Abstract
Food engineering is an important sub-field that requires special attention in the food industry. The application of biochemical process engineering principles in food production often leads to the optimization of certain features of the food production process; similarly, it results in rapid production, improved quality and reduced food losses. Consequently, to address each aspect of food processing including engineering adequately, researchers must have a multidisciplinary approach, using aspects from a number of fields such as microbiology, chemistry, food technology, process engineering and molecular biology. Accordingly, this review focuses on the engineering of various vinegars. Furthermore, cognizance is given to the gaps that need to be addressed in vinegar engineering, particularly to address limitations employed in traditional approaches during vinegar production. Food engineering assessments address numerous functions in integrated systems for which fermentation systems are the primary process. Mathematical models are used to describe the process, simulate future fermentations and describe process performance. Vinegar engineering also includes the use or design of bioreactors intended for improved product yield and rapid production, improved mass or energy transfer efficiencies and the reduction of detrimental hydrodynamics fermentor conditions on the microorganisms used. For vinegar fermentation, bioreactor selection which might include cell immobilization requires that appropriate process control and optimization be conducted using mathematical models, with rates of acetification being influenced by parameters such as the ratio of dissolved oxygen consumption in comparison to acetic acid yield.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
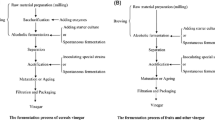
Vinegar is generally defined as a sour or acidic liquid obtained from a two-step fermentation process [10, 18, 40]. The fermentation process utilizes yeast for the anaerobic fermentation of sugar to ethanol and acetic acid bacteria (AAB) for the aerobic oxidation of ethanol to acetic acid (Fig. 1) [10, 40, 86]. As a key metabolite, acetic acid is an important ingredient in vinegar and its concentration defines the organoleptic characteristics of vinegars. Typically, vinegar is not generally classified as food, but as a food-flavouring agent [74, 86], an important feedstock in the food industry [74] and a food preservative [29, 49].
Vinegar is made from numerous carbohydrate sources or food products that contain fermentable (reducible) sugar for yeast to metabolize [10, 86]. Currently, there are numerous types of vinegars produced globally and most of these are produced from cheap raw materials, which is why most vinegars are lowly priced. These raw materials can include by-products obtained from food processing, low-quality fruit, agricultural surpluses and fruit waste [10, 63, 86, 90]. Some vinegars are obtained from non-fermentation processes, such as distilling alcohol, which is subsequently oxidized to acetic acid [86].
There are two distinct production methods for fermentation-based vinegars, namely the traditional and submerged methods. The traditional method relies on surface culture fermentations, whereby oxygen is obtained from the air. In simpler terms, this method applies low technological inputs, and as a result, the fermentation period is longer and the vinegars are therefore expensive [23, 90]. These expensive vinegars are usually those made in certain areas with regional and seasonal input of raw materials. Examples include oxos vinegar from Greece, sherry vinegar from Spain and the Traditional Balsamic Vinegar (TBV) from the provinces of Reggio Emilia and Modena, Italy [86]. The second method is the submerged tank method, which entails the use of technologically advanced systems such as the use of spargers, coolers, antifoams, stainless steel fermentors and automated control systems [23, 90]. The submerged method is typically used by large producers for the production of commercial vinegars, which are in high demand [90]. An example of a typical process distinction can be made between traditional wine vinegar fermentation processes that takes up to 2 months to achieve the required final product quality concentrations, and the industrial wine vinegar fermentation using the Frings acetator (submerged method) that only takes up to 20–24 h [7].
For these reasons, the application of biochemical engineering principles in vinegar production is important for large-scale producers, not only because vinegar is a food flavouring agent found in virtually every household [9] but also due to its widespread use in the food industry [5, 48, 71, 95]. In the context of this review, vinegar engineering refers to food, biochemical, bioprocess and chemical reaction engineering technology. This will bring about an understanding of vinegar engineering and address some challenges, which customary microbiological and chemistry methods do not resolve. These parameters include bioreactor selection (design), dissolved oxygen mass transfer efficiency, effect of fluid mechanics on microorganisms and kinetic modelling of co-, by- and final products. In this review, some concepts in vinegar engineering system analysis are introduced. However, the introduction of new technologies can lead to authentication doubts, as some vinegars are produced following well-defined traditional approaches. Therefore, these new approaches are more fitting to vinegar production methods, which are not protected by legislation. There is a growing interest in vinegar production utilizing a variety of fruit, agricultural waste or raw materials. Some unusual vinegars include those produced from onion [8, 32, 41, 43], hawthorn [94, 99] and wood [2, 69, 96].
According to statistical data published by the Vinegar Institute (Vinegar Institute, 2005), global shares of various vinegars were balsamic vinegar (34%), red wine vinegar (17%), cider vinegar (7%), rice vinegar (4%) and white vinegar (2%), while other vinegars were 36% (Fig. 2). Furthermore, in the USA, white distilled vinegars have the highest unit shares (68%), while in China, brewed and white fruit vinegars are the most popular.
Global shares of various vinegars published by the Vinegar Institute in 2005. Adapted from https://versatilevinegar.org/market-trends/
Vinegar Engineering: Patents and Future Outlooks
Patents are fundamental to vinegar engineering research. As a result, a substantial amount of vinegar patents have been filed during the 19th and twentieth century (summarized in Table 1). Nevertheless, a brief outline of the history of vinegar engineering patents includes inventions that are primarily associated with bioreactor/acetifier design and configurations. One of the earliest examples of inventions in the twentieth century was made by [38, 39]). These inventions entailed the cooling of the infusion mash/wort in order to prevent thermal disturbances that arise due to large generators used in the acetifiers. Other inventions entailed the addition of temperature-responsive elements in the bioreactor with the aim of regulating temperature and flow of the wort according to the required conditions of various vinegar products.
Another critical invention was aimed at preventing yield losses through evaporation by employing vats made from earthenware, wood and non-corrosive metals in rare cases [6]. The latter was achieved by altering bioreactor designs to include cooling devices on the gas outlets aimed at cooling and condensing vinegar vapours exiting the exhaust outlets. Further improvements by Ernst and Hunt Foods Inc [22] were made in the submerged methods for vinegar production (continuous system) whereby continuous aeration using an air dispersing device was used. Bioreactors also contained cooling and heating coils for temperature control and a rotor for efficient liquid circulation within the system. The super-oxygenated atmosphere was also created in another invention by controlling oxygen demand in the production system in the second phase of fermentation according to the system’s oxygen requirements. Foam accumulation during vinegar fermentations was controlled using rotational mechanical devices [97]. This particular invention allowed the accumulated foam to be broken down into gas and liquid vinegars that are subsequently transferred back into the vinegar broth inside the bioreactor.
Overall, these aforementioned inventions are evidence that researchers have mostly paid special attention/focused on bioreactor design-based parameters. Moreover, in vinegar engineering, there are several areas that can be explored in this regard. Some important inventions and patents not discussed in this section are also listed in Table 1.
Vinegar Engineering and Bioreactors
Bioreactors: Importance and General Overview
Bioreactor studies have been conducted for various vinegar production systems using a diverse range of bioreactors (Table 2). Industrial vinegars generally employ the acetators/bioreactors (submerged approach) for vinegar production, due to the high yields obtained in such systems [36]. The use of bioreactors normally offers a controlled environment, which leads to faster production, reduced product losses and possibilities for optimization [4, 92, 98]. Most bioreactors are normally operated using optimized conditions and parameters. These include pH, temperature [12, 36], substrate/nutrient concentrations [36, 53], airflow rates [64], agitation and pressure [26]. Optimization of the aforementioned parameters has been extensively studied for some fermentation systems. However, there is insubstantial research on the optimization of process parameters for bioreactors used in vinegar production. As bioreactors come in various designs to suit various biological systems in order to meet the demands of the process [93], they are generally operated using different operational modes, which include batch, fed batch, resting/immobilized cells and continuous systems [26].
Desired Features in Bioreactors
The most notable mutual and desired feature in most of the bioreactors used for vinegar production is the ability to permit high oxygen transfer and sufficient agitation. Agitation should sufficiently homogenize bioreactor contents and prevent low-oxygen areas, thus preventing dead zones and stuck fermentations. Additionally, there should be minimal oxygen transfer interruption, as this can slow down the process or render the AAB non-viable [36, 83]. To serve this purpose, the most commonly used bioreactor for commercial vinegar production was designed by Heinrich Frings ‘Frings acetator’ [25] and it achieves high product yields (95%) [24, 79]. It is recommended because of low energy requirements (400 W/L) compared to other bioreactors and also contains a Frings alkolograph that quantifies alcohol concentration during fermentation [79]. Importantly, the acetator also has a self-aspirating system that replaces system oxygenation by compressed air [25]. The homogenous dispersion of very fine air bubbles is also another desired feature in industrial bioreactors; hence, it is common to find bioreactors equipped with special turbines designed for this purpose [83].
Bioreactor Design and Configurations
The design and configurations of a bioreactor play a critical role in the performance of the vinegar fermentation process. Bioreactor configurations include height to diameter ratio (aspect ratio), the surface area to volume ratio [37], shape of vessel [14, 37], stainless steel features [15], impeller configurations (size, number of blades, location) [33], turbines and gas inlets and outlets [34]. In order to emphasize the importance of design and configuration, a graphical depiction of how height to diameter ratio influences gaseous (O2 and CO2) exchange is demonstrated in Fig. 3. In this figure, a small perforation diameter (Dmin) and larger height to liquid–air interface area (H/Smax) favours alcoholic fermentation, while a larger perforation diameter (Dmax) and smaller height to liquid–air interface area (H/Smin) favours the acetification process. These are important factors to consider when designing a bioreactor for vinegar production. In addition, vinegar production systems which separate the alcoholic and acetification process can employ both geometric designs to suit the needs of each fermentation process. Previously, a 10-L bioreactor equipped with a sparger located 2 cm from the bottom of the bioreactor for air diffusion was reported as a suitable design for vinegar production [34]. The bioreactor had two impeller flat blade Rushton turbines with a gap of 1.45 cm between the sparger and the first impeller, with each impeller having six blades (Fig. 4). Proximity placement of the sparger and impeller is not advisable, as this would cause flooding and bubble coalescence. Similarly, if they are too far apart, this would impair foam mitigation. Temperature control can be maintained with the use of a water jacket, and an oxygen probe must be installed to monitor dissolved oxygen.
An illustration of the influence of geometric characteristics on gaseous exchange. a favours alcoholic fermentation, b favours acetification, diffusion of gases is indicated by arrows, H/S ratio of container headspace (i.e. H = height to liquid–air interface area (S)), D = perforation diameter of the reactor lid [37].
Mehaia and Cheryan [65] used a membrane recycle bioreactor for the production of date extract vinegar. The design of this type of bioreactor included a hollow-fibre module with a polysulphane membrane, through which sterilized air was sparged at a rate of 0.4 L min−1, achieving a high acetic acid production rate of 10.8 g L−1 h−1 (Table 2). Huang et al. [45] studied vinegar production with fructose as a substrate, while using a fibrous-bed bioreactor (Table 2). The bioreactor was constructed using a glass column and contained a spiral-wound terry cloth. The quality and acetic acid concentration of vinegars are strain-, bioreactor- and fermentation type-dependent. Acetic acid production rates for different types of bioreactors (fed-batch and continuous systems), including free-floating and immobilized cells, are shown in Table 2.
Wine vinegar production, on the other hand, was studied using a non-commercial 100-L bubble column reactor (Table 2) [24]. The bioreactor was equipped with a novel gas–liquid dynamic sparger, which allowed the distribution of fine bubble sizes. This bioreactor showed increased acetification rates compared to other industrial bioreactors. An acetic acid production rate of 1.8 g L−1 h−1 coupled with a 94% (v/v) yield was achieved. Horiuchi et al. [41, 42] studied a charcoal pellet bioreactor (Fig. 5) for acetic acid production from ethanol and onion alcohol, respectively, which was later improved for onion vinegar production (Fig. 6). The onion vinegar bioreactor was serially connected to an ethanol jar fermentor with the onion alcohol being separately produced in the jar fermentor while being continuously fed into the charcoal pellet bioreactor whereby acetification took place (Fig. 5), achieving a high acetic acid production rate (Table 2).
Initially, de Ory et al. [13] designed a bioreactor using a cylinder-shaped stainless steel reactor (Fig. 7) with geometric characteristics including an internal diameter of 0.47 m and a height of 1.4 m for a 225-L working volume. Temperature control was conducted by an interior heat exchanger which was attached to a thermostatic bath. Under this configuration, the reactor re-circulated the outlet gas streams through the bottom of the fermentation vessel with the use of an air pump and stainless steel diffusers. In this design, the stainless steel diffusers were also responsible for pneumatic stirring and maximum oxygen distribution. Furthermore, an industrial oxygen cylinder was connected to the reactor by means of an electro-valve (refer to Fig. 7), which can be switched off to maintain optimum dissolved oxygen (2 mg L−1).
Reproduced industrial acetifier: [1] reactor, [2] gas recycling pump, [3] expansion tank, [4] air diffusers, [5] heat exchanger, [6] thermostatic bath, [7] oxygen cylinder, [8] electro valve, [9] dissolved oxygen sensor and [10] feed inlet and effluent outlet pump. de Ory et al. [13] as reported in de Ory et al. [14]
Furthermore, an aerated-stirred pilot bioreactor was proposed to be an improvement of existing fermenters by González-Sáiz et al. [34]. The study proposed the replacement of plate tops with dish tops in industrial fermenters while achieving an acetification rate of 13.2 and 11.1 g L−1 h−1 for the batch and continuous system, respectively.
Overall Remarks on Bioreactor Design for Vinegar Production
Despite the large number of vinegars produced worldwide, bioreactors have only been studied for a few vinegars, and it is evident that there are several designs (geometric characteristics) of bioreactors depending on the type of vinegar produced and the distinct needs of the fermentation process. However, some basic conditions need to be defined, such as the bacteria used, optimum stirring speed and dissolved oxygen transfer rates.
Immobilized Versus Free-Floating Cells for Vinegar Fermentation
The Importance of Immobilized Cell Systems
Acetic acid bacteria are sensitive bacteria, and it appears that the immobilization of AAB cells improves their efficiency [55, 58, 60, 100]. Generally, the immobilization of cells refers to restricting the motion of cells during fermentation. This can be done by using several techniques, which include entrapping the cells in a carrier, adsorbing the cells on a solid surface and mechanical containment behind a barrier [14]. The most commonly used method in fermentation systems is the adsorption of cells onto a solid surface, which can be performed using the static, dynamic batch, reactor loading and electrode positioning process [1]. Cell immobilization offers defence against harsh environmental conditions such as low pH, osmotic stress and temperature including shearing due to agitation, which consequently increases biomass concentration [45, 55, 58, 87]. Furthermore, cell immobilization provides the advantage of cellular longevity; however, it is also crucial to choose an inexpensive material to use for cell immobilization in order to minimize production costs [55, 87]. This technique can be classified as a bioengineering technique which augments bioreactor design, while improving the total biomass surface area to carry out biochemical reactions. It is an appealing approach that would benefit vinegar producers if implemented due to the rapid acetification rates obtained with immobilized cells [52, 55, 88].
The Choice of a Support Material: Important Factors to Consider
According to the literature reviewed, there are always several reasons for selecting a specific support material for cell immobilization. The selection criteria include availability, benignity, influence of material on product quality, cost, physical properties and oxygen transfer efficiency in cell entrapping gels taking into account the radius and total surface area of the materials [41, 52, 55,56,57,58, 87]. Figure 8 depicts cell immobilization materials that are usually preferred during vinegar production. For example, the fibrous bed (Fig. 8a) was reported to promote high cell density [88], with the charcoal pellets (Fig. 8d) being selected for their high surface area and their microbial affinity [42]. The loofa sponge (Fig. 8c) was described as an edible and fibrous agricultural material with no safety concerns [58]. Other carriers might be oak and/wood shavings (Fig. 8e) [91] which might impart certain flavours into the final product [61, 62] and alginate beads (Fig. 8f) [47, 55].
Cell immobilization materials used in vinegar studies. a cited from Krusong et al. [56]. a Corncobs. b Sugarcane bagasse. c Sliced loofa sponge. d Charcoal pellets. e Wood shavings. f Alginate beads
Cell Immobilization: Comparative Analysis
As aforementioned, cell immobilization techniques vary in terms of which protocols to follow and materials to use. Table 3 lists several studies on the subject of AAB cell immobilization and acetification rates. Hydrous titanium(IV) oxide and hydrous titanium(IV) chelated cellulose were used to immobilize two Acetobacter strains for the conversion of ethanol (produced by fermenting wort) into acetic acid [52]. Improved conversion rates were obtained; however, the performance of the two bacteria strains varied when using titanium oxide. This was attributed to the fact that one specie formed a slime (extracellular polysaccharide substances) while the other strain did not form a slime. The slime-forming bacteria resulted in a 67% and a 24% relative difference increase in acetification rates when immobilized in hydrous titanium(IV) oxide using small (2.5 L) and large (8.0 L) bioreactors, respectively. The non-slime-forming bacteria performed better when the cells were freely floating in contrast to immobilized cells when using the smaller fermenter. In the larger bioreactors, titanium cellulose chelate was added for the immobilization of cells, and there was a relative increase in acetification rates compared to free-floating cells. Kennedy et al. [52] showed that immobilized cells and the aggregation of cells is an efficient strategy compared to free-floating cells for vinegar production. However, producers interested in employing this method would have to optimize the concentration of titanium salts required and evaluate the residual salts in the final product for quality and gustatory perception purposes.
Fibrous bed matrices are widely employed for cell immobilization in the vinegar industry due to their robustness. Additionally, fibrous beds allow efficient mass transfer and do not undergo productivity reduction when immobilized cells are used repeatedly. Non-active immobilized cells can also be scraped off from fibrous beds and replaced with active cells instead of re-starting the immobilization process [46]. Huang et al. [45] immobilized Clostridium formicoaceticum cells for the production of acetic acid using fructose. In this study, the cells were adsorbed in the fibrous matrix by pumping 25 mL min−1 of the fermentation broth into the fibrous bed. The cells were immobilized after 36–48 h of continuous broth pumping. The immobilized cell fermentations were compared to free-floating cell fermentations in batch, fed-batch and continuous systems, and all acetification rates are shown in Table 3. Nonetheless, the highest acetic acid concentration achieved in free cell and immobilized cell fermentation was 46.4 and 78.2 g L−1, respectively. Here, it was concluded that cell immobilization by adsorption is one of the most employed cell immobilization techniques due to its simplicity and it is often cheaper, depending on the material used. Talabardon et al. [88] also immobilized AAB cells by adsorption onto a fibrous bed matrix. This was also a comparative study between free-floating and immobilized cell fermentations for the production of acetic acid from lactose and milk permeate using Clostridium thermolacticum and Moorella thermoautotrophica. Free-floating cells resulted in acetification rates of 0.06 and 0.08 g L−1 h−1 using lactose and milk permeate, respectively. As for the immobilized cells, acetic acid production was increased to 0.54 and 0.30 g L−1 h−1 using lactose and milk permeates respectively. The studies used the adsorption of cells into a fibrous bed technique for the immobilization of cells, resulting in improved acetification rates.
The cell immobilization by entrapment technique is generally classified as expensive due to the cost of alginate and the cost of special equipment required to make gel beads [81]. Nevertheless, this technique was evaluated and was found to compare well with the adsorption techniques during the production of sugarcane vinegar [55]. Acetobacter aceti cells were entrapped using a 4% (w/v) sodium alginate solution to form a cell paste, followed by expulsion through a syringe into a 0.2-M CaCl2 solution, consequently resulting in the formation of calcium alginate beads. Cell adsorption can also be employed using three different materials, i.e. corncobs (Fig. 8a), bagasse (Fig. 8b) and wood shavings (Fig. 8e). Comparative analysis of the gel entrapment and adsorption method for vinegar production is crucial because, apart from broadening the vinegar engineering knowledge, it also allows producers to make an informed decision concerning which technique to employ. Nonetheless, the alginate gel–entrapped and free cell fermentations showed relatively lower acetification rates compared to adsorbed cell fermentations (Table 3), an outcome that has been reported to occur in various studies [14]. In these studies, it was concluded that the lower acetification rates in entrapped cells were due to the lower surface area available for cell immobilization; and as a result, dissolved oxygen and substrate mass transfer across the beads was limited [55]. Lower acetification rates when using the entrapment technique could also be attributed to the growth of cells near the gel surface or the gel radius being too large. For high acetification rates, the recommended gel radius is 1.0–1.4 mm with dissolved oxygen concentration of 0.031–0.14 mg L−1 [87]. Interestingly, positive results were observed with the gel entrapment technique for balsamic-styled vinegar production using beads sized 4.5–8.5 mm (Table 3) [47]. Their observations could be attributed to the microbial consortium of acetic acid bacteria used, the inoculum size, temperature treatments and several other factors.
In a different study, a loofa sponge (Luffa cylindrica) was used to immobilize cells for the adsorption of Acetobacter aceti WK cells for corn vinegar production [58]. The sponge was cut into small pieces of 1-in. thickness, washed with water and then sterilized by submerging into 4% (v/v) acetic acid for 24 h prior to being used as a cell support material. The loofa sponge is an environmentally benign porously structured material composed of lignin, cellulose and hemicellulose [11], which allows for high dissolved oxygen diffusion that eventually makes it an ideal material for cell immobilization [58]. For this particular reason, it is widely grown in the subtropical regions of Korea, China, Brazil, Japan and some areas of Central and South America [11, 89].
Overall Remarks on Cell Immobilization
Overall, it appears that there are no optimal procedures for the immobilization of AAB cells. However, it is evident that cell immobilization using adsorption is the most common method. The gel entrapment method is also common, albeit at a laboratory scale. Additionally, recent studies focusing on cell immobilization for vinegar engineering are gravely lacking. Based on the reviewed studies, the immobilization of AAB cells by both entrapment and adsorption improves production rate compared to free-floating cells, and in most instances, the selection of the materials to use in a fermentation is important. However, according to most of the reviewed studies, the adsorption of AAB cells to a surface is more effective compared to gel entrapment. Although the materials or methods for adsorption could vary, the type of the vinegar being produced and bacteria used is of paramount importance. Employing cell immobilization for vinegar production also requires an appropriate bioreactor design since immobilization materials such as alginate beads have a low mechanical strength and can easily be damaged by impellers and high agitation rates; therefore, this design parameter would have to be taken into consideration. Ideally, materials used for cell immobilization must be able to resist operating conditions and mechanical influences. An example is fibrous bed matrices, which have been widely studied and are well understood in the process engineering industry. Hence, it is reasonable to conclude that fibrous beds offer a convenient cell immobilization approach.
Aeration During Vinegar Production
Importance of Aeration During Vinegar Production
Aeration is reasonably recognized and well understood as an important bioreactor performance parameter for industrial vinegar production. However, this knowledge is frequently directed to a handful of spirit vinegars and not other varieties. Nonetheless, several studies have reported that oxygen transfer is the rate-limiting step for vinegar production [30, 36, 78, 90]. This is attributed to AAB being strictly aerobic and requiring high dissolved oxygen levels to grow optimally and carry out all the essential activities [36]. The stoichiometry behind the use of oxygen by AAB is that 1 mol of oxygen is required for the oxidation of ethanol to produce 1 mol of acetic acid [30, 78]. This means that, in most vinegar production processes, a continuous supply of oxygen is a necessity [26, 27]. García-García et al. [25] reported that vinegar fermentation exhibits an extremely high demand for oxygen to the extent that a 25-m3 vessel at 20 °C with an acetification rate of 2 g L−1 h−1 requires 20 m3 of gaseous oxygen transfer per hour. If the oxygen concentration is very low, it can slow down the acetification process. Consequently, the oxygen uptake rate for the AAB used must be known, as this will allow proper control of oxygen in the fermentor. The primary challenge is the low solubility of oxygen in the fermentation medium during aerobic system operations such as those used for vinegar fermentation; therefore, it is critical to design a fermentation system that can improve oxygen mass transfer between gaseous and liquid phases [30].
It was also reported that a momentary interruption of oxygen transfer during fermentation could cause the inhibition of the acetification process. However, at higher acetic acid concentrations above 5%, the interruption of oxygen transfer was reported to be less detrimental [36], which effectively highlights the need for adequate oxygen at the initiation of the vinegar engineering process. Schlepütz et al. [83] proposed the use of a respiration activity monitoring system (RAMOS) which can assist in preventing the interruption of oxygen supply. The transfer of oxygen during a fermentation process requires hydrostatic pressure monitoring and reduced mechanical influences such as stirring speed on the cells. Other factors to consider when transferring oxygen for vinegar production are the microorganisms used and their oxygen uptake rate [36, 82].
Influence of Volumetric Mass Transfer Coefficient (k La)
The volumetric mass transfer coefficient (kLa) is an important factor that is generally used to assess the competency of a bioreactor. Furthermore, kLa is used as an important tool when scaling up for vinegar production or any aerobic fermentation system [34]. The determination of kLa depends on several factors such as the geometric parameters of the bioreactor, hydrodynamics, airflow rates, media properties, morphology of microorganisms used and properties [68]. Several methods are used to determine kLa; these include among others the sodium sulphite oxidation method and the gassing in method [85]. kLa in shake flasks is determined by the inclusion of shaking parameters (shaking frequency, shaking diameter) as well as the flask size and working volume [84]. This means that scaling up from conventional shake flasks to fermenters is possible. However, several laboratory-scale studies for vinegar production often neglect this factor; subsequently, results from these studies are not very useful for industrial scale fermentations because of the inconsistency of findings when scaling up.
A high value of kLa is one of the primary goals when designing a bioreactor because it is proportional to the reactor productivity. The aeration capacity of a bioreactor is fundamentally dependent on kLa. For this reason, several studies have investigated the relationship between airflow rates and kLa during vinegar production, with Fregapane et al. [24] reporting improved kLa when airflow rates were increased for all vessel geometric parameters studied. The highest kLa values of 75–170 h−1 were obtained when airflow rates of 0.06–0.25 vvm min−1 were employed. Similar correlations were reported by Krusong et al. [59], with an airflow rate of 1.1 min−1 in a 25-L bioreactor resulting in a kLa of 106 h−1, while an airflow rate of 3.1 min−1 in a 75-L bioreactor resulting in a kLa of 93 h−1. These studies accentuate the importance of sufficient airflow rates for high kLa or vice versa, notwithstanding the geometric characteristics of the bioreactor. Furthermore, kLa for industrial fermenters such as the Frings acetator has been reported to range between 100 and 900 h−1 [25].
Aeration: Comparative Studies
Several oxygen transfer studies were conducted for vinegar production (Tables 4 and 5). These studies paid special attention towards oxygen pressure, uptake and acetification rates.
These include improving oxygen transfer during vinegar production by using a fixed-bed bioreactor with pulse flow due to its high oxygen transfer rate [30]. Fluctuating oxygen partial pressure (Po2) during the process was achieved by mixing nitrogen and oxygen. The overall gas–liquid mass transfer coefficient kLa was determined when the cells were at a steady state with the oxygen uptake rate being determined using the mass balance method, whereby dissolved oxygen is measured at the liquid and gas phases of the reactor. However, the mass balance method on its own can be inaccurate; therefore, using stoichiometry for computations can be a solution, since it uses the acetic acid productivity rate to calculate the oxygen uptake rate. Furthermore, since oxygen was termed as the rate-limiting step, consequently increasing inlet oxygen partial pressure (Po2) can lead to increased dissolved oxygen uptake rate and increased acetic acid production rate (see Table 4) [30].
The optimum oxygen concentrations were defined for different functions by Park et al. [76], which are 3–7 mg L−1 for effective oxygen consumption, and 2–15 mg L−1 for essential enzymes, such as alcohol dehydrogenase and acetaldehyde dehydrogenase. For acetification, the optimum oxygen was reported to be 1–3 mg L−1, and it was concluded that the electron transfer step during ethanol oxidation was the most sensitive to oxygen during the acetification process.
In a different study, the influence of oxygen partial pressures on wine vinegar production was investigated [80]. Fermentations were conducted with varying air compositions ranging from 21 to 63% enriched oxygen content. The aeration rate was 0.06 vvm min−1, with the results showing higher acetification rates when oxygen-rich air, containing 30% oxygen, was used. Fermentation time decreased from 65 to 35 h with an overall acetic acid yield of 96–99%. It is important to note that excessive aeration rates can result in excessive foam formation and loss of medium even when an antifoam system is used. Furthermore, excessive aeration also leads to the loss of ethanol due to evaporation which eventually leads to reduced yields [59]. Some other studies reporting on oxygen transfer include that of Qi et al. [78] who introduced a model describing the ratio of oxygen consumption versus acetic acid yield, which could be used to evaluate fermentation efficiency. Overall, all of the aforementioned studies show the significance of efficient oxygen transfer during vinegar production, a key parameter that cannot be achieved without effective bioreactor design and configuration considerations. Table 5 shows several other studies which investigated aeration during vinegar production, with evidence that adequate aeration ranges from 0.05–1.00 vvm min−1.
Mathematical Computations Used for Assessing Vinegar Engineering
Mathematical models and computations are predominant in bioprocess engineering systems; they normally include the use of equations and computer software for adequate process evaluation and optimization. Mathematical applications offer several advantages, such as process optimization, measuring efficiency, risk assessment, data interpretation, quality assessment and kinetic modelling or simulation. Table 6 lists some mathematical applications in 12 vinegar engineering studies. Virtually every element of the fermentation system requires the use of mathematics to be properly understood, and this includes mass transfer [33, 35, 78], hydrodynamic effects [34], chemical developments [31], microbial growth [31, 33, 72, 87], distillation [3], cell immobilization [87], continuous or batch system efficiency analyses and bioreactor design and configuration. Furthermore, kinetic modelling of a fermentation process is one of the most common and extensively studied mathematical applications which can be applied in vinegar engineering; it assists in understanding the behaviour of a fermentation process. The kinetic models normally simulate and describe the relationship between microbial growth kinetics and chemical developments in the fermentation vessel over a certain period [34, 70].
Modelling microbial growth, substrate consumption and product formation have been done by several researchers [16, 28, 31, 33, 72, 77, 87]. However, the models differ to some degree. For instance, Garrido-Vidal et al. [28] used quadratic models which included the stoichiometric rates of oxidation and yield factors for more accurate and realistic process prediction results. González-Sáiz et al. [34] also included stoichiometric coefficients when calculating yield factors during fermentation. Ghosh et al. [31] modelled palm juice vinegar using common mathematical equations such as the Monods and Luedeking-Piret models to describe microbial proliferation during the fermentation process. Some other models include those proposed by Monteagudo et al. [66] to model substrate utilization rates. These models are reliable, and they can easily be rearranged or manipulated to best describe any system, even a continuous fermentation system. In continuous systems, growth rates, substrate consumption and product formation are modelled or calculated with the inclusion of dilution rates, substrate feeding and effluent rates [28, 76, 87]. Additionally, some models on microbial growth include those developed by Pochat-Bohatier et al. [77] which can be used to demonstrate mechanical shearing influences on microbial behaviour.
During industrial vinegar fermentation, stirring is one of the most important mechanical inputs; however, it can have a positive or detrimental effect. Stirring can affect the microorganisms, while influencing oxygen transfer during the acetification process. This effect can be studied and demonstrated, mathematically. This crucial part of the fermentation process has been understudied in vinegar engineering, and the stirring speed (rpm) for vinegar production has not been properly defined. Thus far, a handful of studies were performed in which mathematical computations were used to demonstrate the effect of agitation during the fermentation process. González-Sáiz et al. [34, 35] reported on a range of stirring speeds and their effect. The agitation range was between 200 and 1000 rpm. The models showed that maximum microbial growth was achieved at a high agitation speed of 800 rpm. Additionally, it was reported that agitation higher than 800 rpm caused an inhibitory shearing effect and cell damage. Heat transferred into the system was calculated while taking into account the microbial heat generated during ethanol oxidation. It was concluded that the models proposed in the study could be applied in various aerobic processes with varying agitation systems. González-Sáiz et al. [35] further reported that conditions with very low agitation resulted in failed fermentations for both experimental and predicted results. In another investigation, Garrido-Vidal et al. [28] studied agitation at a much lower range (50 rpm) in comparison to 1000 rpm; quadratic models, which include agitation, were used, and these models showed that agitation, aeration rate and overpressure have an effect on oxygen transfer. It is also important to note that aeration has been reported to be more important in increasing oxygen transfer compared to agitation. Additionally, sparging and very high agitation speeds result in very high economical costs due to high energy inputs [33].
Modelling of fermentation processes is normally done using laboratory-scale experiments; however, it is also crucial to study the scaling-up of the models for large-scale industrial applications. González-Sáiz et al. [34] studied models in a pilot fermenter and the scaling-up of the models for an industrial fermentation process. The response surfaces generated by the models led to the conclusion that superficial air velocity, aerated mechanical power input, hydrostatic pressure, temperature and concentrations of compounds must be similar to the pilot fermentor. Garcia-Ochoa and Gomez [26] reported that oxygen transfer rate is the most important parameter for the scaling-up of bioreactors. It is crucial to ensure homogenous oxygen concentration in the fermentation medium for adequate growth and acetification rates. Therefore, agitation and aeration are also important parameters in ensuring homogenous oxygen concentration.
Fortunately, several studies have mathematically studied oxygen mass transfer for vinegar engineering (Table 6). These studies include one by González-Sáiz et al. [32] which modelled oxygen transfer in an oxygen-saturated fermentation medium for vinegar production. They described oxygen concentration using the balance method, which is the balance between the oxygen transferred and the oxygen uptake rate. Dobre et al. [16] proposed two models, the first which presumes that acetic acid formation is influenced by oxygen transfer from the gas to liquid phase, while the other model was similar to the Monods model, which presumed that acetic acid formation is influenced by substrate consumption. This study concluded that both models should be used simultaneously. Some other studies modelled the influence of oxygen transfer on specific growth rate of AAB [28].
Another important process parameter to model during vinegar production is the purification of the product. In most vinegar production processes, pure acetic acid must be obtained at the end of the process. A comprehensive study conducted by Nayak et al. [72] involved microfiltration and nanofiltration membranes to purify the product. The models used in this study included the influence of the filtration methods. Furthermore, the models also incorporated some other important parameters such as cross flow rates, dilution rates, pH and recycling of materials in correlation with substrate-product inhibition. It was concluded that the proposed models accurately predicted the performance of the process.
A unique study conducted by Bakar [3] entailed the application of mathematical equations to demonstrate the effect of purifying the fermentation product, using batch distillation columns. The proposed equations included the calculation of boil-up rate during batch distillation, the average composition of the total material distilled and component balance for the hold-up tank.
Conclusions
Overall, based on the reviewed studies, a number of conclusions can be made. (1) The descriptions regarding the design and configurations of a bioreactor have rather flexible design parameters; hence, the diversity with respect to bioreactors used for vinegar production. [2] Oxygen transfer and agitation seem to be major factors to be taken into consideration when designing a bioreactor for improved production. (3) Cell immobilization studies have been conducted for a variety of vinegars at laboratory scale. However, information on cell immobilization techniques is lacking for industrial-scale vinegar production. (4) Some parameters still need to be optimized such as oxygen transfer rates, stirring speed, efficient cell immobilization methods and materials. [5] Although there may be no one size fits all, these parameters can be defined for selected microorganisms. [6] Mathematical computations have mostly been done on commercial-industrial vinegars, and these applications are gravely lacking in traditional vinegars. Several laboratory-scale studies have not investigated scaling up procedures. [7] Studies demonstrating the effect of hydrodynamics and mechanical influences/fermentation vessel design during vinegar production are scanty; therefore, these topics require more attention. Moreover, there is a necessity for studies, which will define a standard method for the production of different types of vinegar.
References
Anonymous (n.d.) Enzyme immobilization methods and applications (Biotechnology notes). https://www.easybiologyclass.com/enzyme-cell-immobilization-techniques/ Accessed 11 April 2019
Baimark Y, Niamsa N (2009) Study on wood vinegars for use as coagulating and antifungal agents on the production of natural rubber sheets. Biomass Bioenergy 33:994–998
Bakar FB (2014) Modelling and simulation of fermentation product purification for local vinegar using batch distillation. CHEMICA: Jurnal Teknik Kimia 1(2):49–55
Boehme P, Stellenberger T, Zhang W, Schulz E, Bergmann T, Jing L, Grebbing M, Baiker A, Ehrhardt A (2014) Feasible and sufficient small-scale amplification of high-capacity adenovirus. Mol Ther 22:1
Branen AL, Davidson PM, Salminen S, Thorngate J eds. (2001). Food additives. CRC Press
Brun KEJ (1945) Manufacture of vinegar. U.S., Patent 2,390-717
Budak HN, Guzel-Seydim ZB (2010) Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J Sci Food Agric 90:2021–2026
Cheun KS, Kang SG, Kang SK, Jung ST, Park YK (2005) Changes of the flavonoids in onion vinegar fermented with onion juice and ethanol. Korean J Food Preserv 12:650–655
Davis EA, Wong WH, Harman WN (2015) Distilled white vinegar (5% acetic acid) as a potential decontamination method for adult zebra mussels. Manag Biol Invasions 6:423–428
De Leonardis A, Macciola V, Iorizzo M, Lombardi SJ, Lopez F, Marconi E (2018) Effective assay for olive vinegar production from olive oil mill wastewaters. Food Chem 240:437–440
Demir H, Top A, Balköse D, Ülkü S (2008) Dye adsorption behavior of Luffa cylindrica fibers. J Hazard Mater 153:389–394
Dendooven L, Magaña IP, De la Torre M (2000) Optimization of gibberellic acid production by immobilized Gibberella fujikuroi mycelium in fluidized bioreactors. J Biotechnol 76:147–155
de Ory I, Romero LE, Cantero D (1999) Maximum yield acetic acid fermenter. Bioprocess Eng 21:187–190
de Ory I, Romero LE, Cantero D (2004a) Operation in semi-continuous with a closed pilot plant scale acetifier for vinegar production. J Food Eng 63:39–45
de Ory I, Romero LE, Cantero D (2004b) Optimization of immobilization conditions for vinegar production. Siran, wood chips and polyurethane foam as carriers for Acetobacter aceti. Process Biochem 39:547–555
Dobre T, Sandu IU, Stroescu M, Stoica AN (2007) Modelling of acetic acid biosynthesis at low acid concentration. Revista de Chimie-Bucharest-original edition 58:251
Ebner H, Enenkel A, Firma FH, (1978) Two stage process for the production of vinegar with high acetic acid concentration. U.S. Patent 4,076,844
Ebner H, Follmann H, Sellmer S, (2000) Vinegar. Ullmann's Encyclopedia of Industrial Chemistry. vol A27
Ebner H, Firma FH, (1969) Process for acetic acid fermentation. U.S. Patent 3,445,245
Ebner H, Firma FH, (1985) Process for the production of vinegar with more than 12 gms/100 ml acetic acid. U.S. Patent 4,503,078
Enenkel A, Firma FH, (1988) Control arrangement for a vinegar fermentation process. U.S. Patent 4,773,315
Ernst M, Hunt Foods Inc, (1961) Process for making vinegar. U.S. Patent 2,997,424
Fernández-Pérez R, Torres C, Sanz S, Ruiz-Larrea F (2010) Strain typing of acetic acid bacteria responsible for vinegar production by the submerged elaboration method. Food Microbiol 27:973–978
Fregapane G, Rubio-Fernández H, Nieto J, Salvador MD (1999) Wine vinegar production using a noncommercial 100-litre bubble column reactor equipped with a novel type of dynamic sparger. Biotechnol Bioeng 63:141–146
García-García I, Santos-Dueñas IM, Jiménez-Ot C, Jiménez-Hornero JE, Bonilla-Venceslada JL (2009) Vinegar engineering. In: Vinegars of the World. Springer, Milan, pp 97–120
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27:153–176
Garcia-Ochoa F, Gomez E, Santos VE, Merchuk JC (2010) Oxygen uptake rate in microbial processes: an overview. Biochem Eng J 49:289–307
Garrido-Vidal D, Pizarro C, González-Sáiz JM (2003) Study of process variables in industrial acetic fermentation by a continuous pilot fermentor and response surfaces. Biotechnol Prog 19:1468–1479
Ghatak PD, Sen CK, (2016) Antioxidant additives in food preservation and human health. Food Toxicol, 377
Ghommidh C, Navarro JM, Messing RA (1982) A study of acetic acid production by immobilized Acetobacter cells: product inhibition effects. Biotechnol Bioeng 24:1991–1999
Ghosh S, Chakraborty R, Chatterjee G, Raychaudhuri U (2012) Study on fermentation conditions of palm juice vinegar by response surface methodology and development of a kinetic model. Braz J Chem Eng 29:461–472
González-Sáiz JM, Esteban-Díez I, Sánchez-Gallardo C, Pizarro C (2008a) Monitoring of substrate and product concentrations in acetic fermentation processes for onion vinegar production by NIR spectroscopy: value addition to worthless onions. Anal Bioanal Chem 391:2
González-Sáiz JM, Pizarro C, Garrido-Vidal D (2008b) Modelling gas–liquid and liquid–gas transfers in vinegar production by genetic algorithms. J Food Eng 87:136–147
González-Sáiz JM, Garrido-Vidal D, Pizarro C (2009a) Scale up and design of processes in aerated-stirred fermenters for the industrial production of vinegar. J Food Eng 93:89–100
González-Sáiz JM, Garrido-Vidal D, Pizarro C (2009b) Modelling the industrial production of vinegar in aerated-stirred fermentors in terms of process variables. J Food Eng 91:183–196
Gullo M, Verzelloni E, Canonico M (2014) Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem 49:1571–1579
Halladj F, Boukhiar A, Amellal H, Benamara S (2016) Optimization of traditional date vinegar preparation using full factorial design. J Am Soc Brew Chem 74:137–144
Heinrich F, Firma FH, (1932) Manufacture of vinegar. U.S. Patent 1,880,381
Heinrich F, Standard Brands Inc, (1937) Apparatus for the production of vinegar. U.S. Patent 2,094,592
Ho CW, Lazim AM, Fazry S, Zaki UK, Lim SJ (2017) Varieties, production, composition and health benefits of vinegars: a review. Food Chem 221:1621–1630
Horiuchi JI, Kanno T, Kobayashi M (2000a) Effective onion vinegar production by a two-step fermentation system. J Biosci Bioeng 90:289–293
Horiuchi JI, Tabata K, Kanno T, Kobayashi M (2000b) Continuous acetic acid production by a packed bed bioreactor employing charcoal pellets derived from waste mushroom medium. J Biosci Bioeng 89:126–130
Horiuchi JI, Tada K, Kobayashi M, Kanno T, Ebie K (2004) Biological approach for effective utilization of worthless onions—vinegar production and composting. Resour Conserv Recycl 40:97–109
Hsu EJ, Hsu Edward J, (1989) Automated method for a semi-solid fermentation used in the production of ancient quality rice vinegar and/or rice wine. U.S. Patent 4,808,419
Huang YL, Mann K, Novak JM, Yang ST (1998) Acetic acid production from fructose by clostridium formicoaceticum immobilized in a Fibrous-Bed bioreactor. Biotechnol Prog 14:800–806
Huang YL, Wu Z, Zhang L, Cheung CM, Yang ST (2002) Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour Technol 82:51–59
Hutchinson UF, Ntwampe SK, Ngongang MM, Du Plessis HW, Chidi BS, Saulse CK, Jolly NP, (2018) Cell immobilization by Gel entrapment in Ca-alginate beads for balsamic-styled vinegar production. In Proceedings of the 10th International Conference on Advances in Science, Engineering, Technology and Healthcare (ASETH-18) November. 19–20, 2018 Cape Town (South Africa)
Hyuseinov EE, Ludwig WP, WTI Inc (2016) Concentrated natural food additive and methods of preparing the same. U.S. Patent Application 15/177,408
Johnston CS, Gaas CA (2006) Vinegar: medicinal uses and antiglycemic effect. Medscape Gen Med 8:61
Kaur P, Kocher GS, Phutela RP (2011) Production of tea vinegar by batch and semicontinuous fermentation. J Food Sci Technol 48:755–758
Kaushal N, Phutela RP (2017) Vinegar production from barley malt using immobilized Gluconobacter oxydans. Int J Pure Appl Biosci 5:264–271
Kennedy JF, Humphreys JD, Barker SA, Greenshields RN (1980) Application of living immobilized cells to the acceleration of the continuous conversions of ethanol (wort) to acetic acid (vinegar)—hydrous titanium (IV) oxide-immobilized Acetobacter species. Enzym Microb Technol 2:209–216
Kim JN, Choo JS, Wee YJ, Yun JS, Ryu HW (2005) Culture medium optimization for acetic acid production by a persimmon vinegar-derived bacterium. Appl Biochem Biotechnol 123:861–869
Kocher GS, Dhillon HK (2013) Fermentative production of sugarcane vinegar by immobilized cells of Acetobacter aceti under packed bed conditions. Sugar Tech 15:71–76
Kocher GS, Kalra KL, Phutela RP (2006) Comparative production of sugarcane vinegar by different immobilization techniques. J Inst Brew 112:264–266
Krusong, W., Petch-nom, P. and Pinviset, P., 2010. Semicontinuous production process of corn vinegar in stirred tank reactor using fixation of Acetobacter aceti WK on surface of loffa sponge. Kasetsart J Nat Sci 44:201–207
Krusong W, Tantratian S (2014) Acetification of rice wine by Acetobacter aceti using loofa sponge in a low-cost reciprocating shaker. J Appl Microbiol 117:1348–1357
Krusong W, Vichitraka A, (2011) An air-lift acetifier with mash recycling system for corn vinegar production by adsorbed cells of Acetobacter aceti WK on surface of loofa sponge. In 2nd International conference on biotechnology and food science IPCBEE 86–90
Krusong W, Yaiyen S, Pornpukdeewatana S (2015) Impact of high initial concentrations of acetic acid and ethanol on acetification rate in an internal Venturi injector bioreactor. J Appl Microbiol 118:629–640
Kumar G, Mudhoo A, Sivagurunathan P, Nagarajan D, Ghimire A, Lay CH, Lin CY, Lee DJ, Chang JS (2016) Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresour Technol 219:725–737
Kyraleou M, Kallithraka S, Chira K, Tzanakouli E, Ligas I, Kotseridis Y (2015) Differentiation of wines treated with wood chips based on their phenolic content, volatile composition, and sensory parameters. J Food Sci 80:C2701–C2710
Laqui-Estaña J, López-Solís R, Peña-Neira Á, Medel-Marabolí M, Obreque-Slier E (2019) Wines in contact with oak wood: the impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J Sci Food Agric 99:436–448
Mazza S, Murooka Y (2009) Vinegars through the ages. In: Vinegars of the World. Springer, Milan, pp 17–39
Mazutti MA, Zabot G, Boni G, Skovronski A, de Oliveira D, Di Luccio M, Rodrigues MI, Treichel H, Maugeri F (2010) Kinetics of inulinase production by solid-state fermentation in a packed-bed bioreactor. Food Chem 120:163–173
Mehaia MA, Cheryan M (1991) Fermentation of date extracts to ethanol and vinegar in batch and continuous membrane reactors. Enzym Microb Technol 13:257–261
Monteagudo JM, Rodríguez L, Rincón J, Fuertes J (1997) Kinetics of lactic acid fermentation by Lactobacillus delbrueckii grown on beet molasses. J Chem Technol Biotechnol: International Research in Process, Environmental and Clean Technology 68:271–276
Mounir M, Shafiei R, Zarmehrkhorshid R, Hamouda A, Thonart P, Delvigne F, Alaoui MI (2016) Optimization of biomass production of Acetobacter pasteurianus KU710511 as a potential starter for fruit vinegar production. Afr J Biotechnol 15:1429–1441
Moutafchieva D, Popova D, Dimitrova M, Tchaoushev S (2013) Experimental determination of the volumetric mass transfer coefficient. J Chem Technol Metall 48:351–356
Mun SP, Ku CS (2010) Pyrolysis GC-MS analysis of tars formed during the aging of wood and bamboo crude vinegars. J Wood Sci 56:47–52
Mitchell DA, von Meien OF, Krieger N, Dalsenter FD (2004) A review of recent developments in modeling of microbial growth kinetics and intraparticle phenomena in solid-state fermentation. Biochem Eng J 17:15–26
Nair M, Upadhyaya I, Amalaradjou MR, Venkitanarayanan K (2017) Antimicrobial food additives and disinfectants: mode of action and microbial resistance mechanisms. In: Om V (ed) Singh ed. Food Borne Pathogens and Antibiotic Resistance, Wiley-Blackwell, pp 275–301
Nayak J, Pal M, Pal P (2015) Modeling and simulation of direct production of acetic acid from cheese whey in a multi-stage membrane-integrated bioreactor. Biochem Eng J 93:179–195
Nayak J, Pal P (2013) Transforming waste cheese-whey into acetic acid through a continuous membrane-integrated hybrid process. Ind Eng Chem Res 52:2977–2984
Nie Z, Zheng Y, Wang M, Han Y, Wang Y, Luo J, Niu D (2013) Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour Technol 148:325–333
Otto H, Heinz E, (1955) Method for the production of vinegar acids by oxidative fermentation of alcohols. U.S. Patent 2,707,683
Park YS, Ohtake H, Fukaya M, Okumura H, Kawamura Y, Toda K (1989) Effects of dissolved oxygen and acetic acid concentrations on acetic acid production in continuous culture of Acetobacter aceti. J Ferment Bioeng 68:96–101
Pochat-Bohatier C, Bohatier C, Ghommidh C (2003) Modelling the kinetics of growth of acetic acid bacteria to increase vinegar production: analogy with mechanical modelling. http://www.math.ucsd.edu/~helton/MTNSHISTORY/CONTENTS/2000PERPIGNAN/CDROM/articles/B57.pdf Accessed: 12 March 2019
Qi Z, Yang H, Xia X, Xin Y, Zhang L, Wang W, Yu X (2013) A protocol for optimization vinegar fermentation according to the ratio of oxygen consumption versus acid yield. J Food Eng 116:304–309
Qi Z, Yang H, Xia X, Quan W, Wang W, Yu X (2014) Achieving high strength vinegar fermentation via regulating cellular growth status and aeration strategy. Process Biochem 49:1063–1070
Rubio-Fernández H, Salvador MD, Fregapane G (2004) Influence of fermentation oxygen partial pressure on semicontinuous acetification for wine vinegar production. Eur Food Res Technol 219:393–397
Saudagar PS, Shaligram NS, Singhal RS (2008) Immobilization of Streptomyces clavuligerus on loofah sponge for the production of clavulanic acid. Bioresour Technol 99:2250–2253
Schlepütz T, Büchs J (2013) Investigation of vinegar production using a novel shaken repeated batch culture system. Biotechnol Prog 29:1158–1168
Schlepütz T, Gerhards JP, Büchs J (2013) Ensuring constant oxygen supply during inoculation is essential to obtain reproducible results with obligatory aerobic acetic acid bacteria in vinegar production. Process Biochem 48:398–405
Seletzky JM, Noak U, Fricke J, Welk E, Eberhard W, Knocke C, Büchs J (2007) Scale-up from shake flasks to fermenters in batch and continuous mode with Corynebacterium glutamicum on lactic acid based on oxygen transfer and pH. Biotechnol Bioeng 98:800–811
Sobotka M, Prokop A, Dunn IJ, Einsele A, (1982) Review of methods for the measurement of oxygen transfer in microbial systems. In Ann Rep Ferment Proc Vol. 5, 127–210. Elsevier
Solieri L, Giudici P (2009) Vinegars of the world. In: Vinegars of the World. Springer, Milan, pp 1–16
Sun Y, Furusaki S (1990) Continuous production of acetic acid using immobilized Acetobacter aceti in a three-phase fluidized bed bioreactor. J Ferment Bioeng 69:102–110
Talabardon M, Schwitzguébel JP, Peringer P, Yang ST (2000) Acetic acid production from lactose by an anaerobic thermophilic coculture immobilized in a fibrous-bed bioreactor. Biotechnol Prog 16:1008–1017
Tanobe VO, Flores-Sahagun TH, Amico SC, Muniz GI, Satyanarayana KG (2014) Sponge gourd (Luffa cylindrica) reinforced polyester composites: preparation and properties. Def Sci J 64:273–280
Tesfaye W, Morales ML, Garcıa-Parrilla MC, Troncoso AM (2002) Wine vinegar: technology, authenticity and quality evaluation. Trends Food Sci Technol 13:12–21
Thiripurasundari G, Usharani G (2011) Comparative production of vinegar using cashew apple juice by different immobilization techniques. Current Botany 2:31–33
Van Noort D, (2016) Bioreactors ona Chip. In Carl-Fredrik Mandenius. ed. Bioreactors: Design, Operation and Novel Applications. Wiley-VCH
Van't Riet K, Tramper J, (1991) Basic bioreactor design. CRC Press
Wenye Z, Junsong Z, Xin Z (2003) Study on the technology condition of making hawthorn fruit vinegar by one-step fermentation [J]. Chinese Condiment 7:005
Wiley RC, (2017) Preservation methods for minimally processed refrigerated fruits and vegetables. In Minimally Processed Refrigerated Fruits and Vegetables . Springer US. 187–237
Wititsiri S (2011) Production of wood vinegars from coconut shells and additional materials for control of termite workers, Odontotermes sp. and striped mealy bugs, Ferrisia virgata. Songklanakarin J Sci Technol 33(3):349–354
Wittler R, Firma FH (1991) Method and apparatus for controlling foam in a vinegar fermentation process. U.S. Patent 4,997,660
Yan Q, Zheng P, Dong JJ, Sun ZH (2014) A fibrous bed bioreactor to improve the productivity of succinic acid by Actinobacillus succinogenes. J Chem Technol Biotechnol 89:1760–1766
Zheng Y, Zhang K, Wang C, Liu H, Luo J, Wang M (2010) Improving acetic acid production of Acetobacter pasteurianus AC2005 in hawthorn vinegar fermentation by using beer for seed culture. Int J Food Sci Technol 45:2394–2399
Żur J, Wojcieszyńska D, Guzik U (2016) Metabolic responses of bacterial cells to immobilization. Molecules 21:958
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hutchinson, U., Jolly, N., Chidi, B. et al. Vinegar Engineering: a Bioprocess Perspective. Food Eng Rev 11, 290–305 (2019). https://doi.org/10.1007/s12393-019-09196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-019-09196-x