and antioxidant capacity in milk and model systems after HPH treatments. The results revealed reduced microbial load, almost unaffected concentration of ascorbic acid immediately after the treatment vs. rapid degradation during storage. The degradation rate of riboflavin, a light-sensitive molecule, was reduced by ~ 50%, depending on the homogenization pressure. The authors suggested that this outcome is an indirect effect of the (U)HPH on the degradation due to increased light scattering and absorbance of the wavelengths related to photo-oxidation of riboflavin. (U)HPH is known to disrupt plant matrices affecting both the functional properties of the products and the release of some bioactive compounds in the matrices. Juice beverage industry is facing several quality defects during storage, such as cloud loss, enzymatic browning, flavor changes, color loss, and deterioration of bioactive compounds [207]. Recent works have focused on these properties and the optional improvement by (U)HPH using different numbers of passes on some fruit beverages and nectars [85, 129, 156, 173, 180, 202, 207, 209]. Moscovici-Joubran et al. [129] studied the effect of different (U)HPH pressures and the number of cycles on the physicochemical, structural, and functional attributes of strawberry nectar. The study also tested the effect of a filtration step that partially removed large particles. As expected, the (U)HPH treatment reduced the particle size, but contrary to some previous publications on juices [96, 114, 156, 202], the stability against separation was also negatively affected, likely due to the destruction of a weak network. The total polyphenol concentration was not affected by the homogenization pressure itself but significantly enhanced with an increasing number of cycles at 200 MPa, whereas the color and the anthocyanins content were only slightly affected. Moscovici-Joubran et al. [129] demonstrated that the number of cycles has the potential of enhancing the extractable content of health-promoting compounds in processed matrices. The effect of (U)HPH on the antioxidant capacity of bioactive compounds was mainly investigated in food matrices, and specifically in fruit juices, and was reviewed before [81, 210]. In general, compared with the heat treatment, (U)HPH processing of juices was reported to better preserve antioxidant capacity [66, 85, 180, 191], total polyphenol content [66, 85, 180, 191], and bioactive compounds such as ascorbic acid [120, 167, 180, 191], carotenoids [66, 106, 180, 191], flavonoids [15, 191], and vitamins [19]. Toro-Funes et al. [184] investigated the effect of (U)HPH and various inlet temperatures on soy drink bioactive compounds, phytosterols, isoflavones, and tocopherols. The total phytosterols and isoflavones content in soy drink increased with increasing pressures and temperatures. As the inlet temperature increased, the total tocopherols decreased while an increase in the pressure level increased their content. Liu et al. [113] investigated the impact of HPH and different inlet temperature on physical stability and carotenoid degradation kinetics of carrot beverage during storage. The use of lower inlet temperature combined with the HPH preserved the color during storage better than the same pressure level with higher inlet temperature. The physical stability of all the tested samples decreased during storage. Yet, the stability of the samples treated at a higher temperature and high pressure decreased more than samples treated with lower inlet temperature combined with the high pressure. The combination of high pressure and high inlet temperature also assisted in improving the preservation of carotenoids during storage when compared with the other tested treatments. A study carried out by Loira et al. [115] tested the application of (U)HPH to process grape must before fermentation. They demonstrated that (U)HPH processing led to an elimination of grape microflora allowing the reduction of the added sulfite levels needed for controlling oxidation and thus improving wine’s aroma compared with sulfited wine.
Fernandez-Avila et al. [52] reviewed the potential and the current application of (U)HPH utilization for the production of nanostructures based on lipid, carbohydrates, protein, or protein-polysaccharide complexes loaded with biologically bioactive compounds such as vitamins and phenolic compounds. The bioaccessibility of bioactive compounds in model suspensions and food matrices as affected by HPH was reported in previous works and reviewed elsewhere [16, 27, 92, 122]. Some works published recently investigated the influence of HPH processing of food products on the bioaccessibility of some bioactive compounds such as carotenoids [110, 114, 201]. In general, HPH improved the bioaccessibility of total carotenoids [110, 201] and total polyphenols [201]. The addition of oil or emulsion before HPH treatment also enhanced the bioaccessibility of carotenoids without affecting the bioaccessibility of polyphenols [110, 201]. Liu et al. [110] studied the effects of HPH and the addition of oil on carotenoid bioaccessibility in carrot juice. They reported that the total carotenoids bioaccessibility was significantly higher when carrot juice was treated at pressures above 60 MPa and inlet temperature 70 °C. Liu et al. [114] studied the influence of HPH, number of cycles, and different inlet temperatures on the bioaccessibility of carotenoids in carrot juice, reporting that HPH increased the total carotenoids bioaccessibility. The physical stability was also improved while the effect of pressure level and number of cycles were more significant than inlet temperatures.
(U)HPH Effects on Polysaccharides
In the food industry, polysaccharides are extensively exploited to modify textural properties of fluids and semi-solid foods and used as stabilizers, thickeners, binders, suspending agents, emulsifiers, and gelling agents, depending on the functional properties of a given polymer [2, 133, 179]. When added to food, polysaccharides can modify hydration, solubility, rheological, and interfacial properties and can be used as texture enhancers [2, 70, 133, 179]. The rheological characteristics of food products are essential both for the process design and optimization (e.g., flow in pumps and pipelines) and for the final product itself as it affects product stability, quality prediction, sensory characteristics, and consequently consumer acceptance [146, 192]. Physicochemical properties, such as apparent viscosity, are associated with the polysaccharide molecular weight (Mw) distribution that can be affected by food processing [70]. Thus, many researchers have attempted to control polysaccharides molecular weight and make the distribution more uniform, mainly by the degradation of polysaccharides by enzymatic [87, 94, 97, 132], chemical [3, 41, 60, 67, 84, 93, 94, 151, 172], physical [1, 34, 38] methods, and also (U)HPH [70]. The latter showed a large effect on polysaccharides and their functionality. In (U)HPH processing, polysaccharides are subjected to the turbulent and shear forces [89]. Therefore, (U)HPH could influence the structure and the rheological properties of polysaccharides, which would thus influence their application in food systems [200]. The degree of physical change of polysaccharides during (U)HPH is influenced by processing parameters such as pressure and the number of homogenization cycles, and parameters related to the polysaccharide and the fluid such as solution pH and the polymer structure and concentration [89, 194]. Several studies explored the influence of (U)HPH on the rheological properties and the degradation of polysaccharides solutions. Studies investigating the influence of (U)HPH on polysaccharides and their techno-functional properties are summarized and presented in Table 1.
(U)HPH impacts on polysaccharides described in Table 1 indicate that the structure and conformation of polysaccharides (e.g., linear, branched) have a larger impact on the outcome of (U)HPH treatment than the polymer charge. Linear and stiff polysaccharides undergo depolymerization resulting in reduced polydispersity while globular-branched structures are less affected [194]. Also, pH has an impact on depolymerization, possibly reflecting the polymer conformation in solution during homogenization. At pH 6.3, (U)HPH induced conformational changes in pectins resulting in a more compact structure, while at pH 4.4, no conformational changes were noticed [170]. Besides, larger chitosan molecules were more susceptible to chain scission resulting in a narrower molecular weight distribution than that of original chitosan, indicating that large macromolecules were preferentially fragmented [86]. Similar results were reported also for pectins [170]. For a specific homogenization pressure, the molecular weight reduction occurs until a critical molecular weight is achieved and no further reduction occurs if the homogenization pressure is kept constant or reduced [71]. These results also suggest that additional homogenization cycles at constant pressure would only reduce polysaccharide molecular weight polydispersity by depolymerizing remaining polysaccharide strands having a molecular weight above the critical molecular weight for the specific homogenization pressure. In the case of rheological properties of the polysaccharide containing system, the thickening properties of polymers were also reduced due to the (U)HPH treatment likely related to the reduction in molecular weight [58].
Effect on Proteins
Enzymes in food matrices and their activity are an important parameter influencing shelf life, characteristics of the product, and the fate of bioactive compounds. (U)HPH was previously reported to activate, inactivate, and in some cases, not to affect enzyme activity, and the data has been summarized in previous reviews [49, 122, 210]. Proteins in food are technologically valuable due to their diverse techno-functional properties such as solubility, swelling, water holding capacity, foaming, emulsifying, and gelling capacity [211]. The use of proteins as emulsifying and foaming agents is based on their amphiphilic characteristics, and their ability to migrate to the interface depends on the conformation and the flexibility of the molecule [65, 121, 147]. Research on processing technologies changing the functionality of polymers, including proteins, is growing, partially due to the opportunity to reduce the use of stabilizers and emulsifier agents. (U)HPH is one of the technologies capable of achieving physical protein modifications [70]. The effect of (U)HPH on the protein’s secondary or tertiary structure is controversial. (U)HPH was reported as a useful tool for the disruption of protein quaternary structure [70]. Some studies suggest that (U)HPH up to 400 MPa has little impact on the secondary or tertiary structure when the solutions are immediately cooled after the homogenization [50, 64, 138,139,140] while others led to different findings [37, 203]. Various studies were conducted to understand the influence of (U)HPH on the techno-functional properties of proteins in suspension, such as foaming, solubility, and particle size distribution [26, 44, 109, 158, 205]. In this section, the focus will be mainly on the effect of (U)HPH on the techno-functional properties of plant-based proteins and more recent works dealing with proteins that possess poor water solubility. Protein unfolding can expose the inner hydrophobic regions increasing the potential for hydrophobic interactions between proteins, fat globules, and small particles finely distributed in the aqueous phase, creating a new oil-water interface that may favor the formation of particle aggregates [53, 150, 198]. (U)HPH impact on proteins, described in Table 2, indicate that (U)HPH has the potential to improve and modify techno-functional properties of proteins such solubility, emulsifying and foaming properties, particle size distributions, zeta potential, and rheological properties of proteins. Several works demonstrated that homogenization above a specific pressure might induce further unfolding of the protein and exposure of hydrophobic regions leading to protein aggregation and thus a reduction in some of the techno-functional properties [157, 158]. (U)HPH was also reported as a tool for enhancing proteins solubility [26, 168, 204, 205, 208].
Polysaccharides and Proteins Mixed Systems
Proteins and polysaccharides in the food matrix can improve physicochemical properties such as stability, rheological properties, and mouthfeel [48, 149, 160]. When these two ingredients are mixed in an aqueous phase, the interaction between them can result in segregation and/or association, depending on their concentration and net charge. If an electrostatic repulsive force occurs, phase separation of a protein and polysaccharide-rich phases will occur. In the case of attractive electrostatic force, phase separation of protein and polysaccharide-rich phase and a solvent-rich phase will occur [149]. Some physical processes can induce changes in protein structure, altering the ability to interact, and among them is (U)HPH. (U)HPH impact on polysaccharides and proteins mixed systems is also described in Table 2. The impact of (U)HPH on the interaction between electrostatically charged polysaccharides and neutral polysaccharide and protein was demonstrated to effect the complexes [83]. Such studies may help to understand how (U)HPH can manipulate protein-polysaccharide interactions and open the way for further research on the formation of new functional polysaccharide-protein aggregates\complexes, and opportunities in the stabilization of hydrophobic proteins in an aqueous medium.
Improvement of Techno-Functionality of Complete Food Systems by (U)HPH
Many studies investigated the possibility of processing liquid food products by (U)HPH to reduce the microbial load, improve techno-functional and sensory properties, reduce fat content, and reduce the use of stabilizers [6, 28, 119, 122, 138, 139, 143, 166, 210]. The application of (U)HPH in dairy products, including for pasteurization and improvement of functional properties, has been comprehensively reviewed [9, 46, 49, 63, 122, 135, 166, 187, 210]. Recent works on bovine milk developed a method for monitoring and predicting the structural and functional changes in milk during (U)HPH at different inlet temperatures by monitoring the formation of Maillard compounds using face fluorescence spectroscopy [111, 112]. The influence of (U)HPH milk treatment on the properties of cheese has been previously reviewed [46, 135, 187, 210]. For dairy-based yogurt, several studies investigated the utilization of (U)HPH on skim and whole milk for the production of set or stirred yogurt and its influence on yogurt properties. They reported that (U)HPH treatment resulted in the reduction of particle size, the formation of finer dispersion, as well as the denaturation of whey proteins and dissociation of casein micelles. Thus, yogurt properties such as water holding capacity, firmness, syneresis, consistency, and water retention were improved [75, 102, 162, 164, 165]. The impact of HPH on techno-functional properties of some complete food systems is presented in Table 3. HPJ was studied and shown to change the physiochemical properties of skim milk, skim milk powder, and whole milk [76, 77, 185]. These findings may allow the future utilization of HPJ to manufacture novel functional ingredients to minimize/replace the addition of emulsifiers or stabilizers to food products. (U)HPH was also reported as a tool for changing during shelf life the physiochemical properties, texture, and sensorial properties of dairy products such as cream and yogurt [28, 148, 163].
The demand for plant-based protein beverages and yogurt substitutes as an alternative to milk and milk-based yogurt is increasing in recent years [82, 118]. Plant-based dispersions often suffer from techno-functional limitations such as poor aqueous solubility, off-flavor, color and physical instability [4, 39, 82]. Thus, recent works have focused on the utilization of (U)HPH as a preservation technology for such products and studied the outcome on techno-functional characteristics of plant-based drinks, e.g., particle size, physical stability, color, and volatile compounds. As expected, (U)HPH considerably reduced the microbial load and particle size and extended the product shelf life [31, 32, 35, 55, 56, 68, 69, 171], improved product color, usually luminosity, compared with the one observed for conventional heat treatment [32, 56], and improved physical stability, mainly against creaming, due to the particle size reduction [31, 55]. Also, (U)HPH processing resulted in a similar, or sometimes decreased the off-flavors when compared with conventional thermal treatments [32, 56]. (U)HPH was found to improve characteristics of yogurt alternative prepared from the treated plant-based beverage, such as greater firmness, higher water holding capacity, and similar color compared with conventional heat treatment [36, 54].
Conclusion
(U)HPH is an emerging technology with potential applications in various areas of the food industry including pasteurization and sterilization, emulsion and suspension stabilization, and modification of the structure of whole matrices and specific biopolymers for the production of functional foods and novel ingredients. Major effects due to (U)HPH treatment are observed for polysaccharides, proteins, and their combination, leading to the formation of physically modified biopolymers with novel functionalities. The extent of these effects depends on a range of controllable processing parameters such as pressure, inlet temperature, valve design, solution pH, and others. To transform the current rich scientific data obtained from laboratory and pilot-scale work into commercial applications, the understanding of scaling up, energy consumption, and process cost estimation must improve. An additional possible driving force for the implementation of this technology could stem from a better understanding of (U)HPH effects on the bioaccessibility of bioactive compounds and the benefits of the modification of matrices and biopolymers when engineering the digestive fate of foods. From an engineering point of view, a more comprehensive understanding of the effect of valve type and geometry on the techno-functionality is also vital.
References
Aida TM, Yamagata T, Watanabe M, Smith RL (2010) Depolymerization of sodium alginate under hydrothermal conditions. Carbohydr Polym 80:296–302. https://doi.org/10.1016/j.carbpol.2009.11.032
van Aken GA (2016) Polysaccharides in food emulsions. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications, 2nd edn. CRC/Taylor & Francis, Boca Raton, pp 521–540
Akpinar O, Erdogan K, Bostanci S (2009) Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr Res 344:660–666. https://doi.org/10.1016/j.carres.2009.01.015
Alonso-Miravalles L, O’Mahony J (2018) Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 7:1–17. https://doi.org/10.3390/foods7050073
Alves Filho EG, de Brito ES, Rodrigues S (2020) Effects of cold plasma processing in food components. In: Bermudez-Aguirre D (ed) Advances in cold plasma applications for food safety and preservation. Academic Press, London, pp 253–268
Amador Espejo GG, Hernández-Herrero MM, Juan B, Trujillo AJ (2014) Inactivation of Bacillus spores inoculated in milk by ultra high pressure homogenization. Food Microbiol 44:204–210. https://doi.org/10.1016/j.fm.2014.06.010
Augusto PED, Ibarz A, Cristianini M (2012) Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: time-dependent and steady-state shear. J Food Eng 111:570–579. https://doi.org/10.1016/j.jfoodeng.2012.03.015
Augusto PED, Ibarz A, Cristianini M (2012) Effect of high pressure homogenization (HPH) on the rheological properties of a fruit juice serum model. J Food Eng 111:474–477. https://doi.org/10.1016/j.jfoodeng.2012.02.033
Balasubramaniam VM(B), Martínez-Monteagudo SI, Gupta R (2015) Principles and application of high pressure–based technologies in the food industry. Annu Rev Food Sci Technol 6:435–462. https://doi.org/10.1146/annurev-food-022814-015539
Balasubramaniam VM, Barbosa-Cánovas GV, Lelieveld HLM (2016) High-pressure processing equipment for the food industry. In: Balasubramaniam VM, Barbosa-Cánovas GV, Lelieveld HLM (eds) High pressure processing of food principles, technology and applications, 1st edn. Springer, New York, pp 63–65
Barba FJ, Grimi N, Vorobiev E (2014) New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng Rev 7:45–62. https://doi.org/10.1007/s12393-014-9095-6
Berk Z (2018) Mixing. In: Berk Z (ed) Food process engineering and technology, 3rd edn. Academic Press, London, pp 193–217
Bermúdez-Aguirre D, Barbosa-Cánovas GV (2011) An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Eng Rev 3:44–61. https://doi.org/10.1007/s12393-010-9030-4
Bernaerts TMM, Verstreken H, Dejonghe C, Gheysen L, Foubert I, Grauwet T, Van Loey AM (2020) Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J Funct Foods 65:103770. https://doi.org/10.1016/j.jff.2019.103770
Betoret E, Betoret N, Carbonell JV, Fito P (2009) Effects of pressure homogenization on particle size and the functional properties of citrus juices. J Food Eng 92:18–23. https://doi.org/10.1016/j.jfoodeng.2008.10.028
Betoret E, Betoret N, Rocculi P, Dalla Rosa M (2015) Strategies to improve food functionality: structure-property relationships on high pressures homogenization, vacuum impregnation and drying technologies. Trends Food Sci Technol 46:1–12
Betoret E, Sentandreu E, Betoret N, Fito P (2012) Homogenization pressures applied to citrus juice manufacturing. Functional properties and application. Journal of Food Engineering 111:28–33. https://doi.org/10.1016/j.jfoodeng.2012.01.035
van Boekel M, Fogliano V, Pellegrini N, Stanton C, Scholz G, Lalljie S, Somoza V, Knorr D, Jasti PR, Eisenbrand G (2010) A review on the beneficial aspects of food processing. Molecular Nutrition and Food Research 54:1215–1247. https://doi.org/10.1002/mnfr.200900608
Briviba K, Gräf V, Walz E, Guamis B, Butz P (2016) Ultra high pressure homogenization of almond milk: physico-chemical and physiological effects. Food Chem 192:82–89. https://doi.org/10.1016/j.foodchem.2015.06.063
Bury D, Jelen P, Kaláb M (2001) Disruption of Lactobacillus delbrueckii ssp. bulgaricus 11842 cells for lactose hydrolysis in dairy products: a comparison of sonication, high-pressure homogenization and bead milling. Innov Food Sci Emerg Technol 2:23–29. https://doi.org/10.1016/S1466-8564(00)00039-4
Campos FP, Cristianini M (2007) Inactivation of Saccharomyces cerevisiae and Lactobacillus plantarum in orange juice using ultra high-pressure homogenisation. Innov Food Sci Emerg Technol 8:226–229. https://doi.org/10.1016/j.ifset.2006.12.002
Carbonell JV, Navarro JL, Izquierdo L, Sentandreu E (2013) Influence of high pressure homogenization and pulp reduction on residual pectinmethylesterase activity, cloud stability and acceptability of Lane Late orange juice: a study to obtain high quality orange juice with extended shelf life. J Food Eng 119:696–700. https://doi.org/10.1016/j.jfoodeng.2013.06.041
Cerdán-Calero M, Izquierdo L, Sentandreu E (2013) Valencia Late orange juice preserved by pulp reduction and high pressure homogenization: sensory quality and gas chromatography-mass spectrometry analysis of volatiles. LWT Food Sci Technol 51:476–483. https://doi.org/10.1016/j.lwt.2012.11.016
Chandonnet S, Korstvedt H, Siciliano AA (1985) Preparation of microemulsions by microfluidization. Soap, Cosmetics, Chemical Specialities 61:37–38
Chen J, Liang RH, Liu W, Liu CM, Li T, Tu ZC, Wan J (2012) Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll 28:121–129. https://doi.org/10.1016/j.foodhyd.2011.12.018
Chen X, Xu X, Zhou G (2016) Potential of high pressure homogenization to solubilize chicken breast myofibrillar proteins in water. Innov Food Sci Emerg Technol 33:170–179. https://doi.org/10.1016/j.ifset.2015.11.012
Cilla A, Bosch L, Barberá R, Alegría A (2018) Effect of processing on the bioaccessibility of bioactive compounds – a review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J Food Compos Anal 68:3–15. https://doi.org/10.1016/j.jfca.2017.01.009
Ciron CIE, Gee VL, Kelly AL, Auty MAE (2012) Modifying the microstructure of low-fat yoghurt by microfluidisation of milk at different pressures to enhance rheological and sensory properties. Food Chem 130:510–519. https://doi.org/10.1016/j.foodchem.2011.07.056
Clark A, Prescott T, Khan A, Olabi AG (2010) Causes of breakage and disruption in a homogeniser. Appl Energy 87:3680–3690. https://doi.org/10.1016/j.apenergy.2010.05.007
Clark A V., Theodore R., Rejimbal J, Gomez CMC (1993) Ultra-high pressure homogenization of unpasteurized juice. US Patent 5232726
Codina-Torrella I, Guamis B, Ferragut V, Trujillo AJ (2017) Potential application of ultra-high pressure homogenization in the physico-chemical stabilization of tiger nuts’ milk beverage. Innov Food Sci Emerg Technol 40:42–51. https://doi.org/10.1016/j.ifset.2016.06.023
Codina-Torrella I, Guamis B, Zamora A, Quevedo JM, Trujillo AJ (2018) Microbiological stabilization of tiger nuts’ milk beverage using ultra-high pressure homogenization. A preliminary study on microbial shelf-life extension. Food Microbiol 69:143–150. https://doi.org/10.1016/j.fm.2017.08.002
Corredig M, Wicker L (2001) Changes in the molecular weight distribution of three commercial pectins after valve homogenization. Food Hydrocoll 15:17–23. https://doi.org/10.1016/S0268-005X(00)00044-8
Cote GL, Willet JL (1999) Thermomechanical depolymerization of dextran. Carbohydr Polym 39:119–126. https://doi.org/10.1016/S0144-8617(98)00165-9
Cruz N, Capellas M, Hernández M, Trujillo AJ, Guamis B, Ferragut V (2007) Ultra high pressure homogenization of soymilk: microbiological, physicochemical and microstructural characteristics. Food Res Int 40:725–732. https://doi.org/10.1016/j.foodres.2007.01.003
Cruz N, Capellas M, Jaramillo DP, Trujillo AJ, Guamis B, Ferragut V (2009) Soymilk treated by ultra high-pressure homogenization: acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll 23:490–496. https://doi.org/10.1016/j.foodhyd.2008.03.010
Datta N, Hayes MG, Deeth HC, Kelly AL (2005) Significance of frictional heating for effects of high pressure homogenisation on milk. J Dairy Res 72:393–399. https://doi.org/10.1017/S0022029905001056
David B, Boldo P (2008) A statistical thermodynamic approach to sonochemical reactions. Ultrason Sonochem 15:78–88. https://doi.org/10.1016/j.ultsonch.2007.02.001
Day L (2013) Proteins from land plants - potential resources for human nutrition and food security. Trends Food Sci Technol 32:25–42. https://doi.org/10.1016/J.TIFS.2013.05.005
Deliza R, Rosenthal A, Abadio FBD, Silva CHO, Castillo C (2005) Application of high pressure technology in the fruit juice processing: benefits perceived by consumers. J Food Eng 67:241–246. https://doi.org/10.1016/j.jfoodeng.2004.05.068
Diaz JV, Anthon GE, Barrett DM (2007) Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: effects of pH, temperature, and degree of methyl esterification. J Agric Food Chem 55:5131–5136. https://doi.org/10.1021/jf0701483
Diels AMJ, Callewaert L, Wuytack EY, Masschalck B, Michiels CW (2004) Moderate temperatures affect Escherichia coli inactivation by high-pressure homogenization only through fluid viscosity. Biotechnol Prog 20:1512–1517. https://doi.org/10.1021/bp0499092
Diels AMJ, Michiels CW (2006) High-pressure homogenization as a non-thermal technique for the inactivation of microorganisms. Crit Rev Microbiol 32:201–216. https://doi.org/10.1080/10408410601023516
Dong X, Zhao M, Yang B, Yang X, Shi J, Jiang Y (2011) Effect of high-pressure homogenization on the functional property of peanut protein. J Food Process Eng 34:2191–2204. https://doi.org/10.1111/j.1745-4530.2009.00546.x
Donsì F, Annunziata M, Ferrari G (2013) Microbial inactivation by high pressure homogenization: effect of the disruption valve geometry. J Food Eng 115:362–370. https://doi.org/10.1016/j.jfoodeng.2012.10.046
Donsì F, Ferrari G, Maresca P (2009) High-pressure homogenization for food sanitization. In: Barbosa-Cánovas G (ed) Global issues in food science and technology. Springer/AP, Amsterdam, pp 309–352
Donsì F, Sessa M, Ferrari G (2012) Effect of emulsifier type and disruption chamber geometry on the fabrication of food nanoemulsions by high pressure homogenization. Ind Eng Chem Res 51:7606–7618. https://doi.org/10.1021/ie2017898
Doublier J-L, Garniera C, Renarda D, Sanchez C (2000) Protein-polysaccharide interactions. Current Opinion in Colloid and Interface Science 5:202–204. https://doi.org/10.1016/S1359-0294(00)00054-6
Dumay E, Chevalier-Lucia D, Picart-Palmade L, Benzaria A, Gràcia-Julià A, Blayo C (2013) Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci Technol 31:13–26. https://doi.org/10.1016/j.tifs.2012.03.005
Escobar D, Clark S, Ganesan V, Repiso L, Waller J, Harte F (2011) High-pressure homogenization of raw and pasteurized milk modifies the yield, composition, and texture of queso fresco cheese. J Dairy Sci 94:1201–1210. https://doi.org/10.3168/jds.2010-3870
Fantin G, Fogagnolo M, Guerzoni ME, Lanciotti R, Medici A, Pedrini P, Rossi D (1996) Effect of high hydrostatic pressure and high pressure homogenization on the enantioselectivity of microbial reductions. Tetrahedron Asymmetry 7:2879–2887. https://doi.org/10.1016/0957-4166(96)00379-5
Fernandez-Avila C, Hebishy E, Donsì F, Arranz E, Trujillo AJ (2019) Production of food bioactive-loaded nanostructures by high-pressure homogenization. In: Jafari SM (ed) Nanoencapsulation of food ingredients by specialized equipment, 1st edn. Elsevier, San Diego, pp 251–340
Fernandez-Avila C, Trujillo AJ (2016) Ultra-high pressure homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem 209:104–113. https://doi.org/10.1016/j.foodchem.2016.04.019
Ferragut V, Cruz NS, Trujillo A, Guamis B, Capellas M (2009) Physical characteristics during storage of soy yogurt made from ultra-high pressure homogenized soymilk. J Food Eng 92:63–69
Ferragut V, Hernández-Herrero M, Veciana-Nogués MT, Borras-Suarez M, González-Linares J, Vidal-Carou MC, Guamis B (2015) Ultra-high-pressure homogenization (UHPH) system for producing high-quality vegetable-based beverages: physicochemical, microbiological, nutritional and toxicological characteristics. J Sci Food Agric 95:953–961. https://doi.org/10.1002/jsfa.6769
Ferragut V, Valencia-Flores D, Pérez-González M, Gallardo J, Hernández-Herrero M (2015) Quality characteristics and shelf-life of ultra-high pressure homogenized (UHPH) almond beverage. Foods 4:159–172. https://doi.org/10.3390/foods4020159
Floury J, Bellettre J, Legrand J, Desrumaux A (2004) Analysis of a new type of high pressure homogeniser. A study of the flow pattern. Chemical Engineering Science 59:843–853. https://doi.org/10.1016/j.ces.2003.11.017
Floury J, Desrumaux A, Axelos MAV, Legrand J (2002) Degradation of methylcellulose during ultra-high pressure homogenisation. Food Hydrocoll 16:47–53. https://doi.org/10.1016/S0268-005X(01)00039-X
Friedrich I, Müller-Goymann CC (2003) Characterization of solidified reverse micellar solutions (SRMS) and production development of SRMS-based nanosuspensions. Eur J Pharm Biopharm 56:111–119. https://doi.org/10.1016/S0939-6411(03)00043-2
Garna H, Mabon N, Nott K, Wathelet B, Paquot M (2006) Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chem 96:477–484. https://doi.org/10.1016/j.foodchem.2005.03.002
Geciova J, Bury D, Jelen P (2002) Methods for disruption of microbial cells for potential use in the dairy industry- a review. Int Dairy J 12:541–553. https://doi.org/10.1016/S0958-6946(02)00038-9
Georget E, Miller B, Aganovic K, Callanan M, Heinz V, Mathys A (2014) Bacterial spore inactivation by ultra-high pressure homogenization. Innov Food Sci Emerg Technol 26:116–123. https://doi.org/10.1016/j.ifset.2014.08.004
Georget E, Miller B, Callanan M, Heinz V, Mathys A (2014) (Ultra) high pressure homogenization for continuous high pressure sterilization of pumpable foods – a review. Frontiers in Nutrition 1:15. https://doi.org/10.3389/fnut.2014.00015
Grácia-Juliá A, René M, Cortés-Muñoz M, Picart L, López-Pedemonte T, Chevalier D, Dumay E (2008) Effect of dynamic high pressure on whey protein aggregation: a comparison with the effect of continuous short-time thermal treatments. Food Hydrocoll 22:1014–1032. https://doi.org/10.1016/j.foodhyd.2007.05.017
Graham D, Phillips M (1979) Proteins at liquid interfaces: kinetics of adsorption and surface denaturation. Journal of Colloid and Interfaces Science 70:403–414
Guan Y, Zhou L, Bi J, Yi J, Liu X, Chen Q, Wu X, Zhou M (2016) Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innov Food Sci Emerg Technol 36:320–329. https://doi.org/10.1016/j.ifset.2016.07.009
Guilloux K, Gaillard I, Courtois J, Courtois B, Petit E (2009) Production of arabinoxylan-oligosaccharides from flaxseed (Linum usitatissimum). J Agric Food Chem 57:11308–11313. https://doi.org/10.1021/jf902212z
Gul O, Atalar I, Mortas M, Saricaoglu FT, Yazıcı F (2018) Application of TOPSIS methodology to determine optimum hazelnut cake concentration and high pressure homogenization condition for hazelnut milk production based on physicochemical, structural and sensory properties. Journal of Food Measurement and Characterization 12:2404–2415. https://doi.org/10.1007/s11694-018-9857-6
Gul O, Saricaoglu FT, Mortas M, Atalar I, Yazici F (2017) Effect of high pressure homogenization (HPH) on microstructure and rheological properties of hazelnut milk. Innov Food Sci Emerg Technol 41:411–420. https://doi.org/10.1016/j.ifset.2017.05.002
Harte F (2016) Food processing by high-pressure homogenization. In: Balasubramaniam VM, Barbosa-Cánovas GV, Lelieveld HLM (eds) High pressure processing of food principles, technology and applications, 1st edn. Springer, New York, pp 123–141
Harte F, Venegas R (2010) A model for viscosity reduction in polysaccharides subjected to high-pressure homogenization. J Texture Stud 41:49–61. https://doi.org/10.1111/j.1745-4603.2009.00212.x
Harte FM, Martinez MC, Mohan MS (2016) Foaming and emulsifying properties of high pressure jet processing pasteurized milk. US Pat No 10,390,543
Hayes MG, Kelly AL (2003) High pressure homogenisation of raw whole bovine milk (a) effects on fat globule size and other properties. J Dairy Res 70:297–305. https://doi.org/10.1017/S0022029903006320
Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K (2005) Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm 299:167–177. https://doi.org/10.1016/j.ijpharm.2005.05.014
Hernández A, Harte FM (2008) Manufacture of acid gels from skim milk using high-pressure homogenization. J Dairy Sci 91:3761–3767. https://doi.org/10.3168/jds.2008-1321
Hettiarachchi CA, Corzo-Martínez M, Mohan MS, Harte FM (2018) Enhanced foaming and emulsifying properties of high-pressure-jet-processed skim milk. Int Dairy J 87:60–66. https://doi.org/10.1016/j.idairyj.2018.06.004
Hettiarachchi CA, Voronin GL, Harte FM (2019) Spray drying of high pressure jet-processed condensed skim milk. J Food Eng 261:1–8. https://doi.org/10.1016/j.jfoodeng.2019.04.007
Innings F, Trägårdh C (2007) Analysis of the flow field in a high-pressure homogenizer. Exp Thermal Fluid Sci 32:345–354. https://doi.org/10.1016/j.expthermflusci.2007.04.007
Innocente N, Biasutti M, Venir E, Spaziani M, Marchesini G (2009) Effect of high-pressure homogenization on droplet size distribution and rheological properties of ice cream mixes. J Dairy Sci 92:1864–1875. https://doi.org/10.3168/jds.2008-1797
Iordache M, Jelen P (2003) High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innov Food Sci Emerg Technol 4:367–376. https://doi.org/10.1016/S1466-8564(03)00061-4
Jacobo ÁS, Saldo J, Gervilla R (2014) Influence of high-pressure and ultra-high-pressure homogenization on antioxidants in fruit juice. In: Processing and impact on antioxidants in beverages. Elsevier Inc., pp 185–193
Jeske S, Zannini E, Arendt EK (2018) Past, present and future: the strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res Int 110:42–51. https://doi.org/10.1016/j.foodres.2017.03.045
Jiao B, Shi A, Liu H, Sheng X, Liu L, Hu H, Adhikari B, Wang Q (2018) Effect of electrostatically charged and neutral polysaccharides on the rheological characteristics of peanut protein isolate after high-pressure homogenization. Food Hydrocoll 77:329–335. https://doi.org/10.1016/j.foodhyd.2017.10.009
Jinglin Y, Shujun W, Fengmin J, Sun L, Yu J (2009) The structure of C-type Rhizoma dioscorea starch granule revealed by acid hydrolysis method. Food Chem 113:585–591. https://doi.org/10.1016/j.foodchem.2008.08.040
Karacam CH, Sahin S, Oztop MH (2015) Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. Ananassa) juice. LWT Food Sci Technol 64:932–937. https://doi.org/10.1016/j.lwt.2015.06.064
Kasaai MR, Charlet G, Paquin P, Arul J (2003) Fragmentation of chitosan by microfluidization process. Innov Food Sci Emerg Technol 4:403–413
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: areview. Bioresour Technol 77:215–227. https://doi.org/10.1016/S0960-8524(00)00118-8
Keck CM, Müller RH (2006) Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm 62:3–16. https://doi.org/10.1016/j.ejpb.2005.05.009
Kivelä R, Pitkänen L, Laine P, Aseyev V, Sontag-Strohm T (2010) Influence of homogenisation on the solution properties of oat β-glucan. Food Hydrocoll 24:611–618. https://doi.org/10.1016/j.foodhyd.2010.02.008
Kleinig AR, Middelberg APJ (1998) On the mechanism of microbial cell disruption in high-pressure homogenisation. Chem Eng Sci 53:891–898. https://doi.org/10.1016/S0009-2509(97)00414-4
Kluge J, Muhrer G, Mazzotti M (2012) High pressure homogenization of pharmaceutical solids. J Supercrit Fluids 66:380–388. https://doi.org/10.1016/j.supflu.2012.01.009
Kopec RE, Failla ML (2017) Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J Food Compos Anal 68:16–30. https://doi.org/10.1016/j.jfca.2017.06.008
Krall SM, McFeeters RF (1998) Pectin hydrolysis: effect of temperature, degree of methylation, pH, and calcium on hydrolysis rates. J Agric Food Chem 46:1311–1315. https://doi.org/10.1021/jf970473y
Kravtchenko TP, Penci M, Voragen AGJ, Pilnik W (1993) Enzymic and chemical degradation of some industrial pectins. Carbohydr Polym 20:195–205. https://doi.org/10.1016/0144-8617(93)90151-S
Kroh LW (1994) Caramelisation in food and beverages. Food Chem 51:373–379. https://doi.org/10.1016/0308-8146(94)90188-0
Kubo MTK, Augusto PED, Cristianini M (2013) Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Res Int 51:170–179. https://doi.org/10.1016/j.foodres.2012.12.004
Kumar ABV, Gowda LR, Tharanathan RN (2004) Non-specific depolymerization of chitosan by pronase and characterization of the resultant products. Eur J Biochem 271:713–723. https://doi.org/10.1111/j.1432-1033.2003.03975.x
Lagoueyte N, Paquin P (1998) Effects of microfluidization on the functional properties of xanthan gum. Food Hydrocoll 12:365–371. https://doi.org/10.1016/S0268-005X(98)00004-6
Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr CM, Hoffman M, Maincent P (1999) Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm 184:97–105. https://doi.org/10.1016/S0378-5173(99)00107-6
Lanciotti R, Gardini F, Sinigaglia M, Guerzoni ME (1996) Effects of growth conditions on the resistance of some pathogenic and spoilage species to high pressure homogenization. Lett Appl Microbiol 22:165–168. https://doi.org/10.1111/j.1472-765X.1996.tb01134.x
Lanciotti R, Sinigaglia M, Angelini P, Guerzoni ME (1994) Effects of homogenization pressure on the survival and growth of some food spoilage and pathogenic micro-organisms. Lett Appl Microbiol 18:319–322. https://doi.org/10.1111/j.1472-765X.1994.tb00878.x
Lanciotti R, Vannini L, Pittia P, Guerzoni ME (2004) Suitability of high-dynamic-pressure-treated milk for the production of yoghurt. Food Microbiol 21:753–760. https://doi.org/10.1016/j.fm.2004.01.014
Lander R, Manger W, Scouloudis M, Ku A, Davis C, Lee A (2000) Gaulin homogenization: a mechanistic study. Biotechnol Prog 16:80–85. https://doi.org/10.1021/bp990135c
Leite TS, Augusto PED, Cristianini M (2014) The use of high pressure homogenization (HPH) to reduce consistency of concentrated orange juice (COJ). Innov Food Sci Emerg Technol 26:124–133. https://doi.org/10.1016/j.ifset.2014.08.005
Leite TS, Augusto PED, Cristianini M (2016) Frozen concentrated orange juice (FCOJ) processed by the high pressure homogenization (HPH) technology: effect on the ready-to-drink juice. Food Bioprocess Technol 9:1070–1078. https://doi.org/10.1007/s11947-016-1688-z
Lemmens L, Tchuenche ES, van Loey AM, Hendrickx ME (2013) Beta-carotene isomerisation in mango puree as influenced by thermal processing and high-pressure homogenisation. Eur Food Res Technol 236:155–163. https://doi.org/10.1007/s00217-012-1872-y
Li X, Farid M (2016) A review on recent development in non-conventional food sterilization technologies. J Food Eng 182:33–45. https://doi.org/10.1016/j.jfoodeng.2016.02.026
Liu HH, Chien JT, Kuo MI (2013) Ultra high pressure homogenized soy flour for tofu making. Food Hydrocoll 32:278–285. https://doi.org/10.1016/j.foodhyd.2013.01.005
Liu HH, Kuo MI (2016) Ultra high pressure homogenization effect on the proteins in soy flour. Food Hydrocoll 52:741–748. https://doi.org/10.1016/j.foodhyd.2015.08.018
Liu J, Bi J, Liu X, Zhang B, Wu X, Wellala CKD, Zhang B (2019) Effects of high pressure homogenization and addition of oil on the carotenoid bioaccessibility of carrot juice. Food Function 10:458–468. https://doi.org/10.1039/c8fo01925h
Liu J, Zamora A, Castillo M, Saldo J (2018) Modeling the effect on skim milk during ultra-high pressure homogenization using front-face fluorescence. Innov Food Sci Emerg Technol 47:439–444. https://doi.org/10.1016/j.ifset.2018.04.009
Liu J, Zamora A, Castillo M, Saldo J (2018) Modeling of the changes in bovine milk caused by ultra-high pressure homogenization using front-face fluorescence spectroscopy. J Food Eng 233:88–97. https://doi.org/10.1016/j.jfoodeng.2018.04.010
Liu X, Liu J, Bi J, Cao F, Ding Y, Peng J (2019) Effects of high pressure homogenization on physical stability and carotenoid degradation kinetics of carrot beverage during storage. J Food Eng 263:63–69. https://doi.org/10.1016/j.jfoodeng.2019.05.034
Liu X, Liu J, Bi J, Yi J, Peng J, Ning C, Wellala CKD, Zhang B (2019) Effects of high pressure homogenization on pectin structural characteristics and carotenoid bioaccessibility of carrot juice. Carbohydr Polym 203:176–184. https://doi.org/10.1016/j.carbpol.2018.09.055
Loira I, Morata A, Bañuelos MA, Puig-Pujol A, Guamis B, González C, Suárez-Lepe JA (2018) Use of ultra-high pressure homogenization processing in winemaking: control of microbial populations in grape musts and effects in sensory quality. Innov Food Sci Emerg Technol 50:50–56. https://doi.org/10.1016/j.ifset.2018.10.005
Lopez-Sanchez P, Svelander C, Bialek L, Schumm S, Langton M (2011) Rheology and microstructure of carrot and tomato emulsions as a result of high-pressure homogenization conditions. J Food Sci 76:E130–E140. https://doi.org/10.1111/j.1750-3841.2010.01894.x
Lopez BG, Mesa AJT, Perez VF, Terre JMQ, Pedemonte TJL, Dunat MNB (2010) Continuous system and procedure of sterilization and physical stabilization of pumpable fluids by means of an ultra-high pressure homogenization. US Pat. 9192190B2
Loveday SM (2019) Food proteins: technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu Rev Food Sci Technol 10:311–339. https://doi.org/10.1146/annurev-food-032818-121128
Lucey JA (2001) The relationship between rheological parameters and whey separation in milk gels. Food Hydrocoll 15:603–608. https://doi.org/10.1016/S0268-005X(01)00043-1
Maresca P, Donsì F, Ferrari G (2011) Application of a multi-pass high-pressure homogenization treatment for the pasteurization of fruit juices. J Food Eng 104:364–372. https://doi.org/10.1016/j.jfoodeng.2010.12.030
Martin AH, Grolle K, Bos MA, Cohen Stuart MA, Van Vliet T (2002) Network forming properties of various proteins adsorbed at the air/water interface in relation to foam stability. J Colloid Interface Sci 254:175–183. https://doi.org/10.1006/jcis.2002.8592
Martínez-Monteagudo SI, Yan B, Balasubramaniam VM (2017) Engineering process characterization of high-pressure homogenization—from laboratory to industrial scale. Food Eng Rev 9:143–169. https://doi.org/10.1007/s12393-016-9151-5
Martínez KD, Ganesan V, Pilosof AMR, Harte FM (2011) Effect of dynamic high-pressure treatment on the interfacial and foaming properties of soy protein isolate-hydroxypropylmethylcelluloses systems. Food Hydrocoll 25:1640–1645. https://doi.org/10.1016/j.foodhyd.2011.02.013
Meyer RS (2001) Ultra high pressure, high temperature food preservation process. US Pat 6,177,115
Middelberg APJ (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13:491–551. https://doi.org/10.1016/0734-9750(95)02007-P
Miller J, Rogowski M, Kelly W (2002) Using a CFD model to understand the fluid dynamics promoting E. coli breakage in a high-pressure homogenizer. Biotechnol Prog 18:1060–1067. https://doi.org/10.1021/bp020010z
Mogol BA, Gökmen V (2016) Thermal process contaminants: acrylamide, chloropropanols and furan. Curr Opin Food Sci 7:86–92. https://doi.org/10.1016/j.cofs.2016.01.005
Möschwitzer J, Achleitner G, Pomper H, Müller RH (2004) Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm 58:615–619. https://doi.org/10.1016/j.ejpb.2004.03.022
Moscovici Joubran A, Katz IH, Okun Z, Davidovich-Pinhas M, Shpigelman A (2019) The effect of pressure level and cycling in high-pressure homogenization on physicochemical, structural and functional properties of filtered and non-filtered strawberry nectar. Innov Food Sci Emerg Technol 57:102203. https://doi.org/10.1016/j.ifset.2019.102203
Mújica-Paz H, Valdez-Fragoso A, Samson CT, Welti-Chanes J, Torres A (2011) High-pressure processing technologies for the pasteurization and sterilization of foods. Food Bioprocess Technol 4:969–985. https://doi.org/10.1007/s11947-011-0543-5
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm 50:161–177. https://doi.org/10.1016/S0939-6411(00)00087-4
Nikolić MV, Mojovic L (2007) Hydrolysis of apple pectin by the coordinated activity of pectic enzymes. Food Chem 101:1–9. https://doi.org/10.1016/j.foodchem.2005.12.053
Nishinari K (2016) Polysaccharide rheology and in-mouth perception. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications. CRC/Taylor & Francis, Boca Raton, pp 541–588
Nunes L, Tavares GM (2019) Thermal treatments and emerging technologies: impacts on the structure and techno-functional properties of milk proteins. Trends Food Sci Technol 90:88–99. https://doi.org/10.1016/j.tifs.2019.06.004
Paquin P (1999) Technological properties of high pressure homogenizers: the efect of fat globules, milk proteins, and polysaccharides. Int Dairy J 9:329–335. https://doi.org/10.1016/S0958-6946(99)00083-7
Paquin P, Lacasse J, Subirade M, Turgeon S (1999) Continuous process of dynamic high-pressure homogenization for the denaturation of proteins. US6511695B1
Patrignani F, Lanciotti R (2016) Applications of high and ultra high pressure homogenization for food safety. Front Microbiol 7:1132. https://doi.org/10.3389/fmicb.2016.01132
Pereda J, Ferragut V, Buffa M, Guamis B, Trujillo AJ (2008) Proteolysis of ultra-high pressure homogenised treated milk during refrigerated storage. Food Chem 111:696–702. https://doi.org/10.1016/j.foodchem.2008.04.040
Pereda J, Ferragut V, Quevedo JM, Guamis B, Trujillo AJ (2007) Effects of ultra-high pressure homogenization on microbial and physicochemical shelf life of milk. J Dairy Sci 90:1081–1093. https://doi.org/10.3168/jds.S0022-0302(07)71595-3
Pereda J, Ferragut V, Quevedo JM, Guamis B, Trujillo AJ (2009) Heat damage evaluation in ultra-high pressure homogenized milk. Food Hydrocoll 23:1974–1979. https://doi.org/10.1016/j.foodhyd.2009.02.010
Perez-Locas C, Yaylayan VA (2010) The Maillard reaction and food quality deterioration. In: Skibsted LH, Risbo JLM (eds) Chemical deterioration and physical instability of food and beverages. Woodhead Publishing, Oxford, pp 70–94
Picart L, Thiebaud M, René M, Guiraud JP, Cheftel JC, Dumay E (2006) Effects of high pressure homogenisation of raw bovine milk on alkaline phosphatase and microbial inactivation. A comparison with continuous short-time thermal treatments. Journal of Dairy Research 73:454–463. https://doi.org/10.1017/S0022029906001853
Poliseli-Scopel FH, Hernández-Herrero M, Guamis B, Ferragut V (2012) Comparison of ultra high pressure homogenization and conventional thermal treatments on the microbiological, physical and chemical quality of soymilk. LWT Food Sci Technol 46:42–48. https://doi.org/10.1016/j.lwt.2011.11.004
Poliseli-Scopel FH, Hernández-Herrero M, Guamis B, Ferragut V (2014) Sterilization and aseptic packaging of soymilk treated by ultra high pressure homogenization. Innov Food Sci Emerg Technol 22:81–88. https://doi.org/10.1016/j.ifset.2014.01.001
Popper L, Knorr D (1990) Applications of high-pressure homogenization for food preservation. Food Technol 44:84–89
Rao MA (2014) Rheological properties of fluid foods. In: Rheology of fluid, semisolid, and solid foods principles and applications, 3rd edn. Springer, New York, pp 121–177
Ridout MJ, Mackie AR, Wilde PJ (2004) Rheology of mixed β-casein/β-lactoglobulin films at the air-water interface. J Agric Food Chem 52:3930–3937. https://doi.org/10.1021/jf034854p
Rodarte D, Zamora A, Trujillo AJ, Juan B (2018) Effect of ultra-high pressure homogenization on cream: shelf life and physicochemical characteristics. LWT 92:108–115. https://doi.org/10.1016/j.lwt.2018.02.020
Rodriguez Patino JM, Pilosof AMR (2011) Protein-polysaccharide interactions at fluid interfaces. Food Hydrocoll 25. https://doi.org/10.1016/j.foodhyd.2011.02.023
Roesch RR, Corredig M (2003) Texture and microstructure of emulsions prepared with soy protein concentrate by high-pressure homogenization. LWT Food Sci Technol 36:113–124. https://doi.org/10.1016/S0023-6438(02)00208-6
Rota C, Liverani L, Spelta F, Mascellani G, Tomasi A, Iannone A, Vismara E (2005) Free radical generation during chemical depolymerization of heparin. Anal Biochem 344:193–203. https://doi.org/10.1016/j.ab.2005.06.043
Samarasinghe N, Fernando S, Lacey R, Faulkner WB (2012) Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew Energy 48:300–308. https://doi.org/10.1016/j.renene.2012.04.039
Sandra S, Dalgleish DG (2005) Effects of ultra-high-pressure homogenization and heating on structural properties of casein micelles in reconstituted skim milk powder. Int Dairy J 15:1095–1104. https://doi.org/10.1016/j.idairyj.2004.11.015
dos Santos Aguilar JG, Cristianini M, Sato HH (2018) Modification of enzymes by use of high-pressure homogenization. Food Res Int 109:120–125. https://doi.org/10.1016/j.foodres.2018.04.011
Saricaoglu FT (2020) Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int J Biol Macromol 144:760–769. https://doi.org/10.1016/j.ijbiomac.2019.11.034
Saricaoglu FT, Atalar I, Yilmaz VA, Odabas HI, Gul O (2019) Application of multi pass high pressure homogenization to improve stability, physical and bioactive properties of rosehip (Rosa canina L.) nectar. Food Chem 282:67–75. https://doi.org/10.1016/j.foodchem.2019.01.002
Saricaoglu FT, Gul O, Besir A, Atalar I (2018) Effect of high pressure homogenization (HPH) on functional and rheological properties of hazelnut meal proteins obtained from hazelnut oil industry by-products. J Food Eng 233:98–108. https://doi.org/10.1016/j.jfoodeng.2018.04.003
Saricaoglu FT, Gul O, Tural S, Turhan S (2017) Potential application of high pressure homogenization (HPH) for improving functional and rheological properties of mechanically deboned chicken meat (MDCM) proteins. J Food Eng 215:161–171. https://doi.org/10.1016/j.jfoodeng.2017.07.029
Sauer T, Robinson CW, Glick BR (1989) Disruption of native and recombinant Escherichia coli in a high-pressure homogenizer. Biotechnol Bioeng 33:1330–1342. https://doi.org/10.1002/bit.260331016
Schmitt C, Sanchez C, Desobry-Banon S, Hardy J (1998) Structure and technofunctional properties of protein-polysaccharide complexes: a review. Crit Rev Food Sci Nutr 38:689–753. https://doi.org/10.1080/10408699891274354
Sentandreu E, Gurrea MADC, Betoret N, Navarro JL (2011) Changes in orange juice characteristics due to homogenization and centrifugation. J Food Eng 105:241–245. https://doi.org/10.1016/j.jfoodeng.2011.02.027
Serra M, Trujillo AJ, Guamis B, Ferragut V (2009) Evaluation of physical properties during storage of set and stirred yogurts made from ultra-high pressure homogenization-treated milk. Food Hydrocoll 23:82–91. https://doi.org/10.1016/j.foodhyd.2007.11.015
Serra M, Trujillo AJ, Guamis B, Ferragut V (2009) Flavour profiles and survival of starter cultures of yoghurt produced from high-pressure homogenized milk. Int Dairy J 19:100–106. https://doi.org/10.1016/j.idairyj.2008.08.002
Serra M, Trujillo AJ, Jaramillo PD, Guamis B, Ferragut V (2008) Ultra-high pressure homogenization-induced changes in skim milk: impact on acid coagulation properties. J Dairy Res 75:69–75. https://doi.org/10.1017/S0022029907003032
Serra M, Trujillo AJ, Quevedo JM, Guamis B, Ferragut V (2007) Acid coagulation properties and suitability for yogurt production of cows’ milk treated by high-pressure homogenisation. Int Dairy J 17:782–790. https://doi.org/10.1016/j.idairyj.2006.10.001
Sevenich R, Mathys A (2018) Continuous versus discontinuous ultra-high-pressure systems for food sterilization with focus on ultra-high-pressure homogenization and high-pressure thermal sterilization: a review. Compr Rev Food Sci Food Saf 17:646–662. https://doi.org/10.1111/1541-4337.12348
Sharabi S, Okun Z, Shpigelman A (2018) Changes in the shelf life stability of riboflavin, vitamin C and antioxidant properties of milk after (ultra) high pressure homogenization: direct and indirect effects. Innov Food Sci Emerg Technol 47:161–169. https://doi.org/10.1016/j.ifset.2018.02.014
Shi X, Zou H, Sun S, Lu Z, Zhang T, Gao J, Yu C (2019) Application of high-pressure homogenization for improving the physicochemical, functional and rheological properties of myofibrillar protein. Int J Biol Macromol 138:425–432
Shirgaonkar IZ, Lothe RR, Pandit AB (1998) Comments on the mechanism of microbial cell disruption in high-pressure and high-speed devices. Biotechnol Prog 14:657–660. https://doi.org/10.1021/bp980052g
Shpigelman A, Kyomugasho C, Christiaens S, Van Loey AM, Hendrickx ME (2015) The effect of high pressure homogenization on pectin: importance of pectin source and pH. Food Hydrocoll 43:189–198. https://doi.org/10.1016/j.foodhyd.2014.05.019
Sidhu J, Singh R (2016) Ultra high pressure homogenization of soy milk: effect on quality attributes during storage. Beverages 2:15. https://doi.org/10.3390/beverages2020015
Sila DN, Smout C, Elliot F, Van Loey A, Hendrickx M (2006) Non-enzymatic depolymerization of carrot pectin: toward a better understanding of carrot texture during thermal processing. J Food Sci 71:E1–E9. https://doi.org/10.1111/j.1365-2621.2006.tb12391.x
Silva VM, Sato ACK, Barbosa G, Dacanal G, Ciro-Velásquez HJ, Cunha RL (2010) The effect of homogenisation on the stability of pineapple pulp. Int J Food Sci Technol 45:2127–2133. https://doi.org/10.1111/j.1365-2621.2010.02386.x
Singha S, Bhattacharya B, Basu S (2016) Process technology of nanoemulsions in food processing. In: Grumezescu AM (ed) Nanotechnology in the agri-food industry. Volume 2, : encapsulation. Academic Press, London, pp 831–871
Slavov AM, Denev PN, Denkova ZR, Kostov GA, Denkova-Kostova RS, Chochkov RM, Deseva IN, Teneva DG (2019) Emerging cold pasteurization technologies to improve shelf life and ensure food quality. In: Galanakis CM (ed) Food quality and shelf life. Academic Press, pp 55–123
Song X, Zhou C, Fu F, Chen Z, Wu Q (2013) Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind Crop Prod 43:538–544. https://doi.org/10.1016/j.indcrop.2012.08.005
Souto EB, Anselmi C, Centini M, Müller RH (2005) Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN®). Int J Pharm 295:261–268. https://doi.org/10.1016/j.ijpharm.2005.02.005
Stang M, Schuchmann H, Schubert H (2001) Emulsification in high-pressure homogenizers. Engineering in Life Sciences 1:151. https://doi.org/10.1002/1618-2863(200110)1:4<151::aid-elsc151>3.0.co;2-d
Stephen AM, Churms SC (2016) Indroduction. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications, 2nd edn. CRC/Taylor & Francis, Boca Raton, pp 1–24
Suárez-Jacobo Á, Rüfer CE, Gervilla R, Guamis B, Roig-Sagués AX, Saldo J (2011) Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem 127:447–454. https://doi.org/10.1016/j.foodchem.2010.12.152
Taylor TM, Roach A, Black DG, Davidson PM, Harte F (2007) Inactivation of Escherichia coli K-12 exposed to pressures in excess of 300 MPa in a high-pressure homogenizer. J Food Prot 70:1007–1010. https://doi.org/10.4315/0362-028X-70.4.1007
Thiebaud M, Dumay E, Picart L, Guiraud JP, Cheftel JC (2003) High-pressure homogenisation of raw bovine milk. Effects on fat globule size distribution and microbial inactivation. Int Dairy J 13:427–439. https://doi.org/10.1016/S0958-6946(03)00051-7
Toepfl S, Mathys A, Heinz V, Knorr D (2006) Review: potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Reviews International 22:405–423. https://doi.org/10.1080/87559120600865164
Toro-Funes N, Bosch-Fusté J, Veciana-Nogués MT, Vidal-Carou MC (2014) Effect of ultra high pressure homogenization treatment on the bioactive compounds of soya milk. Food Chem 152:597–602. https://doi.org/10.1016/j.foodchem.2013.12.015
Tran M, Roberts R, Felix TL, Harte FM (2018) Effect of high-pressure-jet processing on the viscosity and foaming properties of pasteurized whole milk. J Dairy Sci 101:3887–3899
Trotta M, Gallarate M, Pattarino F, Morel S (2001) Emulsions containing partially water-miscible solvents for the preparation of drug nanosuspensions. J Control Release 76:119–128. https://doi.org/10.1016/S0168-3659(01)00432-1
Trujillo AJ, Roig-Sagués AX, Zamora A, Ferragut V (2016) High-pressure homogenization for structure modification. In: Knoerzer K, Juliano P, Smithers G (eds) Innovative food processing technologies: extraction, separation, component modification and process intensification. Woodhead Publishing, Amsterdam, Netherlands, pp 315–344
Uchiyama H, Tozuka Y, Asamoto F, Takeuchi H (2011) α-glucosyl hesperidin induced an improvement in the bioavailability of pranlukast hemihydrate using high-pressure homogenization. Int J Pharm 410:114–117. https://doi.org/10.1016/j.ijpharm.2011.03.017
Valencia-Flores DC, Hernández-Herrero M, Guamis B, Ferragut V (2013) Comparing the effects of ultra-high-pressure homogenization and conventional thermal treatments on the microbiological, physical, and chemical quality of almond beverages. J Food Sci 78:E199–E205. https://doi.org/10.1111/1750-3841.12029
Vannini L, Lanciotti R, Baldi D, Guerzoni ME (2004) Interactions between high pressure homogenization and antimicrobial activity of lysozyme and lactoperoxidase. Int J Food Microbiol 94:123–135. https://doi.org/10.1016/j.ijfoodmicro.2004.01.005
Velázquez-Estrada RM, Hernández-Herrero MM, Rüfer CE, Guamis-López B, Roig-Sagués AX (2013) Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov Food Sci Emerg Technol 18:89–94. https://doi.org/10.1016/j.ifset.2013.02.005
Vélez-Ruíz J (2002) Relevance of rheological properties in food process engineering. In: Welti-Chanes J, Barbosa-Cánovas GV, Aguilera JM (eds) Engineering and food for the 21st century, 1st edn. CRC Press, Boca Raton, pp 307–326
Verduyn C, Suksomcheep A, Suphantharika M (1999) Effect of high pressure homogenization and papain on the preparation of autolysed yeast extract. World J Microbiol Biotechnol 15:63–71. https://doi.org/10.1023/A:1008818511497
Villay A, Lakkis de Filippis F, Picton L, Le Cerf D, Vial C, Michaud P (2012) Comparison of polysaccharide degradations by dynamic high-pressure homogenization. Food Hydrocoll 27:278–286. https://doi.org/10.1016/j.foodhyd.2011.10.003
Walastra P (1993) Principle of emulsion formation. Chem Eng Sci 48:333–349. https://doi.org/10.1016/0009-2509(93)80021-H
Wang B, Li D, Wang LJ, Chiu YL, Chen XD, Mao Z h (2008) Effect of high-pressure homogenization on the structure and thermal properties of maize starch. J Food Eng 87:436–444. https://doi.org/10.1016/j.jfoodeng.2007.12.027
Wang B, Li D, Wang LJ, Liu YH, Adhikari B (2012) Effect of high-pressure homogenization on microstructure and rheological properties of alkali-treated high-amylose maize starch. J Food Eng 113:61–68. https://doi.org/10.1016/j.jfoodeng.2012.05.021
Wang JM, Xia N, Yang XQ, Yin SW, Qi JR, He XT, Yuan DB, Wang LJ (2012) Adsorption and dilatational rheology of heat-treated soy protein at the oil-water interface: relationship to structural properties. J Agric Food Chem 60:3302–3310. https://doi.org/10.1021/jf205128v
Wang T, Sun X, Zhou Z, Chen G (2012) Effects of microfluidization process on physicochemical properties of wheat bran. Food Res Int 48:742–747. https://doi.org/10.1016/j.foodres.2012.06.015
Wang Y, Li D, Wang LJ, Xue J (2011) Effects of high pressure homogenization on rheological properties of flaxseed gum. Carbohydr Polym 83:489–494. https://doi.org/10.1016/j.carbpol.2010.08.015
Wellala CKD, Bi J, Liu X, Liu J, Lyu J, Wu X (2019) Juice related water-soluble pectin characteristics and bioaccessibility of bioactive compounds in oil and emulsion incorporated mixed juice processed by high pressure homogenization. Food Hydrocoll 93:56–67. https://doi.org/10.1016/j.foodhyd.2019.02.011
Wellala CKD, Bi J, Liu X, Liu J, Lyu J, Zhou M, Marszałek K, Trych U (2020) Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innovative Food Science and Emerging Technologies 60. https://doi.org/10.1016/j.ifset.2019.102279
Welti-Chanes J, Ochoa-Velasco CE, Guerrero-Beltrán JÁ (2009) High-pressure homogenization of orange juice to inactivate pectinmethylesterase. Innov Food Sci Emerg Technol 10:457–462. https://doi.org/10.1016/j.ifset.2009.05.012
Wu F, Shi X, Zou H, Zhang T, Dong X, Zhu R, Yu C (2019) Effects of high-pressure homogenization on physicochemical, rheological and emulsifying properties of myofibrillar protein. J Food Eng 263:272–279. https://doi.org/10.1016/j.jfoodeng.2019.07.009
Yang J, Liu G, Zeng H, Chen L (2018) Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll 83:275–286. https://doi.org/10.1016/j.foodhyd.2018.05.020
Ye R, Harte F (2014) High pressure homogenization to improve the stability of casein-hydroxypropyl cellulose aqueous systems. Food Hydrocoll 35:670–677. https://doi.org/10.1016/j.foodhyd.2013.08.022
Yi J, Kebede B, Kristiani K, Grauwet T, Van Loey A, Hendrickx M (2018) Minimizing quality changes of cloudy apple juice: the use of kiwifruit puree and high pressure homogenization. Food Chem 249:202–212. https://doi.org/10.1016/j.foodchem.2017.12.088
Yu C, Wu F, Cha Y, Zou H, Bao J, Xu R, Du M (2018) Effects of high-pressure homogenization on functional properties and structure of mussel (Mytilus edulis) myofibrillar proteins. Int J Biol Macromol 118:741–746. https://doi.org/10.1016/j.ijbiomac.2018.06.134
Yuan B, Danao MGC, Stratton JE, Weier SA, Weller CL, Lu M (2018) High pressure processing (HPP) of aronia berry purée: effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov Food Sci Emerg Technol 47:249–255. https://doi.org/10.1016/j.ifset.2018.03.009
Zamora A, Guamis B (2015) Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Eng Rev 7:130–142. https://doi.org/10.1007/s12393-014-9097-4
Zayas JF (1997) Functionality of proteins in food. Springer, Berlin
Zhang HQ, Barbosa-Cánovas GV, Balasubramaniam VM, Dunne CP, Farkas DF, Yuan JTC (2011) Nonthermal processing technologies for food. Wiley-Blackwell, Chichester
Zhou L, Guan Y, Bi J, Liu X, Yi J, Chen Q, Wu X, Zhou M (2017) Change of the rheological properties of mango juice by high pressure homogenization. LWT 82:121–130. https://doi.org/10.1016/j.lwt.2017.04.038
Acknowledgments
The authors acknowledge the support of the EIT Food project # 20032 “HPHC - Development and application of hydrocolloids functionalized by dynamic high pressure.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levy, R., Okun, Z. & Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng Rev 13, 490–508 (2021). https://doi.org/10.1007/s12393-020-09239-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-020-09239-8