Abstract
The present study concerns the histological description of fossilized mammalian bone behavior, under the effect of hydrogen peroxide, acetic acid, and formic acid. These reagents have been applied on such material for decades, mainly for matrix removal and surface cleaning. The material used includes fossil bone parts from two different fossiliferous sites in Greece, Charkadio Cave on Tilos Island (Dodecanese) and Kerassia (Euboea Island). In order to conclude on the extent of histological damage on fossilized bone by the different chemicals and discuss their optimum application on bone, numerous experiments were realized. In each of these, samples from both sites were exposed to different combinations of parameters such as the type and concentration of reagents and the duration of exposure. The methodology applied included the detailed observation of bone histology under a scanning electron microscope (SEM), as well as qualitative chemical analyses through X-ray microanalysis (EDS) and mineralogical analyses by X-ray diffraction (XRD) when needed. pH measurements were collected during each subsequent stage of the experiment. All samples underwent density and porosity measurements before and after treatment. In conclusion, the results of this study confirmed that the initial state of preservation is the determinant factor when deciding upon the conservation strategy to be followed and the type and concentration of the applied chemical on fossilized skeletal remains. Also, it became evident that high concentrations of acetic and formic acid tend to deteriorate the microstructure of fossils and thus render any histological study impossible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone Structure and Diagenesis
The bone is a heterogeneous, highly organized, and complex dynamic system that consists of a mixture of organic and inorganic parts. The inorganic part consists of a poorly crystallized biological analogous of carbonated-hydroxylapatite (Ca10 + (y-x)/2(PO4)6-x(CO3)xOH2-y-z(CO3)yXz, where Χ: Cl, F) (Wopenka and Pasteris 2005). The organic part of bone matrix is mainly composed of type I (30% glycine, 14% proline, 11% hydroxyproline) and type V collagen (Weiner and Traub 1992).
Compact bone is characterized by lamellae, haversian channels, haversian systems (osteons), lacunae, Volkman’s channels, etc. (Cohen and Harriss 1958). The aforementioned histomorphological characteristics can be preserved through geological time, despite the phenomenon of diagenesis, which can certainly alter their microscopic appearance, often even destroy them (Stathopoulou 2006; Tuross et al. 1989). Usually, during bone diagenesis, there is a re-crystallization of biological apatite (Schoeninger et al. 1989) and a decomposition of collagen (Hedges and Millard 1995), due mainly to microbial activity (Hackett 1981; Hanson and Buikstra 1987). Hence, it is important to attribute, properly, the various alterations of bone micromorphology and chemical composition to either diagenesis or chemical preparation techniques.
Palaeontological Preparation
The conservation of fossilized bones has been the objective of numerous studies in the past (Bather 1908; Kummel and Raup 1965), mainly because this type of geological specimen must be preserved in time and stabilized for both scientific research, as well as for museum exhibition. Towards this direction, both mechanical and chemical methods have been applied on fossil bones. Particularly, chemical extraction is an intrusive cleansing process and a direct removal method of hard and semi-hard surrounding sediment. This is the reason why scientists had focused their studies on finding le juste milieu between applied preparation techniques and the maintenance of fossils integrity, by using chemical reagents and simultaneously understanding and respecting the nature of bone.
The results have not always been successful, with many bones being destroyed due to the misapplication of mechanical techniques or the excessive use of chemicals (Hedges 1987; Lopez-Polin 2012; Panagiaris 2001). It soon became obvious that depending on the material and its preservation state, as well as its upcoming research, different types of preparation had to be employed (Wagstafee and Fidler 1968; Wilson 1995). As far as chemical means are concerned, numerous reagents have been applied, such as acetic, formic, citric, nitric, hydrochloric and hydrofluoric acid, hydrogen peroxide, etc. (Cooper and Whittington 1965; Croucher and Wooley 1982; Hodgkinson 1995; McCrae and Potze 2007; Rixon 1976).
In 1908 (Bather 1908), Bather referred to a substance used to reveal fossil bones, called sub-acetone, that later studies suggested its main component to be acetic acid (Lindsay 1995). In 1920, Stromer proposed the use of acetic acid, among other acids, (Stromer 1920), but until 1930, these chemical means were not applied for palaeontological preparation. The first who described the results of the use of acetic acid on fossilized bones was Toombs in 1948 (1948). Besides acetic acid, formic acid, being a weak acid, has also been used in laboratories during the past years (Jeppsson et al. 1985; Lindsay 1987; Lindsay 1995; Rutzky et al. 1994; Toombs and Rixon 1959), whereas hydrogen peroxide has been widely applied on small sized fossils, due to its oxidizing effect on their surrounding material (Hodgkinson 1995; Ward 1984).
Chemical Reagents
Hydrogen Peroxide (Perhydrol)
Hydrogen peroxide (H2O2) is the simplest peroxide, slightly more viscous than water and colorless in dilute solution. It appears as the key component of bone preparation during surgical practices, transplants or veterinary laboratory activities. Because of its vast application, numerous studies have taken place in order to test its effectiveness on bone tissue (Boyde et al. 1985; DePaula et al. 2005) and even its impact on analytical techniques (Freeman and Silva 2002).
In palaeontology, perhydrol has been widely used for the cleaning procedure of invertebrate fossils and vertebrate microfossils. Its concentration ranges from 20 to 60% per volume (Ward 1984), but, for safety reasons, weak solutions are preferred, since it can cause caustic burns on skin and eye irritations. It is a known oxidizing substance, while its results are based on the formation of oxygen bubbles in the voids of the surrounding material (Hodgkinson 1995; Rixon 1976). The rapid reaction of perhydrol to the presence of pyrite can corrode osseous and dental fossils, whereas its effect becomes quite intense by the addition of ammonium hydroxide.
Acetic Acid
Acetic acid (C2H4O2) is a weak synthetic carboxylic acid. It presents a clear transparent color with a pungent vinegar odor. In cases where mechanical preparation is deficient, acetic acid appears to be the most applicable chemical for pretreatment (Lopez Mata 2003). Toombs (Toombs 1948) evolved the chemical properties of acetic acid towards conservation, and Rixon (Rixon 1976) used it on reptilian fossils. Modern cleansing practices suggest either its careful application with cotton, or time limited submersions in low concentration acid solutions (Corral 2012). Acetic acid concentrations rarely exceed 15% v/v (Corral 2012; McCrae and Potze 2007; Ward 1984), except for Bromage (Bromage 1984) who used a 40% solution in order to test its effect on surface topography and biological status of bone.
Acetic acid dissolves calcium carbonate (1) from both the surrounding sediment and bone and, in a minor degree, the calcium phosphate (2).
As it is demonstrated by the first equation, during the reaction some evaporation of acetic acid and water occurs, which results in a pH increase and therefore the neutralization of the solution (Hellawell and Nicholas 2012; Jeppsson et al. 1999).
Formic Acid
Formic acid (systematically called methanoic acid - CH2O2) is the simplest carboxylic acid. It is a colorless liquid with a penetrating odor. It is estimated to be quite dangerous, because even in low concentrations can induce severe caustic burns. The use of formic acid was suggested by Rixon (Rixon 1976), due to the unpleasant odor of acetic acid. Both of these acids are considered to be the most suitable for conservation purposes. Although, the application of formic acid on fossils is identical with the abovementioned of acetic acid, extra attention must be paid in the rinsing process, since it often forms a white crust on the surface of fossils which cannot be easily removed (Cooper and Whittington 1965; Hodgkinson 1995; Lindsay 1995; Rixon 1976). The suggested concentrations are below 15% v/v, whereas it is advisable not to excide 10%, since it has proved to be too destructive for fossils (Corral 2012; Hellawell and Nicholas 2012; Rutzky et al. 1994; Ward 1984). Formic acid has the advantage of faster dissolving calcium carbonate (3) and breaking down dolomite (Hellawell and Nicholas 2012).
Formic acid solution can be saturated with tricalcium diphosphate, in order to protect fossils’ hydroxylapatite (Braillon 1973; Rutzky et al. 1994).
Objective
The objective of this paper is to present the results concerning the alterations of fossilized bone histology, under the influence of hydrogen peroxide, acetic acid and formic acid. These chemicals and their solutions were selected, based on a bibliographic review and the fact that these are some of the most commonly used reagents with fossils to this day.
Via the present experimental procedure, an attempt was made to distinguish the diagenetic from the degraded microstuctural appearance of fossil bones and to correlate certain histological formations with the impact of the abovementioned chemicals on cortical osseous tissue. The ulterior motive was to present a significant number of images demonstrating alterations that result from chemical pretreatment, in order to possibly avoid, in future studies (mostly of histological context), false outcomes due to the choice of a certain reagent and/or its concentration.
Material
The studied material was derived from the collections of the Museum of Palaeontology and Geology of the National and Kapodistrian University of Athens (NKUA). The chosen bone parts originated from two distinct fossiliferous sites in Greece, “Charkadio Cave” on Tilos Island and Kerassia on Euboea Island. The majority of fossils from Kerassia was characterized by a bad preservation state and, therefore, the samples used could not be attributed to a particular species. On the other hand, the excellent preservation of fossil bones from Tilos provided identifiable skeletal remains belonging to Palaeoloxodon tiliensis n.sp. (Mitsopoulou et al. 2015), previously known as Elephas tiliensis (Theodorou et al. 2007). Thus, poorly and exceptionally preserved fossils are equally represented in this study.
Kerassia
Kerassia is an Upper Miocene mammal site, located on the northern part of Euboea Island between Limni-Istiea basin and Agia Anna. The locality of Kerassia is relatively new, with the first biostratigraphic data to be provided by Made and Moyà-Solà in 1989, who ascribed middle Turolian as the estimated age (Made and Moyà-Solà 1989). In their publication on Carnivora, Roussiakis and Theodorou (Roussiakis and Theodorou 2003) confirmed the same age. Studied fossils have revealed the presence of a rich “Pikermian” fauna, which mainly consists of Carnivora, Proboscidea, Perissodactyla and Artiodactyla (Athanassiou et al. 2014; Iliopoulos 2003; Roussiakis and Theodorou 2003; Theodorou et al. 2003). Generally, the preservation state of the fossils is rather poor, making any excavation, cleaning and extraction processes quite challenging and time consuming (Theodorou et al. 1995). Extensive microbial focal destruction was detected, via microscopic examination, and was considered responsible for aggravating the specimen condition (Iliopoulos 2003; Iliopoulos 2004; Stathopoulou 2000).
Theodorou et al. (Theodorou et al. 2003) provided the first preliminary data on the sedimentological setting of the locality. The fossiliferous layers, of the upper Limni-Istiea Basin sequence, belong to the known “reddish-brown fluvial deposits” (Mettos et al. 1991), that include clays, conglomerates, sands and siltstones (Theodorou et al. 2003).
Tilos
Tilos island is located in the Dodecanese, northwest of Rhodes, in the southeast of the Aegean Sea. In 1971, Symeonidis found a very rich endemic fauna in Charkadio cave (Symeonidis 1972). Since then, the continuous research directed towards the recognition of a new dwarf elephant species originally named Elephas tiliensis (Theodorou et al. 2007) and later attributed to Palaeoloxodon (Mitsopoulou et al. 2015). This elephant is considered to be the last in Europe, while its appearance on the cave ranges from 45,000 to 4000–3500 years BP (Mitsopoulou et al. 2015; Stathopoulou and Theodorou 2001; Theodorou et al. 2007). The fauna from Charcadio cave also includes deer (140,000 years old), chelonia, aves and micromammals (Bachmayer et al. 1976; Symeonidis 1972; Theodorou 1983; Theodorou 1988). Based on radiochronological techniques, the age of the fauna is Upper Pleistocene–Holocene.
The overall excavation state of the fossils is excellent (Bachmayer et al. 1976). Microbial activity is mostly absent, while diagenesis has not affected the microstructure of bones considerably (Stathopoulou and Theodorou 2001).
According to Stathopoulou and Theodorou (2001) the surrounding sediment of fossils includes calcite, quartz, feldspars and clay minerals, while a wide compositional range of phosphorous was demonstrated.
Experimental Procedure—Methodology
In order to realize this experiment, three parameters were set; location from which the samples were derived, applied chemical solutions and duration of exposure.
Concerning their location, twenty-seven (27) untreated fossil skeletal remains were selected in total; five (5) long bones (mainly costae) from Tilos and twenty-two (22) unidentified mammalian bone fragments from Kerassia. Their number needed to be sufficient enough both for the requirements of the experiment and for the preparation of counter-samples. Subsequently, all these bone parts were cut into similar sized pieces (approximately 1.5 × 1.5 × 1.5 cm), leading to a total of twenty-three (23) bone samples from Kerassia and twenty-five (25) from Tilos. The cutting process produced bone surfaces of two types, one being the external surface of bone and the other the cut compact part of the bone. Both surfaces were compatible with our study, since diagenesis as well as the consequent chemical treatment of fossils are intrusive and affect the bone significantly. Additionally, palaeontologists do not only collect and use whole and intact bone fossils, thus during chemical conservation it is not only the true surface of bones that interacts with the reagents, but also the inner compact part of fossils, which is exposed by the presence of cracks and clear cut fractures. From these, five (5) samples from Kerassia and seven (7) from Tilos were examined as reference samples, prior to the experimental procedure, in order to be compared firstly between them (intra-regional comparison) and secondly with the chemically processed samples (inter-regional comparison and inter-chemical comparison). The conducted study examined only the cortical bone of the samples and not the trabecular.
In order to describe the histological state of preservation of our samples, the histological index was estimated, according to Hedges and Millard (Hedges and Millard 1995). The values of this index represent the degree of destruction of the histology of compact bone and range from 0 (“No original features identifiable, other than Haversian canals”) to 5 (“Very well preserved, virtually indistinguishable from fresh bone”). This method is followed here in order to describe the initial histological appearance of untreated fossils from both regions.
After thoroughly reviewing the available bibliography and evaluating the empirical data, the chemical means of preparation that were chosen, along with the corresponding concentrations, were the following: Hydrogen peroxide in concentrations of 10%, 20%, and 30%, acetic acid in concentrations of 3%, 9 and 15% and formic acid in concentrations of 5%, 10 and 15%. Towards this direction, an effort was also made to associate any chemical induced changes with gradually increased solution concentrations.
Finally, the exposure of bones to the aforementioned chemicals was examined in relation to time. Two distinct durations of exposure were adopted, at six (6) and twelve (12) hours. Every 1 h pH measurements were realized in order to record any variation of hydrogen ions relative number and to determine, if possible, when the saturation of solutions took place. When the samples were extracted from the solutions, the rinsing procedure that followed included four (4) water changes, one every hour. Distilled water was used during all four rinsing stages. Again, pH measurements applied to ensure both solutions neutralization and therefore the proper rinsing of specimens. For pH monitoring a Consort C561 pH meter was used. The used bone parts were kept in the laboratory, in a dry environment, at room temperature. Between the experiment and the microscopical examination of bones approximately two (2) months intervened.
In order to objectively describe the color of our samples, prior to and post chemical treatment, the Munsell color chart was used as the standard for colors (Munsell Color 1994).
During this study and in order to obtain the best possible images, all samples were examined with a scanning electron microscope (SEM) (Jeol JSM-6390). Electron probe microanalyses were carried out, when needed, on a SEM (Jeol JSM-5600) combined with an energy dispersive microanalysis system (OXFORD LINK ISIS 300), with software ZAF correction analysis. These techniques allowed for the detailed observation of bone micromorphology, as well as the qualitative analysis of the present inorganic phases.
X ray diffraction (XRD) was used to provide the mineralogical profile of the surrounding sediment using a D500 SIEMENS diffractometer.
An attempt to quantify the impact of chemical conservation on bone porosity was also made. The vast majority of porosity based studies, focus their interest in understanding, identifying and quantifying the taphonomic alterations that archaeological and palaeontological skeletal remains exhibit (Jans et al. 2004; Nielsen-Marsh and Hedges 1999; Smith et al. 2008). In the current research, helium gas porosity was selected in order to quantify the impact of chemical conservation on bones. Porosity measurements were collected before and after treating the fossils. Density and volume were measured by using an AccuPyc 1330 V3.03, whereas for porosity (envelope density report) a GeoPyc 1360 V3.00 was used.
Results
Untreated Fossils from Kerassia Region
The fossil bones from Kerassia presented, macroscopically, a poor preservation status. The majority of samples were delicate and friable. The voids of the trabecular bone were filled with a distinct mineral phase (Fig. 1h), which EDS examination identified as calcite (Fig. 1i). The texture of specimens appeared brittle, while their color ranged from “white” (Hue 10YR 8/1) to “very pale brown” (Hue 10YR 8/2). The color of mineral inclusions varied between “light gray” (Hue 2.5Y 7/1) to “gray” (Hue 2.5Y 6/1) (Munsell Color 1994).
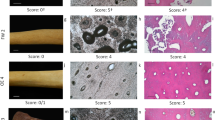
Untreated fossils from Kerassia region. a Haversian systems, where the haversian channels appear empty. b Badly preserved osteon. c Calcite cast of a haversian channel. d Fibrous form of the inside wall of a haversian channel. e Focused image of mineralized collagen of image d. f Microbial activity in the form of small round foci. g Microbial activity in the form of linear longitudinal tunnels. h Mineral phase (calcite) occupying the voids of trabecular bone. i EDS analysis confirming the presence of calcite
The histology of the studied fossils was considerably affected by diagenesis. The characteristic features of cortical bone were not distinguishable. In bulk images, only haversian channels were identifiable (Fig. 1a), whilst the presentation of osteons was rather problematic (Fig. 1b). Calcite growth, inside haversian channels, formed a cast of their inner morphology, providing a naturally created three dimensional structure (Fig. 1c). In longitudinal sections, the fibrous form of mineralized collagen was observed on the inside wall of haversian channels (Fig. 1d, e). The presence of microscopical focal destruction (MFD) was extensive and thus, responsible for inducing extra sensitivity to the bones overall condition. It appeared in the form of small round foci (voids) (Fig. 1f) and linear longitudinal tunnels (Fig. 1g) (Hackett 1981).
According to Hedges and Millard (Hedges and Millard 1995) and after personal evaluation, the histology index of fossils from Kerassia varies between 1 and 0.
Concerning the surrounding sediment of the Kerassia samples, it appeared neither compact nor frail. The conducted XRD analysis showed that the material consisted mainly of calcite, quartz, dolomite and clay minerals such as biotite, montmorillomite etc. (Appendix, Fig. 13).
Kerassia Bones After Treatment with Hydrogen Peroxide
Hydrogen peroxide reinforced the preexisting low consistency of fossils and developed a macroscopically evident fragility. Although, a change in color and texture did not occur, when gradually increasing the hydrogen peroxide concentrations deteriorated the overall condition of specimens significantly.
Microscopically, the prior diagenetic degradation of bones was aggravated. The lack of histological characteristics cannot be attributed to the chosen chemical reagent. Diagenesis and intense microbial activity were the main reasons for the observed poor preservation (Fig. 2a).
Kerassia bones after treatment with hydrogen peroxide (hp). a Further histological aggravation of an already defected preservation (30% hp., 12 h). b Hydrogen peroxides’ impact on naturally occurring bone voids (10% hp., 6 h). c Calcite filling of haversian channel (20% hp., 12 h). d Calcite’s etched surface (10% hp., 12 h)
Nonetheless, hydrogen peroxide was responsible for the enlargement of naturally occurring voids such as haversian channels and microbial borings. In Fig. 2b, the dimensions of the illustrated void exceed 20 μm, while, normally, microbial foci exhibit a diameter range between 0.5 and 10 μm.
According to EDS chemical analysis, the mineral phase found in voids, mainly haversian channels, was calcite (Fig. 2c). The superficial “etching” of calcite was ascribed to the application of hydrogen peroxide (Fig. 2d).
Regarding the duration of exposure to the chemical reagent, the time parameter did not seem to affect the reagents’ impact on bones, neither by accelerating any processes, nor by further deteriorating the abovementioned state.
Kerassia Bones After Treatment with Acetic Acid
Acetic acid induced extra fragility to fossils from Kerassia, that macroscopically could be confirmed by their delicate state. Bones were brittle and, during their placement on SEM stubs, were easily shattered. This phenomenon rendered their standard preparation and examination on SEM quite challenging. The color of specimens remained unaltered.
The absence of well-preserved areas microscopically was associated primarily with diagenesis, microbial activity and secondly with the reaction of acetic acid. The main micromorphological characteristics could not be observed, while Fig. 3a represents a bulk image of the general histological condition of samples. The “pitted” appearance of bone, as Bromage (Bromage 1984) highlights in his publication, is noted here as well (Fig. 3b). Increasing the concentration of acetic acid solution, the “etched” appearance of fossils was intensified, while chemically induced cracks became evident (Fig. 3c). Solutions with 15% acetic acid corroded the observed calcite crystals drastically (Fig. 3d).
Kerassia bones after treatment with acetic acid (aa). a Evident lack of histological structure (9% aa, 6 h). b Fossils’ “pitted” appearance due to acetic acid implementation (3% aa, 12 h). c “Etched” morphology of fossils. White arrow points to a chemically induced crack (3% aa, 12 h). d Corroded calcite crystals (15% aa, 12 h)
Once again, the time parameter did not seem to affect our results.
Kerassia Bones After Treatment with Formic Acid
Macroscopically, fossils that interacted with formic acid maintained their color, but their consistency was deteriorated significantly. Their frailty, complicated the preparation of samples for SEM examination, while their rough surfaces impeded their proper gold coating.
Microscopically, the appearance of bones was rather defective, since poor preservation and microscopical focal destruction coexisted with chemical erosion. Specimens were very susceptible to solutions where formic acid exceeded 5%. In bulk images, the recognition of the basic histological structures was not possible. They displayed a “fused” texture (Fig. 4a) and extensive cracks were also observed. These cracks were considerably deep and encircled the haversian channels (Fig. 4b and c), while their presence was attributed to a combination of diagenetic forces and the corrosive action of reagent.
Kerassia bones after treatment with formic acid (fa). a “Fused” histomorphology of bone (5% fa, 12 h). b Degraded haversian system displaying extensive crevices (15% fa, 6 h). c Enlarged image of an osteon which exhibits deep circumferential cracks (15% fa, 6 h). d Corroded calcite crystals (10% fa, 12 h)
EDS chemical analysis detected calcite, whose crystals were quite affected by the 15% formic acid solutions (Fig. 4d).
Different exposure periods did not alter the predescribed state of the fossils.
Untreated Fossils from Tilos Region
Macroscopically, the excavated condition of fossils was excellent. Preservation acted protectively on bones, which presented a high degree of consistency. Samples displayed a semi-rough texture, while their color was described as “pale yellow” (Hue 2.5 8/2–8/3) (Munsell Color 1994). Inside trabecular bone no secondary mineral phases were observed.
Microscopically, fossils exhibited a complete representation of all histomorphological characteristics. Haversian systems could be observed (Fig. 5a), along with more detailed structures, such as lacunae (Fig. 5b and c). Parallel haversian channels were noted (Fig. 5d), while the mineralized collagen fibers, found on the inside wall of these channels, were observed (Fig. 5e). Microscopical focal destruction was very limited in these samples. In fact, the only areas that displayed the typical form of small round foci and linear longitudinal tunnels are presented in Fig. 5f and g (Hackett 1981). Calcite crystals (Fig. 5i) were found inside cavities and fractures of bones, but mainly inside haversian channels (Fig. 5h). Throughout this examination, it was confirmed that the histology index ranges between 2 and 3, according to Hedges and Millard (Hedges and Millard 1995; Stathopoulou et al. 2008).
Untreated fossils from Tilos region. a Well-preserved Haversian system. b Osteon encircled by lacunae. c Detail of a circumferential lacuna. d Calcite casts of two haversian channels. e Fibrous form of the inner wall of a haversian channel. f, g Microbial activity. h Mineral phase (calcite) occupying bone voids. i EDS analysis confirming the presence of calcite
Concerning the surrounding material of fossils, it is characterized by minor hardness and was removed easily via dilution. XRD analysis demonstrated that it consists mainly of calcite, quartz, anorthite and clay minerals such as illite, montmorillomite, etc. (Appendix, Fig. 14).
Tilos Bones After Treatment with Hydrogen Peroxide
The action of hydrogen peroxide did not seem to affect the state and consistency of fossil bones macroscopically, while, simultaneously, their color and texture remained unaltered.
Microscopically, the former exceptional preservation of the Tilos samples also remained unaltered, allowing for the detailed observation of their histomorphological characteristics (Fig. 6a). Haversian systems, haversian channels, lacunae and even circumferential lamellae were easily noted. Nevertheless, bones that were submerged in solutions with 30% hydrogen peroxide, developed areas with different degrees of destruction. The eroded surface, presented in Fig. 6b, cannot be attributed neither to the limited microbial activity of fossils, nor to diagenetic forces, since similar images were not encountered during the preliminary study of untreated bones. At the same concentration (30%), chemically enlarged cracks were also encountered (Fig. 6d). Solutions with 10 and 20% hydrogen peroxide accomplished solely their cleansing role, without affecting the fossils’ micromorphology.
The crystals found in voids, mainly Haversian channels, were attributed to calcite, according to EDS analyses (Fig. 6c). The time parameter did not further intensify the chemical alterations.
Tilos Bones After Treatment with Acetic Acid
The implementation of acetic acid on fossils did not leave any macroscopical traces.
Microscopically, fossils maintained their optimum structure. The observation of all histomorphological characteristics was possible. Numerous irregular voids were noted in bones that interacted with 9% acetic acid solutions (Fig. 7a). The shape of these voids abstained from the pattern previously encountered. Their appearance cannot be attributed, with certainty, to the reagents action. Hackett (Hackett 1981) describes another expression of microscopical focal destruction, as budded tunnels. “In transverse sections these tunnels appear as rounded, stippled foci” (Hackett1981), description which is in accordance with Fig. 7a. Even their chemical enlargement is doubtful, since the diameter of voids (not exceeding 10 μm) is within the expected range (Hackett 1981; Jans et al. 2004).
Tilos bones after treatment with acetic acid (aa). a Irregular voids, possibly budded appearance of microscopical focal destruction (9% aa, 6 h). b Extensive crevices due to acetic acid implementation (15% aa, 6 h). c Chemically induced fractures (15% aa, 6 h). d Diagenetic circumferential cracks (15% aa, 6 h)
Nevertheless, the increase of the acid concentrations altered the fossils histological appearance. Extensive cracks resulted from the use of 15% C2H4O2 solutions (Fig. 7b). The fractures were located either randomly or geometrically throughout the examined surfaces of bones. Irregular cracks were attributed to the reagent (Fig. 7c). The cracks, that developed perimetrically to the osteons, were assigned to diagenetic and/or strain forces (Fig. 7d), whereas only their enlargement could originate from the chemical reagent.
Calcite crystals, that were encountered in various void types, remained unaltered after chemical treatment.
Duration of exposure to the chosen reagent did not alter the outcome.
Tilos Bones After Treatment with Formic Acid
Fossils were not affected macroscopically by the application of formic acid on them. The initial texture and consistency remained identical, but a worth mentioning change of color was observed. The surface that interacted with the reagent progressively changed its color, which according to the concentration ranged from “yellow” (Hue 10YR 7/6) at 5% CH2O2, to “reddish yellow” (Hue 7.5 6/6) at 15% CH2O2 (Munsell Color 1994). Perhaps, a release of certain metallic components, such as Fe, took place, that led to this alteration.
Microscopically, the well preserved bone tissue maintained its structural characteristics. Formic acid achieved its cleansing goal, without further deteriorating the samples. In Fig. 8a and b two expressions of the same type of crack are presented; one axially, across the inner wall of a haversian channel and the other overpassing the osteon radially. Both of these cracks resulted from diagenesis and, perhaps, post-depositional strain forces. They appear quite shallow and size restricted. Furthermore, when fossils were examined under high magnifications, “bone flakes” were encountered (Fig. 8c). This phenomenon occurred in concentrations of 15% CH2O2 and might be the initial interaction between cortical bone and chemical reagent.
The noted secondary mineral phases were calcite, whose exposed surfaces showed the illustrated corroded texture (Fig. 8d) when subjected to 15% of formic acid solutions.
As observed, exposure time spans did not additionally affect the specimens.
pH Measurements
pH Monitoring During the Experimental Procedure
During the experiment, pH monitoring took place in order to assess its variation throughout the 12 h that it lasted. The first measurements were collected at zero hour (0 h), when the chemical solutions were ready but not yet interacting with the bone samples. After the immersion of the fossils in the reagents, a detailed hourly pH recording followed (Tables 1 and 2). All samples presented the anticipated pH increase within the first hour (Figs. 9 and 10). After the second hour, pH measurements of hydrogen peroxide and formic acid exhibited a relative stabilization. A similar trend appeared in acetic acids’ solutions between the fourth and fifth hour.
pH Monitoring During the Rinsing Procedure
When the rinsing procedure begun, pH was measured every 1 h. Since the major concern is to secure the proper cleansing of the specimen and the minimization of chemical remnants, pH appeared as the mean to establish this objective. The pH of distilled water was measured prior, giving a mean value of 7.7025. According to Tables 3 and 4, at the third stage of the rinsing procedure, pH had been neutralized, in both Kerassia and Tilos samples (Figs. 11 and 12).
Porosity Measurements
The majority of studies that focus on bone porosity are aiming to assess the taphonomical alterations of porosity that occur in fossilized and archaeological skeletal remains. Here, porosity is being used as an auxiliary quantitative method to determine if chemical treatment could affect the initial porosity of specimens by further increasing it. The results of density and porosity analysis are summarized in Tables 5 and 6. By first comparing the selected regions between them, it is instantly observed that Kerassia presented lower mean values than Tilos, concerning average density, specific pore volume and percent porosity. When the measurements are analyzed independently, intra-regional differences emerged. Porosity results from Kerassias did not seem to follow a recognizable or anticipated pattern. Calculations from the untreated samples were not dissimilar from those emerged in chemicals. Both specific pore volume and percent porosity measurements did not vary (inter-chemical comparison). The impact of formic acid was the only exception, since it demonstrated considerable increase in specific pore volume and percent porosity, giving the highest values at 15% CH2O2 concentration. On the other hand, Tilos presented gradually increased specific pore volume values. The untreated samples from Tilos exhibited the lowest measures, followed by those interacted with hydrogen peroxide and acetic acid. Again, the implementation of formic acid increased the values of specific pore volume and percent porosity, giving a maximum at 10% CH2O2 concentration.
Discussion
The Provenance Parameter
During the planning stages of this experiment, one of the main objectives was the equal representation of both exceptionally and poorly preserved fossils. Although cautious treatment is suggested bibliographically, when handling fragile samples, the intention behind the choice of fossils from Kerassia and Tilos was to demonstrate practically the interaction between chemical treatment and preservation state.
The condition of fossils was evident both macroscopically and microscopically, whether they originated from Kerassia or Tilos. These diametrically opposed preservation states could be attributed to various causes that can coexist or act individually. The employment of different taphonomic forces on bones, their susceptibility to weathering, bioerosion and other diagenetic processes, or their potential transportation from the original death site, are only some of the possible reasons that formed their preservation profile. In addition to that, it must be emphasized that the material from Tilos was excavated from Charkadio cave, which constitutes a natural protective fossiliferous site.
XRD analyses of surrounding sediments were quite similar, with Kerassia having a higher participation of clay minerals than Tilos.
Concerning the untreated samples’ porosity results, Kerassia exhibited lower values than Tilos. Although the overall preservation state of Kerassia bones is considered quite bad and therefore high porosity values were expected, the increased filling of these pores by secondary mineral phases seems to have acted reversibly. EDS analysis and SEM observation have confirmed the presence of calcite crystals, which only in Kerassia specimens were also macroscopically observed, since it occupied the voids of trabecular bone too. Porosity results reflect the lack of abundant secondary minerals within the Tilos bone voids and cracks.
Concerning the effect of reagents on the chosen samples in relation to the provenance parameter, it is safe to state that the observed chemically induced changes were aligned with the preexisting preservation canvas. Any noted radical or unexpected degradation should be attributed to the reagents’ concentration and/or reactivity.
The Chemical Reagent Parameter
Macroscopically, the chosen chemical reagents interacted according to the fossils excavated preservation condition. The bones from Kerassia maintained their original shape, color and texture, but samples’ overall consistency was degraded. Chemical corrosion along with an underlying poor preservation rendered them so frail, as to nearly compromise their preparation for SEM examination. On the other hand, Tilos fossils displayed an excellent appearance, with only exception the implementation of formic acid. This acid considerably changed their color, whilst their shape, texture and consistency remained unaltered.
Microscopically, fossils reacted with the chemicals according to their preexisting diagenetic condition once again. Thus, bones from Tilos sustained, in general, their histological appearance, whilst the condition of those from Kerassia was considerably aggravated. Nevertheless, high concentrations of acetic and formic acid were responsible for both enlarging naturally occurring voids and fissures, as well as for inducing new ones.
Specimens from both Tilos and Kerassia presented the highest specific pore volume and porosity values, when submerged in formic acid solutions at 10 and 15% concentrations respectively. This indicates that formic acid is not only a drastic reagent during chemical treatment, but the most severe, compared to hydrogen peroxide and acetic acid. Through porosity monitoring, it is observed that between the highest concentrations of hydrogen peroxide and acetic acid, the second appears to have a more intense effect on Tilos fossils. As aforementioned, the porosity values of Tilos untreated samples reveal a possible susceptibility to direct chemically induced changes. The majority of pores was free of calcite occupation, a fact that facilitated the intrusion of the chemical reagent, which possibly intensified and/or accelerated the reagents impact.
After a brief synopsis of the chemically induced alterations on fossils, it is of high importance to emphasize some key conclusions that originated from this experiment. Primarily, all the chosen reagents successfully removed the surrounding sediment. However, chemical preparation is, still, an alternative option, when mechanical methods are considered insufficient or dangerous for delicate specimens. The submersion of fossils in distilled water is suggested as an intermediate step, between mechanical and chemical methods. These baths can potentially soften the sediment and any chemical application could be avoided. If this proves to be ineffective, then palaeontological conservation can embrace its chemical character. It is sensible to start with low concentration solutions and gradually increase them, according to the samples’ response to the chemical. Nonetheless, high concentrations should be avoided. Without excluding any reagent, the use of formic acid, at its minimum concentration, is considered as a last resort solution. In relation to hydrogen peroxide and acetic acid, concentrations should not exceed 20 and 6% respectively. The reason why Kerassias’ fossils were more sensitive to chemicals than those from Tilos, is mainly because they are characterized by friableness and a high degree of fragmentation. This segmentation increases the fossils’ specific surface area, allowing the reagent to interact more drastically with the specimen. As a result, the reaction, between the chemicals and the brittle fossils, is accelerated and thus their macroscopical and histological condition are significantly aggravated.
Regardless of the applied reagent, the rinsing process must be careful, exhaustive and repetitive. According to pH measurements, three water changes are sufficient enough to assure the neutralization of pH.
Finally, each of the applied conservation techniques must be adapted to the preservation state of the fossil, its physical characteristics and the type of research that will be conducted.
The Time Parameter
In order to test how the extent of chemically induced degradation of fossils changed in time, two different durations of exposure were chosen, at 6 and 12 h respectively. All the immerged samples were examined 2 months after their last rinsing procedure. It soon became apparent that, despite the chosen chemical reagent or its concentration, the observed deterioration on bones could not be associated with the duration of exposure. Their appearance remained unaltered both macroscopically and microscopically. It is highly considered that any induced damage occurs at a certain point, presumably, because the solutions become saturated after a given time period.
As it was highlighted in the introduction, when acids are applied to fossil bones a pH increase takes place, which was confirmed, and inevitably leads to a gradual cessation of calcium carbonate dissolution. According to pH measurements solution saturation took place during the second hour of the experiment for samples immerged in hydrogen peroxide and formic acid, while for specimens immerged in acetic acid saturation occurred between the fourth and fifth hour. After reaching saturation, pH values did not exhibit significant variations, which is visible as a “plateau” formation in Figs. 9 and 10.
Undoubtedly, the exposure span should be correlated with the size of specimen. Nonetheless, a 6-h stay in the chosen reagent is sufficient enough, to test fossils first reaction and to decide whether or not we will continue to apply sequential chemical baths.
Now, exactly the same samples are being kept in a dry environment, at room temperature, inside paper boxes, in order to be examined again.
Conclusions
Concluding, it must be emphasized that the initial condition of fossils will define the type and concentration of the chosen chemical reagent. Although, the preservation state of fossils proved to be the determinant factor of this experiment, not exceeding concentration thresholds is important. Though the use of chemical reagents was a conservators taboo in the recent past, they are, necessary in order to access and cleanse areas that mechanical methods can never approach. Again prudence is imposed, these chemical means cannot be treated as panacea. Their selection and use must be planned carefully, since their misapplication can accelerate their corrosive action and aggravate their impact on bones.
Towards this direction the detailed documentation of every preparation and extraction technique that is implemented on fossils, is additionally recommended. The presence of these records and their constant update are essential in both evaluating the effectiveness of past methodologies and informing the researchers about the treatment that was followed. These type of data can, above all, contribute to the credibility and progress of modern research, since the “conservation history” of specimens is very important when conducting analytical techniques and during histological study.
Finally, the storage and safekeeping of counter samples, from both fossils and sediments, are strongly advised, in order to create a series of intact reference specimens that will facilitate future relevant studies.
References
Athanassiou A, Roussiakis S, Giaourtsakis I, Theodorou G, Iliopoulos G (2014) A new hornless rhinoceros of the genus Acerorhinus (Perissodactyla: Rhinocerotidae) from the Upper Miocene of Kerassia (Euboea, Greece), with a revision of related forms. Palaeontogr Abt A 303:23–59
Bachmayer F, Symeonidis N, Seeman R, Zapfe H (1976) Die Ausgrabungen in der Zwergelefantenhohle ‘Charcadio’ auf der Insel Tilos (Dodekanes, Griechenland) in der Jahren 1974 und 1975. Ann Naturhist Mus Wien 80:113–144
Bather F (1908) The preparation and preservation of fossils. Mus J 8:76–90
Boyde A, Maconnachie E, Reid S, Delling G, Mundy G (1985) Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan Electron Microsc 4:1537–1554
Braillon J (1973) Utilisation de techniques chimiques et physiques dans le dégagement et le triage des fossiles de vertébrés. Bulletin du Museum national d’Hstoire naturelle, 3ème série. Sci Terre 176:141–166
Bromage T (1984) Interpretation of scanning electron microscopic images of abraded forming bone surfaces. Am J Phys Anthropol 64:161–178
Cohen J, Harriss W (1958) The three dimensional anatomy of Haversian systems. J Bone Joint Surg 40:419–434
Cooper G, Whittington H (1965) Use of acids in the preparation of fossils. In: Kummell B, Raup P (eds) Handbook of palaeontological techniques. W. Freeman & Co., San Francisco, pp 294–300
Corral J (2012) Técnicas aplicadas en la preparación de un cráneo cuaternario de Panthera pardus (Linneo, 1758) de Ataun (cueva Allekoaitze, Guipúzcoa, España). Bol Geol Min 123:127–138
Croucher R, Wooley A (1982) Fossils, minerals and rocks. Collection and preservation. British Museum of Natural History, London
DePaula C, Truncale K, Gertzman A, Sunwoo M, Dunn M (2005) Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank 6:287–298
Freeman J, Silva M (2002) Separation of the Raman spectral signatures of bioapatite and collagen in compact mouse bone bleached with hydrogen peroxide. Appl Spectrosc 56:770–775
Hackett C (1981) Microscopal focal destruction (tunnels) in exhumed human bones. Med Sci Law 21:243–265
Hanson D, Buikstra J (1987) Histomorphological alteration in buried human bone from the lower Illinois valley: implications for palaeodietary research. J Archeol Sci 14:549–563
Hedges R (1987) Potential information from archaeological bone, its recovery and preservation. In: Starling K, Watkinson D (eds) Archaeological bone, antler and ivory. United Kingdom Institute for Conservation, London, pp 22–23
Hedges R, Millard A (1995) Measurements and relationships of diagenetic alteration of bone from three archaeological sites. J Archaeol Sci 22:201–209
Hellawell J, Nicholas C (2012) Acid treatment effects on the stable isotopic signatures of fossils. Palaeontology 55, Part 1:1–10
Hodgkinson R (1995) Microfossils. In: Collins C (ed) The care and conservation of paleontological material. Butterworth–Heinemann, London
Iliopoulos G (2003) The Giraffidae (Mammalia, Artiodactyla) and the study of the histology and chemistry of fossil mammal bone from the Late Miocene of Kerassia (Euboea Island, Greece). PhD Dissertation, University of Leicester
Iliopoulos G (2004) Microbial focal destruction in Late Miocene mammal bone from Kerassia (N. Euboea Island, Greece). Paper presented at the 5th International Symposium on Eastern Mediterranean Geology Thessaloniki, Greece
Jans M, Nielsen-Marsh C, Smith C, Collins M, Kars H (2004) Characterisation of microbial attack on archaeological bone. J Archaeol Sci 31:87-95
Jeppsson J, Fredholm D, Mattiasson B (1985) Acetid acid and phosphatic fossil—a warning. J Paleontol 59:952–956
Jeppsson L, Anehus R, Fredholm D (1999) The optimal acetate buffered acetic acid technique for extracting phosphatic fossils. J Paleontol 73:964–972
Kummel B, Raup D (eds) (1965) Handbook of paleontological techniques. Freeman
Lindsay W (1987) The acid technique in vertebrate palaeontology: a review. Geo. Curator 4:455–461
Lindsay W (1995) A review of the acid technique. In: Collins C (ed) The care and conservation of palaentological material. Butterworth- Heinemann, London
Lopez Mata L (2003) Metodos de conservacion del material oseo. In: Isidro A, Malgosa A (eds) Paleopathologia, la enfermedad no escrita. Masson, Barcelona, pp 25–32
Lopez-Polin L (2012) Possible interferences of some conservation treatments with subsequent studies on fossil bones: a conservator’s overview. Quat Int 275:120–127
Made J, Moyà-Solà S (1989) European Suinae (Artiodactyla) from the Late Miocene onwards. B Soc Paleontol Ital 28:329–339
McCrae C, Potze S (2007) A fresh look at chemical fossil extraction. Palaeontol Afr 42:115–116
Mettos A, Rodogianni T, Papadakos G, Pashos P, Georgiou H (1991) New data on the geology of the Neogene sediments of northern Euboea. Bull Geol Soc Greece 25:71–83
Mitsopoulou V, Michailidis D, Theodorou E, Isidorou S, Roussiakis S, Vasilopoulos T, Polydoras S, Kaisarlis G, Spitas V, Stathopoulou E, Provatidis C, Theodorou G (2015) Digitizing, modelling and 3D printing of skeletal digital models of Palaeoloxodon tiliensis (Tilos, Dodecanese, Greece). Quat Int 379:4–13
Munsell Color Company (1994) Munseel Soil Color. Revised Edition. Macbeth Division of Kollmorgen. New Windsor, NY
Nielsen-Marsh C, Hedges R (1999) Bone porosity and the use of mercury intrusion porosimetry in bone diagenesis studies. Archaeometry 41:165–174
Panagiaris G (2001) The influence of conservation treatments on physical anthropology research. In: Williams E (ed) Human remains: conservation, retrieval and analysis. Archaeopress, Oxford, pp 95–102
Rixon A (1976) Fossil animal remains. Athlon Press, London
Roussiakis S, Theodorou G (2003) Carnivora from the Late Miocene of Kerassia (northern Euboea, Greece). Deinsea 10:469–497
Rutzky I, Elvers W, Maisey J, Kellner A (1994) Chemical Preparation Techniques. In: Leiggi P, May P (eds) Vertebrate Paleontological Techniques., vol 1. Cambridge University Press, Cambridge, pp 3-34
Schoeninger M, Moore K, Murray M, Kingston J (1989) Detection of bone preservation in archaeological and fossil samples. Appl Geochem 4:281–292
Smith C, Faraldos M, Fernandez–Jalvo Y (2008) The precision of porosity measurements: Effects of sample pre-treatment on porosity measurements of modern and archaeological bone Palaeogeography, Palaeoclimatology, Palaeoecology 266:175-182
Stathopoulou E (2000) Exploration of the fossilization mechanism and the interaction with the environment of neogene and quaternary vertebrate skeletal remains. MSc Dissertation, National and Kapodistian University of Athens
Stathopoulou E (2006) On the study of the internal micromorphology and fossilization of cenozoic vertebrates by radioanalytical techniques. PhD Dissertation, National and Kapodistian University of Athens
Stathopoulou E, Theodorou G (2001) Observations on the diagenesis of dwarf elephant skeletal remains from the island of Tilos (Dodecanese, Greece). In: “The World of Elephants”. Proceedings of the 1st International Conference. pp 557–562
Stathopoulou E, Psycharis V, Chryssikos G, Gionis V, Theodorou G (2008) Bone diagenesis: new data from infrared spectroscopy and X-ray diffraction. Palaeogeogr Palaeoclimatol Palaeoecol 266:168–174
Stromer E (1920) Paläozoologisches Praktikum. Berlin: Gebrüder Borntraeger:104
Symeonidis N (1972) Die entdeckung von zwergelefanten in der hohle “Charkadio” auf derinsel Tilos (Dodekanes, Griechenland). Ann. Geol. des Pays Hellenique 24:445–461
Theodorou G (1983) The dwarf elephants of the Charkadio cave on the island of Tilos (Dodekanese, Greece). PhD Dissertation, National and Kapodistian University of Athens
Theodorou G (1988) Environmental factors affecting the evolution of island endemics: the Tilos example from Greece. Mod Geol 13:183–188
Theodorou G, Roussiakis S, Athanassiou A (1995) Contribution to the study of the terrestrial neogene, of Greece. Artiodactyla and Rhinocerotidae from the Kerassia and Chalkoutsi localities. Romanian. J Stratigr 76:129–130
Theodorou G, Athanassiou A, Roussiakis S, Iliopoulos G (2003) Preliminary remarks on the Late Miocene herbivores of Kerassiá (northern Euboea, Greece). Deinsea 10:519–530
Theodorou G, Symeonidis N, Stathopoulou E (2007) Elephas tiliensis n. sp. from Tilos island (Dodecanese, Greece). Hellenic J Geosci 42:19–32
Toombs H (1948) The use of acetic acid in the development of vertebrate fossils. Mus J 48:54
Toombs H, Rixon A (1959) The use of acids in the preparation of vertebrate fossils. Curator 2:304–312
Tuross N, Behrensmeyer A, Eanes E, Fisher L, Hare P (1989) Molecular preservation and crystallographic alterations in a weathering sequence of wildebeest bones. Appl Geochem 4:261–270
Wagstafee R, Fidler J (1968) . Witherby Ltd., London
Ward DJ (1984) Collecting isolated microvertebrate fossils. Zool J Linnean Soc 82:245–259
Weiner S, Traub W (1992) Bone structure: from angstroms to microns. FASEB J 6:879–885
Wilson J (1995) Conservation and processing—cleaning and mechanical preparation. In: Collins C (ed) The care and conservation of paleontological material. Butterworth-Heinemann, London
Wopenka B, Pasteris J (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143
Acknowledgments
The authors would like to thank Assistant Professor Roussiakis Socrates for thoroughly reviewing this paper. The Department of Historical Geology and Palaeontology (NKUA) is appreciated for allowing the authors to conduct the experimental procedure at the homonymous laboratory and examine the specimens with SEM, and apply helium gas pycnometry on our samples. Thanks are owed to MSc Geologist Olga Koumoutsakou for her help and advice concerning gas pycnometry and to Dr. Ifigeneia Megremi for her assistance and scientific suggestions concerning the pH measurements. The Department of Economic Geology and Geochemistry (NKUA) is thanked for permitting the authors to analyze the samples with SEM-EDS. The National Center for Scientific Research “Demokritos” is acknowledged for allowing the conduction of XRD analyses. Funding for the realization of the excavations which provided us with our material (Tilos: 10323 and Kerassia: 12301), came from the Greek Ministry of the Aegean (U.O.A. Project 70/3/699 and 70/3/4407), the General Secretariat of Research and Technology of the Greek Ministry of Development (E.U. financed Project 96 ™YN 106 = Project of UOA 70/3/4407), the Special Account for Research Grants of the University of Athens (Research project 70/4/3370) and the local Municipalities of Tilos and Kerassia. Finally, the authors would like to thank Professor Page and the two anonymous reviewers, whose constructive comments significantly improved the final version of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mallouchou, M.S., Stathopoulou, E.T. & Theodorou, G.E. How Do Fossilized Mammalian Bones Behave During Chemical Conservation? The Histological Case Studies of Tilos and Kerassia. Geoheritage 11, 597–614 (2019). https://doi.org/10.1007/s12371-018-0310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12371-018-0310-3