Abstract
The application of ultrasound to conventional dairy processes has the potential to provide significant benefits for the dairy industry such as energy savings and improved product properties. In recent years, the physical and chemical effects of high-intensity ultrasound in liquid and solid media have been extensively studied. Specific dairy processing applications such as emulsification, crystallisation, inactivation of microbes, functionality modifications and fat separation that harness the physical forces of ultrasound are highlighted in the present review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sound waves with a frequency greater than the upper limit of the human hearing range are known as ultrasound (US). Typically, ultrasound has a frequency in the range from 20 kHz to 10 MHz, which can be subdivided into three main regions: low-frequency, high-power ultrasound (20–100 kHz); intermediate-frequency, medium-power ultrasound (100 kHz–1 MHz); and high-frequency, low-power ultrasound (1–10 MHz) [7]. The frequency range selected for food processing depends on the requirements of the process in question.
Low-frequency, high-power ultrasound uses intensities higher than 1 W/cm2, which induce strong cavitation effects that influence the physical, mechanical or chemical/biochemical properties of foods [75, 77]. Examples of suitable applications include emulsification and homogenisation. In contrast, high-frequency low-power ultrasound uses intensities below 1 W/cm2 where the physical effects are comparatively gentle and as such can be utilised for processes such as non-invasive analysis and monitoring food materials, and non-destructive separations of multi-component mixtures [68]. The intermediate frequency range is characterised by peak sonochemical effects and as such can be selected to initiate chemical modifications in food systems.
There are quite a number of review articles published focussing on ultrasonic processing of different food applications in recent years [11, 13, 25–27, 34, 68, 91, 93, 104, 111, 115]. Although many reviews focussed on different food systems, only one review concentrated on US processing of dairy products [11]. Recent advances in understanding ultrasound processing, particularly in dairy systems, have been reported in the last 4 years. In this review, we highlight some of these recent studies with particular attention to the benefits and/or undesirable properties obtained through the use of ultrasound compared with conventional processing. Examples of such applications include emulsification, inactivation of bacteria, crystallisation, functionality improvements and fat separation.
High-Intensity Ultrasound
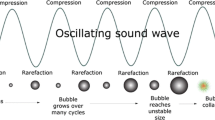
When ultrasound passes through a liquid medium, the interaction between the ultrasonic waves, liquid and dissolved gas leads to a phenomenon known as acoustic cavitation [25]. Several review articles and book chapters describe acoustic cavitation in detail [7, 12, 74] and hence will not be described in significant detail here.
In summary, dissolved gas bubbles within a liquid medium oscillate under the influence of the acoustic field. Over time, these bubbles will grow due to two main growth mechanisms. An individual bubble within a system experiences a fluctuating pressure during bubble oscillation. This can cause dissolved gas and/or solvent vapour to diffuse in and out of the oscillating bubble. If the driving pressure is above a certain threshold, the amount of gas/vapour that diffuses into the bubble during the expansion phase will be greater than the amount that diffuses out of the bubble during the compression phase of the bubble oscillation. This leads to a net growth over time. Such a process is called rectified diffusion. In systems that contain many bubble nuclei (i.e., food and dairy systems), two or more bubbles can combine by bubble–bubble coalescence. This is another possible growth pathway.
Both rectified diffusion and bubble–bubble coalescence lead to the growth of bubbles towards what is known as a resonance size range. This size range varies with the applied frequency of ultrasound and is in general inversely proportional to the ultrasonic frequency. When bubbles reach the resonance size range, they may grow to a maximum size within one or several acoustic cycles and violently collapse. This is known as transient cavitation. In some cases, bubbles can reach the resonance size and continue to oscillate at or near this size for some time. This is known as stable cavitation.
One of the physical phenomena resultant from strong bubble collapse is the generation of temperatures of thousands of degrees in the local vicinity of the bubble [12]. It was found that collapsed bubbles have hot plasma cores with temperatures up to 10,000 K. The temperatures near the surfaces of the bubbles are only few hundreds of degrees [106]. The importance of the medium towards the temperature within bubbles is discussed thoroughly by Ashok kumar [12]. In addition to generating chemical reactions, acoustic cavitation also generates violent physical forces that include micro-jets, shear forces, shock waves and turbulence [7]. Pressures up to several hundred atmospheres can be produced. Highly reactive radicals can be generated within the cavitation bubbles. In food processing, the normal solvent is water, and hence, the extreme conditions generated on collapse of the cavitation bubbles are sufficient to cause rupture of the O–H bonds leading to the formation of H and OH radicals and other recombined products such as hydrogen peroxide [76]. These radicals can participate in a range of oxidation reactions in food media.
High-Intensity Ultrasound Applications in Dairy Processing
The application of ultrasound to liquid-type products in dairy processing leads to the generation of physical forces that include cavitation, acoustic streaming, acoustic radiation, shear, micro-jetting and shockwaves. These physical forces can be used in the generation of emulsions, destruction of bacteria, crystallisation of lactose and separation of fat in dairy systems. In addition, the functional properties of dairy components can be significantly affected by both the physical and chemical effects resultant from acoustic cavitation and other forces of ultrasound.
Formation of Metal Particulates
One of the biggest concerns of using high-intensity ultrasound on food systems is the cavitation-related release of metallic particles into food systems from sonication with ultrasonic transducer probes that come in direct contact with food. Mawson et al. [78] and his CSIRO team investigated the formation of metallic particulates by erosion of a series of transducers in water systems at various frequencies ranging from 18 kHz to 2 MHz. Their study showed that metal leach did occur, but values remained below the accepted drinking water limits, even after long exposure times (7.5-h horn transducers, 7 days for plate transducers). Furthermore, metallic nanoparticles were not detected within the size range of concern to health (i.e., below 80 nm), therefore suggesting no evidence of health hazards from the use of sonotrodes and plate transducers in direct contact with food materials at a range of ultrasonic frequencies.
These long exposure times, as investigated by Mawson et al., [78] are not typically required in food processing applications. It has been shown that ultrasound-induced benefits for various applications in dairy processing can be achieved within the first few seconds of ultrasound application [9, 10]. With the availability of continuous flow-through systems (Prosonix, CA & Hielscher Ultrasonics, Germany), the contact time of product with the acoustic field and sonotrode surfaces can be minimised. Hence, metal particulate generation due to ultrasound application can be considered negligible and not a significant safety concern for ultrasonic dairy processing.
The influence of radicals formed towards the sonochemical production of metal nanoparticles [86] is of another concern in using high-intensity ultrasound on food systems. However, Mawson et al. [78] suggested that the buffering capacity of food materials may prevent the acceleration of production of nanoparticles with the use of radicals.
Furthermore, Ashok kumar et al. [8] showed that unwanted reactions between ultrasonically generated radicals and food ingredients can be minimised by selecting 20 kHz conditions. The addition of radical scavenging compounds like ascorbate can also be used to further reduce the effect radicals. At present, the use of high-intensity ultrasound (especially 20 kHz) on dairy applications can be deemed acceptable for use without any major concerns.
Emulsification
Ultrasonic emulsification offers several benefits over conventional emulsification methods used in dairy systems such as mechanical shaking, high- or ultra-high-pressure homogenising and microfluidising. Improved energy efficiency, higher emulsion stability, lowered requirement of surfactants and controllable size distributions have been reported [1, 55].
Ultrasonic emulsification is primarily driven by cavitation, wherein bubble collapse at or near the oil–water interface causes disruption and mixing of the two phases, resulting in the formation of very fine emulsions [107]. Ultrasound-assisted emulsification is influenced by many variables such as irradiation power [2, 43], position of the ultrasonic source with liquid–liquid interface [102], tip diameter [55], vessel size [55], viscosity of the continuous phase, surfactants concentration [1], hydrostatic pressure and the presence of dissolved gases [14].
The intensity of bubble collapse during cavitation events is the controlling mechanism of power dissipation in an acoustic field. Two important parameters are known to affect this: the hydrostatic pressure of the system and the gas content in the liquid phase.
The hydrostatic pressure may have two effects on the cavitation. Firstly, an increase to the ambient pressure raises the cavitation threshold pressure. This suppresses the likelihood of cavitation by decreasing the parameter space in which cavitation will occur (see Fig. 1). However, it is also known that the intensity of the shock waves generated by collapsing bubbles increases with ambient pressure [101].
Onset cavitation due to local pressure oscillations in a sound field: effect of a shift in ambient hydrostatic pressure (adapted from [14])
The concentration of gas in the liquid phase provides the basis for bubble formation and growth. But at the same time, increasing the amount of gas in the system tends to increase the gas/vapour pressure ratio inside the bubbles, which can cushion the bubble collapse and hence reduce the shock wave intensity. Therefore, overly ‘gassy’ liquids may be less efficiently emulsified.
Behreud and Schubert [14] showed that the application of higher ambient pressure and increased concentration of gas neither influences the magnitude of the cavitational effects per unit volume nor the intensity of the cavitation collapse, although the energy dissipation per unit volume was found to control the particle size distribution of a system. In contrast, Leong et al. [67] showed that pressures up to 400 kPa improved the efficiency of ultrasonic emulsification of food oils, whereas operating with ambient pressures over 450 kPa suppressed the cavitation activity and no emulsification could be achieved.
Shanmugam and Ashokkumar [100] demonstrated the possibility of incorporating novel food oils into milk systems by ultrasonic emulsification. A 20-kHz ultrasound horn was used to emulsify flaxseed oil in a volume of skim milk. No addition of surfactants was required to stabilise the emulsion, as it was found that a small amount of partially denatured whey proteins surrounded the emulsified oil droplets, providing sufficient stability for a minimum of 9 days. The advantage of US is further highlighted since no stable emulsions could be produced when emulsification was instead performed with high-energy mechanical mixing (using matched specific energies).
Another emulsification technique is micro-fluidisation. However, Jafari [51] showed that increasing the micro-fluidisation energy input beyond moderate pressures (40–60 kPa) led to over-processing of emulsion droplets due to re-coalescence and found that decrease in emulsion droplet size <0.5 µm by micro-fluidisation was not possible. In contrast, increased energy input helped to reduce emulsion droplet size with minimum re-coalescence of new droplets with US emulsification [51]. Different reasons for re-coalescence were found including low adsorption rate of the surface active agent, low residence time of the emulsion in the emulsification zone, high rate coalesce frequency and extreme amount of energy density [52].
Homogenisation
High-pressure homogenisation is the most frequently used technique within the dairy industry for homogenisation of milk. Certain dairy products such as milk, yoghurt and ice-cream are homogenised to improve the product stability against creaming during storage. In recent years, the use of ultrasound has attracted much interest for the homogenisation of milk [5, 20, 42, 114] with benefits being similar to those already described in ultrasonic emulsification. However, there remains some uncertainty as to which technique is more efficient for homogenisation and the primary mechanism for its effectiveness.
Koh et al. [61] used a 20-kHz ultrasonic horn and a high-pressure homogeniser to investigate the particle size reduction of whey protein concentrate (WPC) systems in the presence/absence of cavitational conditions. The particle size reduction in WPC systems under high-pressure conditions showed comparable results when subject to ultrasound under similar energy conditions. Notably, cavitation-derived shear forces were found to be absent in high-pressure homogenisation. Hence, the study highlighted the fact that the shear forces generated were mainly responsible for the observed particle size reductions even in the absence of cavitational effects [61]. In the case of ultrasound application, the shear forces are resultant from strong bubble collapse.
The frequency, amplitude and diameter of the ultrasonic probe have a significant influence on the physical properties and variance of milk that is homogenised. Bermúdez-Aguirre et al. [14] showed that ultrasonic homogenisation (400 W, 24 kHz, using a 22-mm probe) of milk at 63 °C for 30 min caused disintegration of the milk fat globule membrane. The diameter of the milk fat globules was reduced to <1 μm compared with the native fat globule dimensions of 4.3 μm and displayed a granular surface morphology due to their interaction with casein micelles. Villamiel and de Jong [110] reported a milk fat globule size reduction of up to 82 % during continuous flow, high-intensity (150 W, 20 kHz, using a Branson sonifier with 18.76 mL cavity) ultrasonication of milk. At either 70 or 75 °C, a monomodal particle size distribution was obtained, whereas a bimodal distribution was observed at lower temperatures. Bosiljkov et al. [20] showed that an increase of the amplitude (20, 60 and 100 %) and time (2–15 min) of ultrasound (30 kHz, using 7 and 10 mm probes) significantly influenced the degree of homogenisation of milk.
Wu et al. [114] reported that high-amplitude ultrasound (90, 225 and 450 W, 20 kHz) not only effectively homogenised milk, but also significantly improved the viscosity and water holding capacity and reduced syneresis of yoghurt produced from ultrasonicated milk. These effects are directly related to the yoghurt structure, which is based on strings or clusters of casein micelles interacting physically with each other and with denatured serum proteins entrapping serum and fat globules. Furthermore, ultrasound could cause some qualitative changes in the fat globule membrane, which would modify the ability of fat globules to interact with themselves and/or casein micelles, improving the gelling properties (unpublished data).
Sonocrystallisation
Sonocrystallisation is the use of power ultrasound to control the crystallisation process commonly used during the nucleation phase of crystallisation. Rapid sonocrystallisation for lactose recovery in the food industry relies on different aspects of ultrasound [26]. The crystallisation of biological soft materials with the use of ultrasound plays a key role in controlling the crystal structure, shape and rate of crystallisation. US is a promising tool for rapid crystallisation of food materials, which will increase the efficiency of some traditional processes, leading to cost-effectiveness [39]. Some of the interesting dairy applications for sonocrystallisation will be highlighted here.
Lactose Crystallisation
In the dairy industry, liquid whey is spray-dried to manufacture WPC as a means of utilising whey waste streams. Whey powders have significant value when incorporated into products such as infant formula and body building powders. In liquid whey, lactose is the most abundant component. However, its presence restricts the spray-drying abilities. The removal of lactose by crystallisation is hence an important process that is required prior to spray-drying liquid whey.
Conventional methods for lactose crystallisation are, however, laden with issues such as long induction times and slow crystallisation rates. A rapid recovery of lactose by ultrasound-assisted crystallisation has been reported recently [21, 22, 89]. In the study by Bund and Pandit [21, 22], the whole process of lactose sonocrystallisation was rapidly completed from reconstituted lactose solutions in the presence of ethanol as an anti-solvent at 30 ± 2 °C. The lactose recovery was much higher (91 %) in the case of sonicated samples when compared to non-sonicated (14 %) samples at the end of 5 min. As ultrasound travels throughout the crystallisation vessel, it mixes the anti-solvent uniformly in the lactose solution. Cavitation events are also likely to be increased due to the lowering of the vapour pressure of the solution as a result of the addition of ethanol. This causes a sharp decrease in the local solubility of lactose. The solution reaches super saturation, causing nucleation and rapid lactose recovery.
Similarly, Patel and Murthy [88, 90] used sonocrystallisation for lactose recovery from whey waste streams. They pointed out that recent increases to worldwide demands for dairy products have led to increased amounts of whey waste, driving an increasing necessity to make use of such waste streams. The crystallisation was reportedly completed with yields in the range of 80–92 % within 4 min of sonication. A recent pilot-scale study by Zisu et al. [120] focussed on crystallisation of lactose in whey systems using a non-contact approach at flow rates of up to 12 L/min, where lactose was concentrated to 32 % prior to sonication at 30 °C. The applied energy densities were varied from 3 to 16 J/mL at a frequency of 20 kHz. It was found that sonication of whey initiated the rapid formation of a large number of lactose crystals in response to acoustic cavitation, which increased the rate of crystallisation. The rate of sonocrystallisation was faster compared with mechanical stirring for processing durations of approximately 120 min. However, once the metastable limit was reached between 120 and 180 min, the rate slowed. A second treatment with ultrasound for 120 min delivering an applied energy density of 4 J/mL stimulated further nuclei formation, and the rate of crystallisation was maintained for >300 min. Yield on the other hand was limited by the solubility of lactose and could not be improved. The crystal size distribution was also narrower than that with stirring, and the overall crystal size was smaller. The study highlighted the importance of the conditions used for promoting crystallisation.
A dramatic shortening of the induction times (along with an increase in the corresponding nucleation rates) observed in the sonicated systems has been attributed to two major factors: ultrasound-stimulated nucleation in the high-pressure vicinity of the collapsing bubbles due to the melting point shift and the consequent increase in supercooling [21, 22]. The cavitation hot spots where bubbles collapse are thought to be privileged nucleation centres, where the critical energy for crystal formation is decreased. The shock wave and micro-jet from local high pressure of the cavitation collapse can also accelerate the motion of the liquid molecules and increase molecular impacts so as to prompt nucleation. In addition, acoustic-induced nucleation is a well-defined initial point for crystallisation, which permits better modulation of crystal growth.
Furthermore, the rapid collapse of bubbles reduces the crystallisation temperature and thereby increases super saturation [60]. Even small changes in super saturation were found to significantly reduce the nucleation rate. In addition, the micro-turbulence governs the growth of the nuclei [83]. The metastable zone width (MZW) provides information for developing a controlled crystallisation process (Fig. 2). The application of ultrasound can reduce the MZW, and this can have many significant benefits such as improved control over crystal size and habit.
Schematic representation of reduction in the metastable zone of lactose with ultrasound [39]
In comparison with mechanical agitation, ultrasound can provide more uniform mixing that can avoid unwanted zones of excessive super saturation in the vessel. Ultrasound can be used to induce nucleation where spontaneous primary nucleation cannot occur, and hence, ultrasound can potentially replace seeding in lactose solution [21, 22].
The effect of cavitation to speed up the nucleation rate is a complex phenomenon and is not yet completely understood. It could be hypothesised that every cavity takes up some part of the solvent, causing instantaneous local super saturation. It could also be speculated that due to hot spot formation as a result of cavity collapse, local solvent evaporation takes place within the cavitation nuclei. On collapse of a cavity, these nuclei are introduced into the crystallisation slurry, causing further cavitation opportunities due to the presence of these solids [45].
Although research on sonocrystallisation of lactose has been reported mostly with the use of the anti-solvents, two recent studies by Zamanipooor et al. [116] and Dincer et al. [41] investigated the effectiveness of ultrasound in enhancing the crystallisation process of lactose in aqueous (i.e., non-solvent) systems. These studies aimed to model the effects of ultrasonic variables on nucleation rate, growth rate and size distribution of lactose crystals. In the study by Zamanipooor et al. [116], ultrasound with a frequency of 20 kHz was applied using an ultrasonic probe to the lactose in water solutions maintained at a constant temperature of 22 ± 1 °C. The variables that significantly (P < 0.05) affected the responses were found to be the lactose concentration and amplitude, with concentration being the most significant. The sonicated sample at the optimal sonication conditions led to nucleation rates 10.6 times higher and yields 5.6 times higher compared with the control. The results of this study by Zamanipooor et al. [116] emphasised the potential effects of ultrasonic treatment in promoting the nucleation rate and yield in the manufacture of lactose without use of solvents. Furthermore, the fact that sonication time had no significant effect (P > 0.05) on the responses implied that sonication duration can be kept to a minimum to save on energy and costs [116].
Furthermore, Dincer et al. [41] found that ultrasound had a significant effect in reducing induction times and narrowing the MZW but had no effect on individual crystal growth rate or morphology. A rapid decrease in induction time was observed up to 0.46 W g−1 power density. Sonication up to 3 min decreased the induction time, but no further reduction was observed beyond 3 min. It was not possible to generate the nucleation rates achieved by sonication using agitation alone. 1-min sonication at 0.46 W g−1 power density followed by continuous stirring was found to be the optimum under the experimental conditions tested [41].
Fat Crystallisation
There has been considerable interest in the application of ultrasound with regards to crystallisation of fats within the food industry [47, 73, 92, 103]. The crystal size and shape of fats within a product play a significant role to the texture and mouth feel. Within the dairy industry, controlling fat crystallisation is a key factor governing the structure and texture of secondary dairy products. Examinations of pure triacylglycerides (TAG’s), confectionary fats, vegetable fats and milk fats indicate that ultrasound affects the rates of polymorph-dependant crystallisation, crystal size and morphology [47, 73, 92, 103]. Martini et al. [73] studied the use of ultrasound as an additional processing condition to alter the crystallisation behaviour of anhydrous milk fat. It was shown that ultrasound decreases induction time of crystallisation, generates smaller crystals and higher viscosities. However, the degree of super cooling and ultrasound settings influenced the degree of ultrasound effect on the crystallisation to a great extent. The studies reviewed above indicate that ultrasound affects the crystallisation behaviour of fats in many ways.
The effectiveness of this technology depends entirely on the processing conditions. However, like sonocrystallisation of lactose, it remains unclear as to the exact mechanisms responsible for these effects. More importantly, this procedure may suggest a rational way of controlling the polymorphism and nucleation rate of various fats/lipids by sonication in dairy-based systems. A primary effect of sonocrystallisation may be due to the high pressure generated when a sonication-induced cavity collapses. The different polymorphic forms have different stabilities that form specifically as a function of the supercooling temperature, mechanism and lifetime of the collapsing bubbles.
Crystallisation of fats is of considerable interest in chocolate manufacture. The crystallisation process can be controlled by means of the amplitude and frequency of the ultrasonic wave along with the exposure time, thus controlling crystal size distribution as well as the point at which crystallisation starts [95]. Cocoa butter exists in a number of polymorphic forms depending on the triglyceride composition. The polymorphic form V is considered the most desirable crystal form in well-tempered chocolate [4]. In order to avoid fat bloom on chocolates, the unstable polymorphic forms of cocoa butter are crystallised during the first cooling process of traditional tempering. In the reheating process, a melt-mediated transformation leads to formation of the stable polymorph form V. This traditional method of tempering chocolates is time-consuming and needs to be precise with respect to the temperature conditions used for obtaining the proper polymorph form and thereby obtain the correct texture and appearance of chocolates. However, in the case of sonocrystallisation (20 kHz, 100–300 W for 3 s) for cocoa butter, it has been found that the stable form is directly crystallised without the formation of subsequent unstable forms [49]. This points to the fact that ultrasound irradiation is an effective tool for controlling polymorphic crystallisation of fats and reducing induction times. Furthermore, a range of crystal structures can be controlled. This means it is possible to tune the desired texture conditions for a particular dairy product. However, the ultrasound-induced cavitation that produces free radicals appears to have restricted the use of sonocrystallisation for systems containing fats and oils, which are susceptible to oxidation by free radicals [97]. The formation of off-flavours prevents using ultrasound as a viable choice for fat crystallisation in dairy systems. On the contrary, Patrick et al. [92] reported that sonication at 66 kHz did not cause any off-flavour production due to oxidative changes to palm oil. Their results indicate that the optimum conditions for obtaining small crystals in the shortest time period are just below the cavitation intensity threshold.
Ice Crystallisation
Ice-creams are designed to be consumed in a frozen stage. The ice crystal size directly influences the texture and taste. For a desired creamy mouth feeling within the frozen product, the ice crystals are required to be as small as possible. When US is applied during the crystal growth phase, fragmentation of large crystals under acoustic stress will occur and lead to crystal size reduction. It has been shown that during freezing of ice-lollipops, the ice crystals that were formed with the application of US were significantly smaller and distributed evenly across the product. The resulting product also adhered more strongly to the supporting stick. But at the same time, smaller crystals made the product harder and difficult to bite [113].
Ice-cream contains up to 50 % by volume of entrapped air. Ultrasonic degassing can occur during the application of ultrasound, and this process can result in undesirable modifications to the ice-cream texture. Acton and Morris [3] overcame this issue by increasing the initial gas content so that the proportion of air lost due to US can be compensated. But the question that remained unanswered was how much extra air needs to be added to obtain the desired texture. Therefore, unique approaches and conditions are needed for each and every product for obtaining desired textures.
Further, high-intensity ultrasound may lead to fat oxidation. Despite the favourable effects of sonication on the crystal structure, the aforementioned effects (degassing and fat crystallisation and oxidation) can lead to off-flavours and improper textures in ice-creams. However, by keeping the cooling regime constant, it has been found that the structure of the crystallised product can be adjusted by varying the ultrasonic intensity [35]. Mortazavi and Tabatabai [81] found that application of 20-min pulsed ultrasound resulted in the best sensory flavour, texture and mouth feel evaluations of ice-creams. Flavour and texture of samples prepared with 5- and 20-min pulse time also had better mouth feel than the control. Hence, the conditions need to be carefully monitored in using ultrasound on ice-cream applications.
Inactivation of Microbes
The dairy industry has typically relied on heat to inactivate microorganisms and enzymes as a means of preservation and ensuring food safety. Thermal treatment typically inactivates enzymes, kills vegetative microorganisms, and destroys some spores but causes significant loss of the nutritional and organoleptic properties of the products. In milk systems, pasteurisation and ultra-high temperature sterilisation often result in changes to the composition and surface properties of the colloidal particles present and alters the physical properties of the milk. While some changes such as those used to improve the texture of products like yoghurt are desirable, others such as gel formation during the manufacture of Ultra-High Temperature milk are highly undesirable [23]. Hence, novel preservation technologies that maintain the nutritional value and organoleptic characteristics of milk unchanged have attracted some interest within the dairy industry. This trend towards the development of ultrasound as an alternative non-thermal technique is further driven by other advantages including reduced energy consumption, ability to target specific organisms and no requirement for the introduction of preservatives [26].
The main mechanism responsible for the ultrasonic deactivation effect is the physical forces generated by acoustic cavitation. The asymmetric collapse of a cavitation bubble leads to a liquid jet rushing through the centre of the collapsing bubble. The speed of this micro-jet can be up to a few hundred metres per second. Microorganisms that have hydrophobic surfaces will promote the collapse of cavitation bubbles on the surface and lead to severe damage of the cell wall. Similarly, micro-streaming effects can lead to the erosion of cell walls, again resulting in inactivation of the microorganisms.
In addition to the cavitation effects discussed above, the bactericidal effect of ultrasound in liquid foods is also attributed to intracellular cavitation [50], which disrupts the structural and functional components up to the point of cell lysis. The effects of localised heating, free radical production causing DNA damage and micro-streaming which causes thinning of cell membranes are crucial in the inactivation [19]. It should be noted that the resistance of different microorganisms to ultrasound varies widely, and many process treatment parameters and ultrasound variables have to be carefully selected to compensate.
When applied alone, intense ultrasound power and long contact times are required to inactivate microorganisms at ambient conditions for microbial inactivation in real food systems. Recent improvements to the use of ultrasonics for microbial inactivation recognise that the combination of more than one established technique in combination with ultrasound provides enhanced benefits. The most effective sonication approaches to inactivate microbes for industrial purposes are the combination of ultrasound with heat thermo sonication (TS), pressure manosonication (MS), or heat and pressure mano-thermo sonication (MTS). The effectiveness of microbial inactivation by these methods is dependent on the amplitude of the ultrasonic waves, exposure/contact time, volume of food being processed, the composition of the food and the treatment conditions.
Several studies have shown additive or even synergistic effects of TS, MS and MTS compared with the individual treatments alone [62, 94]. Some microorganisms, in particular bacterial spores, are more resistant under certain conditions than others, and achieving inactivation can be relatively difficult. Bacillus and Clostridium spores were found to be more resistant to heat and similarly resistant to ultrasound [38]. Raso et al. [96] investigated the inactivation of Bacillus subtilis spores by ultrasonic treatments under static pressure and a combined pressure and heat treatment conditions. They showed that MS treatment at 500 kPa and 117 μm of amplitude for 12 min inactivated ~99 % of the B. subtilis spore population.
The influence of ultrasound amplitude was also reported to be very important to the microbial inactivation. When higher amplitudes were used, higher inactivation rate was observed, which could be due to an increase in the number of bubbles undergoing cavitation per unit time [106] or to an increase in the volume of liquid in which cavitation is liable to occur [106]. While MS treatment (20 kHz, 300 kPa, 70 °C, 12 min) at 90-μm amplitude inactivated 75 % of the B. subtilis spore population, the same treatment at 150-μm amplitude inactivated 99.9 % of this population. The MS treatments at temperatures higher than 70 °C (MTS) also led to more spore inactivation. In the range 70–90 °C, the combination of heat with a MS treatment (20 kHz, 300 kPa, 117 mm, 6 min) had a synergistic effect on spore inactivation.
D’Amico et al. [37] showed that ultrasound treatment combined with mild heat (57 °C) for 18 min resulted in a 5-log reduction of L. monocytogenes in milk and a 5-log reduction in the total aerobic bacteria in raw milk. Juraga et al. [59] investigated the inactivation of Enterobacteriae in raw milk with the use of high-intensity ultrasound. Temperature (20, 40 and 60 °C), amplitude (120, 90 and 60 μm) and time (6, 9 and 12 min) were varied in the study. They found that inactivation of microorganisms using ultrasound depended on the amplitude of the ultrasonic waves, the exposure/contact time with the microorganisms and the temperature of treatment. The results indicated significant inactivation of microorganisms under longer period of treatment with ultrasonic probe particularly in combination with higher temperature and amplitude.
Table 1 highlights some literature that has investigated the influence of different parameters on microbial inactivation for different food systems. TS is considered to increase the lethality values of conventional pasteurisation and sterilisation treatments of liquid foods. Furthermore, the reduction in temperature and/or process time is expected to improve the retention of the nutritional and organoleptic quality of food [87]. Despite its benefits, the effectiveness of TS decreases with increasing temperature. The decrease in effectiveness of TS at increasing temperature is due to a reduction in the cavitation effect as a result of the increase in vapour pressure and the decrease in liquid surface tension [110]. However, this effect can be overcome by MS or MTS. In recent studies, TS has been combined with other emerging technologies for improved microbial inactivation. Some of the highlights of these studies are also presented in Table 1.
Functionality Changes of Proteins in Milk
The use of ultrasound in dairy systems is increasingly being developed for a range of suitable applications, not simply due to the improved processing effectiveness as mentioned in the previous sections, but also for its ability to manufacture products with ‘tailored’ functionality, the ability to preserve food and modulate enzyme activity and the capability of improving the microstructure through component interactions [62]. Manipulating the functional properties of dairy ingredients is interesting, particularly from the point of protein functionality, although the actual mechanisms responsible for the observed effects are still unclear and continue to be investigated.
Recently, Chandrapala et al. [33] investigated the effect of shear on the solubilisation of a range of dairy powders containing casein micelles. In their study, the rate of solubilisation of less soluble milk protein concentrate and micellar casein powders was examined during ultrasonication, high-pressure homogenisation and high-shear rotor–stator mixing and was compared with low-shear overhead stirring. The high-shear techniques were able to greatly accelerate the solubilisation of these powders by physically breaking apart the powder agglomerates and accelerating the release of individual casein micelles into solution. This was achieved without affecting the structure of the solubilised proteins. Furthermore, the effect of high shear on the re-establishment of the mineral balance between the casein micelles and the serum was examined by monitoring the pH of the reconstituted skim milk powder after prior exposure to ultrasonication. Only minor differences in the re-equilibration of the pH were observed after sonication for up to 3 min, suggesting that the localised high-shear forces exerted by sonication did not significantly affect the mass transfer of minerals from within the casein micelles.
Ultrasound has also been used for many years for estimating changes in protein conformation [24, 28, 46, 53, 54]. The protein conformations may be related to functional properties of proteins in foods such as solubility, foaming capacity and flexibility. Jambrak et al. [53] showed that ultrasound with a high-intensity (20 kHz) probe had a major effect on whey protein’s functional properties such as solubility and foam ability. Their results also showed that ultrasound of 40 kHz frequency had less effect on whey protein than a 20 kHz probe. It was explained by the ultrasound mode which was applied to the milk. The 20-kHz ultrasound was applied using a horn inserted directly in solution which favours contact between the tip and the sample. On contrary, the 40-kHz ultrasound was applied using an ultrasound bath with the milk contained in flasks such that there was no direct contact with the irradiating surface.
Treatment of a protein sample with different frequencies has also shown differences in the type of functionality changes. A 40-kHz bath treatment decreased the conductivity of the protein sample, but increased the solubility and foaming ability of the proteins. The larger increases of foaming ability might be due to the homogenisation effect of ultrasound according to the authors. The homogenisation effect of ultrasound usually disperses the protein and fat particles more evenly, which may improve the foaming property. During ultrasound treatment, proteins can become partially unfolded in structure due to physical effects such as shear and temperature, which increase the foaming ability [53, 54]. In contrast, higher-frequency ultrasound of 500 kHz frequency did not impact the foaming ability of whey protein, but it affected solubility and conductivity, due to reduced physical effects at higher frequency.
Whey Proteins
Whey protein powders are commercially produced through membrane filtration, evaporation and spray-drying for application in a range of processed foods. During this process, the aqueous whey protein solutions containing significantly high levels (4–15 % by weight) of protein are subjected to heat treatment. Exposing whey proteins to temperatures ≥70 °C causes denaturation, which in turn leads to protein aggregation through both hydrophobic interactions and the formation of intermolecular disulphide bonds [112]. This can result in excessive thickening or gelling during processing of the dairy product [80]. During the manufacture of secondary dairy products, heat treatment results in further viscosity increases. Ashokkumar et al. [9, 10] and Zisu et al. [117] outlined a novel approach to overcome this problem. The application of ultrasound for a very short duration after such a heating step breaks down these aggregates and prevents their reformation on subsequent heating, thereby reducing the viscosity increase that is usually associated with this process (Fig. 3).
Initially, it was argued that these observed viscosity changes may have been caused by a combination of both the physical and chemical effects of acoustic cavitation. However, it was found that whey solutions sonicated at 20 kHz showed the highest viscosity reductions [9, 10] even though 20 kHz ultrasound formed the least amounts of radicals [40]. The authors therefore attributed the observed viscosity reduction primarily to the physical forces generated during acoustic cavitation. Conversely, whey protein isolate (WPI) solutions were relatively unaffected by sonication, possibly reflecting the absence of larger aggregates in the initial solution, or differences in composition. Adjustment of pH prior to ultrasound treatment did not result in significant differences compared with samples that were sonicated at neutral pH. This suggested that the mechanism for gel promotion is different from effects induced by pH changes [118]. Similarly, Kresic et al. [63] also investigated the rheological and thermophysical properties of WPC and WPI solutions subjected to sonication. According to these results, the use of ultrasound changed the flow behaviour and this was attributed to altered protein structure, namely that the hydrophilic parts of amino acids are opened towards the surrounding aqueous phase, leading to an increased binding of water molecules. There has been some concern that the sonication of proteins in solution can lead to the formation of amyloid-type fragments [105]. This formation was observed when excessively high specific energy was delivered to a small volume. However, a recent study by Chandrapala et al. [24] showed that no significant protein structural changes were observed up to 60 min of sonication, although these minor changes cannot be completely omitted from the observed functional property changes.
A more thorough understanding of the mechanism was investigated by Chandrapala et al. [29]. The three main types of main interactions within protein solutions were investigated: surface charge of the protein aggregates (indicative of electrostatic interactions), reactive thiol groups (indicative of thiol-disulphide interactions) and surface hydrophobicity (indicative of hydrophobic interactions). Interestingly, it was found that surface charge and reactive thiol groups remained unchanged with the sonication step applied in between the preheating and post-heating steps (Fig. 4a, b). However, the surface hydrophobicity of these aggregates was altered markedly (Fig. 4c). Preheating denatures the whey proteins and thereby exposes the hydrophobic groups. This was indicated by an increase in the surface hydrophobicity of these preheated aggregates. This increase in surface hydrophobicity was greatly reduced with the introduction of a sonication step. It was speculated that sonication broke down the protein aggregate networks through physical shear caused by acoustic cavitation, leading to the formation of smaller aggregates with lower surface hydrophobicity. These smaller aggregates are resistant towards further aggregation during post-heating, thereby improving heat stability.
Casein Proteins
Although casein micelles are considered relatively stable particles, their composition and size respond to alterations in pH, temperature and milk protein concentration [72]. It is possible that the localised high temperatures and shear forces created by sonication can physically alter the casein micelles or their interactions with other milk components. Madadlou et al. [71] found that the average size of re-assembled casein micelles could be reduced by exposure to ultrasound (35 kHz frequency) for 6 h provided the pH was above 8. However, it is unclear how this relates to native casein micelles since the re-assembled casein particles were considerably larger (275 nm) and structurally and functionally different [82]. In another study involving true casein micelles in milk, a decrease in particle size resulting from sonication was observed. This particle size decrease was attributed to a reduction in casein micelle size by the authors, although this was not substantiated [84].
A recent study by Chandrapala et al. [31] showed that sonication did not appear to affect casein micelle size or composition or permanently affect the mineral balance in fresh skim milk. Sonication did, however, reduces the size of the already small fat globules remaining in skim milk, appeared to break up whey protein aggregates, and assist in breaking apart casein micelle, non-micellar casein and whey protein aggregates present in reconstituted casein powder systems. The results showed that controlled application of ultrasonic energy can help break up large casein and whey protein aggregates, thereby influencing macroscopic properties such as viscosity, without inducing changes to the casein micelles or mineral balance. Similar results were obtained by Shanmugam et al. [99] when using up to 30 min of sonication, but prolonged sonication resulted in the partial disruption of some whey proteins from the whey–whey aggregates. In contrast, Liu et al. [65] found that casein micelles are disrupted at high pH values with sonication. In their study, reconstituted skim milk at pHs 6.7–8 was sonicated at a specific energy input of 286 kJ/kg using 20 kHz. According to this study, ultrasound caused greater disruption of casein micelles, causing release of proteins from the micellar to the serum phase at high pHs. Furthermore, they showed that the released proteins re-associated to form aggregates of smaller size but with surface charge similar to that of casein micelles in the original milk. However, in other studies, the dissociation of κ-casein was found to be dominant with increase in pH, which can result in an increase to the total protein content in the supernatants [6]. They hypothesised that it is due to a pH-dependent conversion of the native colloidal calcium phosphate (CCP) to an alternative form of calcium phosphate, which is less capable of maintaining the micellar integrity, particularly at higher pH values where the charges of the proteins are greater. Hence, it is arguable as to whether the effects observed by Liu et al. [65] under high pH conditions are just an ultrasound effect or a pH effect and/or a combination of both.
In another study by the same group [66] observed the renneting properties of the aforementioned systems. It was shown that gelation attributes were significantly modified (i.e., faster gelation) in rennet gels made from milk sonicated at pH 8.0 and re-adjusted back to pH 6.7 compared with those made from milk sonicated at pH 6.7. The renneting properties were also modified (i.e., firmer gels) in milk sonicated at pH 6.7 compared with those of non-sonicated control milk. The modified renneting behaviour was attributed to ultrasound-induced changes to the proteins in milk [66].
Chandrapala et al. [30, 32] looked at the phosphate-induced micellar casein gelation and the influence of sonication (20 kHz) to this process. Gels were formed by the addition of 7.6 mM tetra sodium pyro phosphate (TSPP) to 5 wt% micellar casein (MC) solutions. It was shown that sonication at 20 kHz and 31 W for up to 30 min changed the surface hydrophobicity of the proteins, whereas surface charge was unaltered. Sonication before the addition of TSPP formed a firm gel with a fine protein network and low syneresis. Conversely, sonication after TSPP addition led to an inconsistent weak-gel-like structure with high syneresis. Gel strength in both cases increased significantly after short sonication times, while the viscoelastic properties were less affected. Overall, the results showed that sonication can have a significant effect on gelation of micellar casein systems, but the state of the casein micelle prior sonication is a dominant factor and should be carefully controlled. Hence, the effects observed in the study by Liu et al. [65] can be confirmed as a combined effect of pH and ultrasound rather than just simply ultrasound being responsible.
High-power, low-frequency ultrasound (20 kHz) has potential industrial application for lowering the viscosity of concentrated milk prior to spray-drying. A recent study by Zisu et al. [119] investigated the high-intensity low-frequency ultrasound on concentrated skim milk to lower viscosity through the process of acoustic cavitation. Batch sonication for 1 min at 40–80 W and continuous treatment delivering an applied energy density of 4–7 J mL−1 reduced the viscosity of medium-heat skim milk concentrates containing 50–60 % solids. Viscosity was reduced by approximately 10 %, but this has improved to 17 % in highly viscous age-thickened material. Sonication also showed changes in the shear thinning behaviour at shear rates below 150 s−1. Although ultrasound lowered the viscosity of skim milk concentrated to 50 % solids, the treatment could only delay the rate of thickening once the ageing process was established. It was only when ultrasound was activated during concentration that sonication prevented the viscosity of skim milk concentrates from increasing rapidly.
Of the work to date on casein containing systems, a considerable focus has been given to understanding the effects of ultrasound on bulk physical and functional properties. The effect of sonication on milk gels has been reported [30, 32, 84, 97, 98, 109]. Acid gel firmness (G′) was found to be altered when skim milk was ultrasonically treated prior to acidification, although the effect was attributed largely to denaturation of whey protein caused simply by temperature increases (up to about 95 °C) resulting from sonication performed without temperature control [84]. Wu et al. [114] reported that high-intensity ultrasound (90, 225 and 450 W, 20 kHz) not only effectively homogenised milk, but also significantly improved the viscosity and water holding capacity and reduced syneresis of yoghurt produced from sonicated milk. These effects are directly related to the yoghurt structure, which is based on strings or clusters of casein micelles interacting physically with each other and with denatured serum proteins entrapping serum and fat globules. Furthermore, ultrasound could cause some qualitative changes in the fat globule membrane, which would modify the ability of fat globules to interact with themselves and/or casein micelles, thereby improving the gelling properties. However, direct effects of ultrasound on casein micelles or individual caseins cannot be ruled out completely, although negligible effects were found on the individual caseins/casein micelles [31]. This may be because big multimeric protein complexes are more sensitive to the shearing forces created by micro-streaming and bubble implosion than single dissolved monomeric proteins.
Yoghurt
In yoghurt, a key aspect of product quality is associated with the physical properties of the gel. Optimal gel structures will yield yoghurt with a smooth textural character in mouth during consumption and display low serum separation during storage (i.e., syneresis). Vercet et al. [109] studied the use of MTS to obtain tailored functional properties of yoghurt products. The application of ultrasound allowed yoghurts with superior rheological properties such as flow curves, apparent viscosity, yield stress and viscoelastic properties to those of control yoghurts prepared with non-sonicated milk. The authors further showed that MTS yoghurts had more rigid structures, which resulted in higher values of almost all of the many relevant rheological parameters. They suggested that ultrasound effects are mainly related to the cavitation phenomenon. As a result of the cavitation conditions, water molecules can be homolyzed, generating highly reactive free radicals that can react with and modify several molecules. Mechanical stress generated either by shock waves derived from bubble implosion or from micro-streaming derived from bubble oscillations is also able to disrupt large macromolecules or particles. However, the cause of this behaviour was not ascertained. The authors suggested that alterations to the size of the fat globules were not responsible, although a proper comparison of the fat globules was not performed.
Reiner et al. [97] also found that compared with conventional yoghurts, yoghurts from (TS) milk had higher gelation pH values, greater viscosities and higher water holding capacities. The authors further stated that the gel network structure was different; it showed a honeycomb-like network and exhibited a more porous nature. Similarly, Reiner et al. [98] found superior rheological properties of yoghurts prepared from ultrasonicated milk than the yoghurts prepared from conventionally heated milk. Further, Bermudez-Aguirre et al. [18] found only minor changes to the nutritional properties of milk after ultrasound, with the advantage of extending the shelf-life of the product for more than 16 days at 4 °C without the use of intensive heat treatments. These tailored functional properties are desirable in the end products. With the added advantage of minimal nutritional loss compared with thermal treatments but still retaining long stable shelf-lives, the use of ultrasound technology in dairy streams shows promise.
Ultrasonic Separation of Fat
Milk fat separation is essential to the production of dairy products such as butter, cheese, yoghurt and skim milk. Typically performed in large-scale manufacture by centrifugation, high-frequency ultrasound (>400 kHz) has recently been reported as an alternative separation technology that can enhance the rate of milk creaming by ‘natural’ gravitational sedimentation [69, 70].
When an ultrasonic standing wave is set up in a container, the fat globules distributed in the milk experience what are known as acoustic radiation forces that cause them to migrate specifically to the pressure anti-nodes such that ‘banding’ of the fat globules can be observed. This was observed by Miles et al. [79] in a small cuvette container. The milk fat globules have an increased probability to collide together to form collections with a larger effective diameter when disposed into these regions. These larger entities then rise more rapidly to the surface of the container according to Stokes’ Law [64], resulting in faster creaming. The acoustic and buoyancy forces influencing the separation are dependent on the size of the fat globules [68]. The acoustic forces can be manipulated by adjusting the applied frequency and energy density.
Ultrasonically enhanced fat separation in milk has been demonstrated in batch systems ranging in scale from millilitre to litre. Juliano et al. [56] demonstrated the concept of ultrasonic separation for natural whole milk in small test tubes of 7-mL volume and was later successful in scaling this process up to 6 L using a recombined milk emulsion [57]. Leong et al. [69] established suitable ultrasonic parameters that enable successful separation of natural whole milk in large scale, demonstrating the importance of energy density and effectiveness of high-frequency ultrasound (1 and 2 MHz) to separate the small fat globules distributed in milk (~4 µm diameter). Further studies by the same group have demonstrated that operation at moderate temperatures between 25 and 40 °C are more optimal to the fat separation process due to the influence of temperature on the physical properties of the fat globules such as density, viscosity and liquid/solid ratio [70].
One possible concern when using high-frequency ultrasound for the separation of milk fat is the potential for oxidation of fat to occur (i.e., lipolysis). Efforts should be made to limit this as excessive oxidation of fats can lead to rancid off-flavours in the final product. It is noted that peak sonochemical effects have been reported to occur in the frequency range between 400 and 800 kHz, which incidentally is in the range of frequencies reportedly suitable for ultrasonic fat separation. However, Juliano et al. [58] has shown that when sonicating milk within this frequency range, oxidative volatiles derived from sonication was detectable above human sensory threshold limits only when very high specific energies were delivered to the milk. In contrast, Torkamani et al. [108] showed no significant oxidation of fat with sonication of cheddar cheese whey at similar frequencies and energy densities.
Conclusion
Ultrasound is a promising technology suitable for a range of different applications in the dairy industry. In liquid media, the extreme physical forces generated by low-frequency, high-intensity ultrasound induce acoustic streaming, cavitation, shear, micro-jet and shockwaves. These physical forces have been used successfully for the generation of dairy emulsions, functionality improvements of dairy systems, inactivation of microbes, crystallisation of lactose and fat in dairy systems, among several other applications. High-frequency ultrasound on the other hand has been used to initiate rapid creaming of fat from milk. Ultrasound processing has advantages of minimising flavour loss, increasing homogeneity, reducing energy requirements, reducing processing times, enhancing end-product quality, reducing chemical and physical hazards and lowering the environmental impact, when compared to conventional dairy processes. Synergies with pressure and/or temperature have been reported, but caution is advised to minimise nutritional losses and adverse flavour modifications if very high specific energies are to be delivered to the process. Although there are a range of advantages from adopting ultrasonic technology for the dairy industry, more research is needed to improve designs to enable efficient large-scale operations.
References
Abismail B, Conselier JP, Wilhelm AM, Delma H, Gourdon C (1999) Emulsification by ultrasound: droplet size distribution and stability. Ultrason Sonochem 6:75–83
Abismail B, Conselier JP, Wilhelm AM, Delma H, Gourdon C (2000) Emulsification processes: online study by multiple light scattering measurements. Ultrason Sonochem 7:187–192
Acton E, Morris GJ (1992) Methods and apparatus for the control of solidification in liquids. US Patent WO99/20420
Afoakwa EO, Paterson A, Fowler M (2007) Factors influencing rheological and textural qualities in chocolate: a review. Trends Food Sci Techol 18(6):290–298
Al-Hilphy ARS, Niamak AK, Al-Temimi AB (2012) Effect of ultrasonic treatment on buffalo milk homogenization and numbers of bacteria. Int J Food Sci Nutr Eng 2:113–118
Anema SG, Klostermeyer H (1997) Heat induced, pH dependent dissociation of casein micelles on heating reconstituted skim milk at temperatures below 100 °C. J Agric Food Chem 45:1108–1115
Ashokkumar M, Mason TJ (2007) Sonochemistry in kirk-othmer encyclopedia of chemical technology. Wiley, New York
Ashokkumar M, Devi S, Kentish S, Mawson R, Simons L, Vilkhu K Verteg CK (2008) Innvov Food Sci Emerg Technol 9:155–160
Ashokkumar M, Kentish S, Lee J, Zisu B, Palmer M, Augustin M (2009a) Processing of dairy ingredients by ultrasonication. PCT Int Appl WO2009/079691A1
Ashokkumar M, Lee J, Zisu B, Bhaskarcharya R, Kentish S (2009) Sonication increases the heat stability of whey proteins. J Dairy Sci 92:5353–5356
Ashokkumar M, Bhascharya R, Zisu B, Kentish S (2010) The ultrasonic processing of dairy products: an overview. Dairy Sci Technol 90:147–168
Ashokkumar M (2011) The characterization of acoustic cavitation bubbles: an overview. Ultrason Sonochem 18:864–872
Awad TS, Moharram HA, Shaltout OE, Asker D, Youssef MM (2012) Applications of ultrasound in analysis, processing and quality control of food: a review. Food Res Int 48:410–427
Behreud O, Schubert H (2001) Influence of hydrostatic pressure and gas content on continuous ultrasound emulsification. Ultrason Snochem 8:271–276
Bermudez-Aguirre D, Mawson R, Barbosa-Canovas GV (2008) Microstructure of fat globules in whole milk after thermosonication treatment. J Food Sci 73:325–332
Bermudez-Aguirre D, Mobbs T, Barbosa-Canovas GV (2008) Study of butter fat content in milk on the inactivation of L innocua ACC 51742 by thermosonication. Innvov Food Sci Emg Tech 9:176–185
Bermudez-Aguirre D, Mobbs T, Barbosa-Canovas GV, Mawson R, Versteeg K (2009) Composition properties, physicochemical characteristics and shelf life of whole milk after thermal and thermosonication treatments. J Food Qual 32:283–302
Bermudez-Aguirre D, Mobbs T, Barbosa-Canovas GV (2010) Processing of soft Hispanic cheese using thermosonicated milk: a study of physicochemical characteristics and storage life. J Food Sci 75:5548–5558
Bermudez-Aguirre D, Mobbs T, Barbosa-Canovas GV (2011) Ultrasound applications in food processing. In: Barbosa-Canovas GV, Weis J, Feng H (eds) Ultrasound technologies for food and bioprocessing. Springer, New York, pp 65–105
Bosiljkov T, Tripalo B, Brincic M, Jezek D, Karlovic S, Jagust I (2011) Influence of high intensity ultrasound with different probe diameter on the degree of homogenization (variance) and physical properties of cow milk. Afr J Biotechnol 10:34–41
Bund RK, Pandit AB (2007) Sonocrystallisation: effect on lactose recovery and crystal habit. Ultrason Sonochem 14:143–152
Bund RK, Pandit AB (2007) Rapid lactose recovery from paneer whey using sonocrystallisation: a process optimization. Chem Eng Process 46:846–850
Chandrapala J (2009) Effect of concentration, pH and added chelating agents on the colloidal properties of heated reconstituted skim milk. PhD thesis Monash University
Chandrapala J, Zisu B, Kentish S, Ashokkumar M (2011) Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrates. Ultrason Sonochem 18:951–957
Chandrapala JC, Oliver C, Kentish S, Ashokkumar M (2012) Ultrasonics in food processing. Ultrason Sonochem 19:975–983
Chandrapala J, Oliver C, Kentish S, Ashokkumar M (2012) Ultrasonics in food processing: food quality assurance and food safety. Trends Food Sci Technol 26:88–98
Chandrapala J, Oliver C, Kentish S, Ashokkumar M (2012) Use of power ultrasound to improve extraction and modify phase transitions in food processing. Food Rev Int 2:1–25
Chandrapala J, Zisu B, Kentish S, Ashokkumar M (2012) The effects of high intensity ultrasound and heat treatment on the structural and functional properties of α-Lactalbumin, β-Lactoglobulin and their mixtures. Food Res Int 48:940–943
Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M (2012) A possible mechanism to understand the ultrasound induced heat stability of whey protein concentrates. Int Non thermal Workshop, Melbourne
Chandrapala J, Zisu B, Kentish S, Ashokkumar M (2013) Influence of ultrasound on the chemically induced gelation of micellar casein systems. J Dairy Res 1:1–6
Chandrapala J, Martin GJ, Zisu B, Kentish S, Ashokkuamr M (2012) The effect of ultrasound on casein micelle integrity. J Dairy Sci 95:6882–6890
Chandrapala J, Zisu B, Kentish S, Ahokkumar M (2013) Influence of ultrasound on chemically induced gelation of micellar casein systems. J Dairy Res 1:1–6
Chandrapala J, Martin GJ, Kentish S, Ashokkuamr M (2014) Dissolution and reconstitution of casein micelle containing dairy powders by high shear using ultrasonic and physical methods. Ultrason Sonochem 21:1658–1665
Chemat F, Zill-e-Huma S, Khan MK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18:813–835
Chow R, Blindt R, Chivers R, Povey M (2003) The sonocrystallisation of ice in sucrose solutions: primary and secondary nucleation. Ultrasonics 41(8):595–604
Cznak C, Simmer K, Hartmann PE (2010) Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: effect on milk quality. J Dairy Res 77:183–189
D’amico D, Silk TM, Wu J, Guo M (2006) Inactivation of microorganisms in milk and apple cider treated with ultrasound. J Food Protect 69:556–563
Deghani MH (2005) Effectiveness of ultrasound on the destruction of E. coli. Am J Environ Sci 1(3):187–189
Deora NS, Misra NN, Deswal A, Mishra HN, Cillen PJ, Tiwari BK (2013) Ultrasound improved crystallization in food processing. Food Eng Rev 5:36–44
Devi S, Ashokkumar M, Grieser F (2005) The influence of acoustic power on multibubble sonoluminescence in aqueous solution containing organic solutes. J Phys Chem B 109:20044–20050
Dincer T, Zisu B, Vallet CGMR, Jayasena V, Palmer M, Weeks M (2014) Sonocrystallisation of lactose in an aqueous system. Int Dairy J 35(1):43–48
Ertugay MF, Sngul M, Sengul M (2004) Effect of ultrasound treatment on milk homogenization and particle size distribution of fat. Turk J Vet Anim Sci 28:303–308
Freitas S, Hielscher G, Merkle HP, Gauder B (2006) Continuous contact and contamination free ultrasonic emulsification—a useful tool for pharmaceutical development and production. Ultrason Sonochem 13:76–85
Gera N, Doores S (2011) Kinetics and mechanism of bacterial inactivation by ultrasound waves and sonoprotective effect of milk components. J Food Sci 76:M111–M119
Gogate PR, Mujumdar S, Pandit AB (2003) Sonochemical reactors for waste water treatment: comparison using formic acid degradation as a model reaction. Adv Environ Res 7:35–39
Gulseren I, Guzey D, Bruce D, Weis J (2007) Structural and functional changes in ultrasonicated BSA solutions. Ultrason Sonochem 14:173–183
Hartel RW (2013) Advances in food crystallization. Ann Rev Food Sci Technol 4:2770292
Herceg Z, Jambrak AR, Celas V, Thagard SM (2012) The effect of high intensity ultrasound treatment on the amount of S. aureus and E. coli in milk. Food Technol Biotech 50:46–52
Higaki K, Ueno S, Koyano T, Sato K (2001) Effects of ultrasonic irradiation on crystallization behavior of tripalmitoylglycerol and cocoa butter. J Am Oil Chem Soc 78(5):513–518
Hughes DE, Nyborg L (1962) Cell disrupt by ultrasound. Science 138:108–114
Jafari SM (2007) Production of sub micron emulsions by ultrasound and microfluidisation techniques. J Food Sci 82:478–488
Jafari SM, Assadpoor E, He Y, Bhandari B (2008) Re-coalescence of emulsion droplet during high energy emulsification. Food Hydrocoll 22:1191–1202
Jambrak AR, Mason T, Lelas V, Herceg Z, Hereg L (2008) Effect of ultrasound treatment on solubility and foaming properties of whey protein dispersion. J Food Eng 86:281–287
Jambrak AR, Mason T, Lelas V, Kresic G (2010) Ultrasonic effect on physico–chemical and functional properties of α-Lactalbumin. LWT Food Sci Technol 43:254–262
Juang R, Lin K (2004) Ultrasound assisted production of w/o emulsions on liquid surfactant membrane processes. Coll Surf A Physiochem Eng Asp 238:43–49
Juliano P, Kutter A, Cheng LJ, Swiergon P, Mawson R, Augustin M (2011) Enhanced creaming of milk fat globules in milk emulsions by the application of ultrasound and detection by means of optical methods. Ultrason Sonochem 18:963–973
Juliano P, Temmel S, Rout M, Swiergon P, Mawson R, Knoerzer K (2012) Creaming enhancement in a liter scale ultrasonic reactor at selected transducer configurations and frequencies. Ultrason Sonochem 20:52–62
Juliano P, Torkamani AE, Leong T, Kolb V, Watkins P, Ailouni S, Singh TK (2014) Lipid oxidation volatiles absent in milk after selected ultrasound processing. Ultrason Sonochem 21:2165–2175
Juraga E, Salamon BS, Herceg Z (2011) Application of high intensity ultrasound treatment on enterobacteria count in milk. Mljekarstvo 61:125–134
Kickling R (1965) Nucleation of freezing by cavity collapse and its relation to cavitation damage. Nature 206:915–917
Koh LLA, Chandrapala J, Zisu B, Martin GJ, Kentish S, Ashokkumar M (2014) A comparison of the effectiveness of sonication, high shear mixing and homogenization on improving the heat stability of whey proteins solutions. Food Bioprocess Technol 7:556–566
Knorr D, Zenker M, Heinz V, Lee D (2004) Application and potential of ultrasonics in food processing. Trend Food Sci Techol 15:261–266
Kresic G, Lelas V, Jambrak AR, Herceg Z, Brincic SR (2008) Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J Food Eng 87:64–73
Lamb H, Caflisch R (1993) Hydrodynamics. Cambridge University Press, Cambridge
Liu Z, Juliano P, Williams R, Niere J, Augustin M (2014) Ultrasound effects on assembly of casein micelles in reconstitute skim milk. J Dairy Res 81(2):146–155
Liu Z, Juliano P, Williams R, Niere J, Augustin M (2014) Ultrasound improves the renneting properties of milk. Ultrason Sonochem 21(6):2131–2137
Leong T, Wooster T, Kentish S, Ashokkumar M (2009) Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem 16(6):721–727
Leong T, Johansson L, Juliano P, McaRTHUR SL, Manasseh R (2013) Ultrasonic separation of particulate fluids in small and large scale systems: a review. Ind Eng Chem Res 52(47):16555–16576
Leong T, Johansson L, Juliano P, Mawson R, McArthur S, Manasseh R (2014) Design parameters for the separation of fat from natural whole milk in an ultrasonic litre-scale vessel. Ultrason Sonochem 21:1289–1298
Leong T, Johansson L, Juliano P, Mawson R, McArthur S, Manasseh R (2014) Temperature effects on the ultrasonic separation of fat from natural whole milk. Ultrason Sonochem 21:2092–2098
Madadlou A, Mousavi ME, Emam-Djomek Z, Ehsani M, Sheehan D (2009) Sonodisruption of reassembled casein micelles at different pH values. Ultrason Sonochem 16:644–648
Martin GJ, Williams R, Dunstan D (2007) Comparison of casein micelles in raw and reconstituted skim milk. J Dairy Sci 90:4543–4551
Martini S, Suzuki AH, Hartel RW (2008) Effect of high intensity ultrasound on crystallization behavior of anhydrous milk fat. J Am Oil Chem Soc 85:621–628
Mason TJ, Lorimer JP (1988) Sonchemistry: theory, application and uses of ultrasound in chemistry. Ellis Horwood, Chichester
Mason TJ, Luche JL (1996) Ultrasound as a new tool for synthetic chemists. In: Huhbard C, Eldik R (eds) Chemistry under extreme or non classical conditions. Wiley, New York, pp 317–380
Mason TJ, Chemat F, Ashokkumar M (2013) Chapter 22: power ultrasonics for food processing
McClements DJ (1995) Advances in the application of ultrasound in food analysis and processing. Trends Food Sci Technol 6:293–299
Mawson R, Rout M, Swiergon P, Ripoll Munho G, Singh T, Knoerzer K, Juliano P (2014) Production of particulates from transducer erosion: implications on food safety. Ultrason Sonochem 21(6):2122–2130
Miles CA, Morley MJ, Hudson WR, Mackey BM (1995) Principles of separating micro-organisms from suspensions using ultrasound. J Appl Bacteriol 78:47–54
Morr CV, Richter RL (1999) Chemistry of Processing. In: Wang P, Jenness R, Keeney M, Marth EH (eds) Fundamentals of Chemistry, 3rd edn. Aspen Publishers, New York
Mortazavi A, Tabatabai F (2008) Study of ice cream freezing process after treatment with ultrasound. World Appl Sci J 4(2):188–190
Mounsey JS, O’Kennedy BT, Kelly PM (2005) Comparison of re-micellised casein prepared from acid casein with micellar casein prepared by membrane filtration. Lait 85:419–430
Nalojala VS, Moholkar VS (2011) Investigation in the physical mechanism of sonocrystallisation. Ultrason Sonochem 18:345–355
Nguyen NH, Anema SG (2010) Effect of ultrasonication on the properties of skim milk used in the formation of acid gels. Innvov Food Sci Emerg Technol 11:616–622
Noci F, Walking-Ribeiro M, Cronin D, Morgan DJ, Lyng JG (2009) Effect of thermosonication, pulsed electric field and their combination on inactivation of L. innocua in milk. Int Dairy J 19:30–35
Okitsu K, Ashokkumar M, Grieser F (2005) Sonochemical synthesis of gold nanoparticles: effects of ultrasound frequency. J. Phys. Chem. B 109:20673–20675
Ordonoz JA, Aguilera MP, Garcia ML, Sanz B (1987) Effect of combined ultrasonic and heat treatment on the survival of a strain of staphylococcus aureus. J Dairy Res 54:61–67
Patel SR, Murthy VP (2009) Ultrasound assisted crystallization for the recovery of lactose in an anti solvent acetone. Crst Res Technol 44:889–896
Patel SR, Murthy VP (2010) Optimization of process parameters by Tanqueli method in the recovery of lactose from whey using sonocrystallisation. Cryst Res Technol 45:747–752
Patel SR, Murthy VP (2011) Waste valorisation: recovery of lactose from partially deprotonated whey by using acetone as antisolvent. Dairy Sci Technol 91:53–63
Patist A, Bates D (2008) Ultrasonic innovations in the food industry: from the laboratory to commercial production. Innvov Food Sci Emerg Tech 9(2):147–154
Patrick M, Blindt R, Janssen J (2004) The effect of ultrasonic intensity on the crystal structure of plam oil. Ultrason Sonochem 11:251–255
Pingret D, Fabiano-Tixier AS, Chemat F (2013) Degradation during application of ultrasound in food processing a rev. Food Control 3:593–606
Piyasena P, Mohareb E, McKellar RC (2003) Inactivation of microbes using ultrasound: a rev. Int J Food Micro 87:207–216
Povey MJW, Mason TJ (1998) Ultrasound in food processing. Blackie Academic and Professional, London
Raso J, Palop A, Condon S (1998) Inactivation of Bacillus subtilis spores by combining ultrasonic waves under pressure and mild heat treatment. J Appl Microl 85:849–854
Reiner J, Noci F, Cronin DA, Morgan DJ, Lyng G (2009) The effect of thermosonication of milk on selected physicochemical and microstructural properties of yoghurt gels during fermentation. Food Chem 114:905–911
Reiner J, Noci F, Cronin DA, Morgan DJ, Lyng G (2010) A comparison of selected quality characteristics of yoghurts prepared from thermosoicated and conventionally heated milks. Food Chem 119:1108–1110
Shanmugam A, Chandrapala J, Ashokkumar M (2012) The effect of ultrasound on the physical and functional properties of skim milk. Innvov Food Sci and Emg Technol 16:251–258
Shanmugam A, Ashokkumar M (2014) Ultrasonic preparation of stable flax seed oil emulsions in dairy systems–Physicochemical characterization. Food Hydrocoll 39:151–162
Sirotyuk MG (1966) Ultrasonic cavitation processes at elevated hydrostatic pressures. Sov Phys Acoust 12:199–204
Sivakumar M, Senthilkumar P, Majumdar S, Pandit AB (2002) Ultrasound mediated alkaline hydrolysis of methyl benzoate reinvestigation with crucial parameters. Ultrason Sonochem 9:25–30
Sizuki AH, Lee J, Padilla SG, Martini S (2010) Altering functional properties of fats using power ultrasound. J Food Sci 75:208–214
Soria AL, Villameil M (2010) Effects of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol 21(7):323–331
Stathopulos PB, Scholz GA, Hwang YM, Rumfeldt JA, Lepock JR, Meiering EM (2004) Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci 13:3017–3027
Suslick KS (1998) Homogeneous sonochemistry in ultrasound. In: Suslick KS (ed) Its chemical physical and biological effects. VCH, NY
Thompson LH, Doraiswamy LK (1999) Sonochemistry: science and engineering. Ind Eng Chem Res 38:1215–1249
Torkamani AE, Juliano P, Ailouni S, Singh TK (2014) Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason Sonochem 21:951–957
Vercet A, Oria P, Quina P, Crelier S, Lopez P (2002) Rheological properties of yoghurt made with milk submitted and manothermosonication. J Agric Food Chem 50:6165–6171
Villamiel M, de Jong P (2000) Influence of high intensity ultrasound and heat treatment in continuous flow on fat, protein and native enzymes of milk. J Agric Food Chem 48:472–478
Vikhu K, Mawson R, Simon L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry. Innvov Food Sci Emerg Technol 9:161–169
Wang O, Tolkach A, Kulozik U (2006) Quantitative assessment of thermal denaturation of bovine α-Lactalbumin via low intensity ultrasound; HPLC and DSC. J Agric Food Chem 54:6501–6506
Wiltshire M (1992) Presented at Sonochemistry Symp., R.S.C. Annu. Congr., Manchester, UK
Wu H, Hulbert J, Mont JR (2000) Effect of ultrasound on milk homogenization and fermentation with yoghurt starter. Innvov Food Sci and Emg Technol 1:211–218
Zheng L, Sun D-W (2006) Innovative applications of power ultrasound during food freezing processes: a review. Trends Food Sci Technol 17(1):16–23
Zamanipooor M, Dincer T, Zisu B, Jayasena V (2013) Nucleation and growth rates of lactose as affected by ultrasound in aqueous solutions. Dairy Sci Technol 93:595–604
Zisu B, Bhaskarcharya R, Ashokkumar M, Kentish S (2010) Ultrasonics processing of dairy systems in large scale reactors. Ultrason Sonochem 17:1075–1087
Zisu B, Lee J, Chandrapala J, Bhaskarcharya R, Palmer M, Kentish S, Ashokkumar M (2011) Effect of ultrasound on the physical and functional properties of reconstituted whey protein powders. J Dairy Res 78:226–232
Zisu B, Schleyer, Chandrapala J (2012) Application of ultrasound to reduce viscosity and control the rate of age thickening of concentrated skim milk. Int Dairy J 31:1–3
Zisu B, Sciberras M, Jayasena V, Weeks M, Palmer M, Dincer T (2014) Sonocrystallisation of lactose in concentrated whey. Ultrason Sonochem 21(6):2117–2121
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrapala, J., Leong, T. Ultrasonic Processing for Dairy Applications: Recent Advances. Food Eng Rev 7, 143–158 (2015). https://doi.org/10.1007/s12393-014-9105-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-014-9105-8