Abstract
Improving nitrogen fertilizer use by plants and reducing N losses through applying the appropriate nitrogen (N) fertilizer rate with urease inhibitors (UIs) and nitrification inhibitors (NIs) may enhance crop production and the efficiency of N use. Sugarcane and unplanted plots were established in Central Thailand to investigate the effects of fertilizer application based on soil analysis and UIs, NIs and their combination (UINIs) on soil inorganic N, sugarcane productivity and N uptake. Both synthetic and natural inhibitors were investigated: UIs from N-(n-butyl) thiophosphoric triamide (NBPT) and garlic (Allium sativum L.) and NIs from dimethylpyrazole phosphate (DMPP), ground neem seed (Azadirachta indica A. Juss.) and praxelis (Praxelis clematidea (Griseb.) R.M. King & H. Rob). The results indicated the potential of applying UIs, NIs and UINIs along with N fertilizer to obtain a slower decrease in soil NH4+ and improve sugar products. The results indicated enhanced sugarcane growth and an apparent increase (up to 31%) in the cane and sugar yields in all the inhibitory treatments. Notably, despite the synthetic NIs and NIs from plants having similar effects on soil inorganic N, the natural NIs tended to enhance sugarcane yields and N uptake. These findings supported the application of UIs and NIs as a potential solution to reduce N fertilizer rates and N losses from soil, while increasing crop productivity. In addition, NIs from plants might be of interest for further investigation and for promotion to farmers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is one of the most important nutrients for agroecosystems and for its use in fertilizing agricultural land to improve crop production (Rütting et al. 2018). Synthetic N fertilizers, such as urea, are widely used in croplands; but over 50% of the N applied to fields is not utilized by plants and is lost to the environment (Bremner 1990; Hirel et al. 2011; Suter et al. 2016). N losses from fertilizer may be a cause of several forms of environmental degradations, such as increasing nitrogenous gases in the atmosphere (ammonia (NH3) volatilization via the hydrolysis of urea, nitrous oxide (N2O) emission from soil N transformation) and nitrate leaching and run off to waterbodies, leading to lower nitrogen use efficiency by plants (Liu et al. 2017; Chen et al. 2010).
Increased crop yields due to adding a high N fertilizer rate may be not a best solution for farmers. At least one study has reported a negative effect on soil fertility from excessive N fertilization (Sun et al. 2020). Thus, there is a need to enhance more effective management of agricultural land by improved N use by plants, together with increasing agricultural yields and the associated profits by farmers. Best practice management using a science-based approach by adopting the best fertilizer use with the right source at the right time and the right rate and placement could increase both productivity and profitability, while ensuring improved environmental protection (Johnston and Bruulsema 2014).
In addition, agrochemicals input, such as urease inhibitors (UIs) and nitrification inhibitors (NIs), have potential to reduce N losses from added mineral N to the soils and to improve N use by plants (Byrne et al. 2020). UIs, such as N-(n-propyl) thiophosphoric triamide (NPPT) and (N-(n-butyl) thiophosphoric triamide (NBPT) can be used to delay urea hydrolysis, by suppressing the action of the urease enzyme that converts urea to NH4+-N and thereby, protecting against NH3 volatilization and keeping fertilizer in the urea form (Arora and Srivastava 2013). NIs, such as 3, 4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD), basically act on microbial activity in the first step of the nitrification process, which inhibits the activity of ammonia oxidizing bacteria (AOB), with the membrane-associated ammonia monooxygenase (AMO) catalyzing NH3 oxidation to hydroxylamine (NH2OH) before it is oxidized to nitrite (NO2−) by the multiheme enzyme hydroxylamine oxidoreductase (HAO) (Stein and Arp 1998). As a result the remaining NH4+ in the soil can enhance the opportunity to absorb mineral N in the form of NH4+ from the root zone (Subbarao et al. 2012) and subsequently mitigate N2O emission from aerobic soils during the sequential oxidation of NH2OH to NO2− (Poffenbarger et al. 2018). Furthermore, combining UIs and NIs, which work by different mechanisms, may be an effective way of improving nitrogen use efficiency (Drury et al. 2017).
Various synthetic UIs and NIs have been tested to reduce environmental impacts and enhance crop yields; however, there has been no report on the side-effects, ecological impacts, performance stability and benefit-to-cost ratio (Arora and Srivastava 2013). Hence, inhibitors from natural sources are an interesting alternative to improve N use by plants, as well as not being environmentally harmful, while maintaining chemical stability and efficiency at low concentrations, as well as being cost-effective. The potential of allicin extracted from garlic has been reported to inhibit urease activity (Mathialagan et al. 2017) and effective plants acted as NIs, such as neem cake, neem oil (Pengthamkeerati and Modtad 2016), mint (Patra et al. 2007), aromatic plants (Upadhyay et al. 2011) and Brachiaria humidicola pastures (Subbarao et al. 2006). These biological nitrification inhibitors (BNIs) have various functional groups with diverse chemical structural constituents, fatty acids, phenylpropanoids, flavonoids, quinines, diterpenoids and isothiocyanates (Sadhukhan et al. 2022), with different modes of action (Ruser and Schulz 2015).
Praxelis (Praxelis clematidea (Griseb.) R.M. King & H. Rob) is an invasive weed in unmanaged agricultural lands in many countries, including Thailand (Thepphakhun and Intanon 2020). Praxelis contains phenylpropanoid, flavonoids and other compounds (Yang et al. 2020), with these compounds having been evaluated for antibacterial and antifungal activities (Nguyen et al. 2021). It is of interest to investigate the ability of praxelis as a new BNI to reduce losses of N fertilizers to the air and water and to achieve higher crop yields at lower cost.
Ideally, the application of an appropriate fertilizer rate on the basis of soil analysis should be done in an efficient way to meet crop uptake demand. However, when N fertilizer was added to the soil, mineral N was converted to NH4+ and then NO3− forms which are easily losses to the environment. The concept of applying the right rate of fertilizer along with urease and nitrification inhibitors may be an effective strategy to encourage the performance of N fertilizer use in the field and help ensure that plants could uptake most of the N applied to the soil and both economic and environmental protection goals are achieved. Thus, the objective of the current research was to investigate the effects of fertilizer rates (by farmer or soil analysis) and synthetic and natural UIs and NIs on soil inorganic N and crop yields and quality in planted and unplanted plots of sugarcane. Synthetic inhibitors of NBPT and DMPP and inhibitors from plants of garlic, neem and praxelis were investigated.
Materials and Methods
Experimental Design
The study site was conducted during 2019–2020 at the Lop Buri Research Station, Faculty of Agriculture, Kasetsart University, Khok Charoen district, Lop Buri province, Thailand (15°21' N, 100°55' E). Precipitation and air temperature data were obtained from a nearby weather station (Fig. 1). The initial selected soil characteristics of the study site were sand 29%, silt 24%, clay 47% (clayey soil), pH 6.7, organic matter (OM) 2.93%, available P 87 mg kg−1 and available K 394 mg kg−1.
Daily air temperature and rainfall during the cropping season at the Lop Buri Research Station, Faculty of Agriculture, Kasetsart University, Khok Charoen district, Lop Buri province, Thailand. Arrows indicate times of planting, fertilizer and inhibitors applications and harvesting events for the cropping season
The sugarcane plots and unplanted plots were established using a randomized complete block design with 4 and 3 replications, respectively. Each experimental plot size was 28 m2 (4 rows per plot, each 7 m long) with 1.5 × 0.5 m as the row × plant spacing. The sugarcane and unplanted experimental plots consisted of 10 treatments: control (C), only N fertilizer with commonly applied rate by local farmers (F), only N fertilizer with the soil and leaf analysis rate (S) according to the recommendations by the Department of Agriculture in Thailand, DMPP/NBPT (DM/NB), DMPP/DMPP (DM/DM) and DMPP/NBPT + DMPP (DM/NBDM), neem/garlic (NE/GL), neem/neem (NE/NE) and neem/garlic + neem (NE/GLNE) and praxelis/praxelis (PR/PR)). The sugarcane plots were planted with healthy sugarcane plants (Saccharum officinarum L.) variety Khon Kaen 3 and the stems were split with 3–4 joints per piece. The stems were placed horizontally into the furrows, and the basal fertilizer was added when the sprouts first emerged before covering with soil.
After planting for about 3 months, ammonium phosphate fertilizer was applied as basal fertilization at 312.5 kg ha−1 or 50 kg N ha−1for F and at 118.75 kg ha−1 or 19 kg N ha−1for S. The basal (N-P-K) fertilizer formula for F was 16–20–0 and for S was 16–16–8. All inhibitor treatments were added with ammonium phosphate fertilizer at the same rate of S. The inhibitors from plants including garlic, neem and praxelis were grinded before use. The N fertilizer was then well combined with each inhibitor before applied to the soil. Each inhibitor was applied to the plot, with the DM/NB, DM/DM and DM/NBDM treatments added with DMPP 5% of N fertilizer. The NE/GL, NE/NE and NE/GLNE treatments were added with ground neem seed 20% of N fertilizer and praxelis was applied for the PR/PR treatment at a rate of 200% of N fertilizer. Fertilizer was added again with UIs and NIs after basal application for 2 months as dressing fertilization. Urea was added at rates of 312.5 kg N ha−1 or 143.75 kg N ha−1 for the F treatment and 43.75 kg ha−1 or 20.125 kg N ha−1 for the S and other treatments. Inhibitors (NBPT and DMPP) were added at the rate of 5% of N fertilizer for the DM/NB and DM/DM treatments. The DM/NBDM treatment was added with NBPT and DMPP at the rate of 2.5% of N fertilizer. Garlic, neem and praxelis were added with rates of 10%, 20% and 200% of N fertilizer for the NE/GL, NE/NE and PR/PR treatments, respectively. The NE/GLNE treatment was added with garlic and neem at rates of 5 and 10% of N fertilizer, respectively. For dressing fertilization, 0–0–60 fertilizer was added at rates of 125 kg ha−1 for F and 50 kg ha−1 for S and the rest. Watering was applied as necessary and monitoring was carried out for pests and diseases until maturation. The unplanted plot also had inputs of basal and dressing fertilizers at the same rates as the sugarcane plot.
Field Measurements
A soil sample was taken from each plot at 3 days after fertilization, at 1 month and then once every 2 months thereafter until maturation, for 1st and 2nd fertilization. Each sample was extracted with 2 M KCl solution at a 1:10 soil-to-solution ratio and the solution was filtered for analysis of NH4+–N and the NO3−–N using standard methods (Greenberg et al. 1992) using a UDK 132 semi-automatic distillation unit and the colorimetric method (Greenberg et al. 1992) using a UV spectrophotometer, respectively.
The sugarcane was harvested after maturation from along 7 m of the 2 middle rows from 4 rows plot. Sugarcane yield was determined by cutting the sugarcane at the base close to the ground and about 25–30 cm below the leaf base at the top. Both the leaves and leaf sheaths were removed from 2 rows and weighed. A subsample of 3 stalks from the 2 middle rows was collected, weighed and then determined for oven-dry moisture (at 60 °C) to obtain the sugarcane yield and aboveground biomass. The total N content of the cane and sugarcane leaves were subsampled and analyzed using the Kjeldahl method for N uptake (Bhanuvally et al. 2017), where:
The sugar yield was determined using commercial cane sugar (CCS) and the sugarcane yield. The CCS value was derived from juice sucrose (%Pol), total soluble solids content (%Brix) and fiber content measurement using a polarimeter, refractometer and total solids determination in sugarcane, respectively.
Statistical Analysis
Analysis of variance (ANOVA) was determined to evaluate the effects of fertilizer rate, UI and NI applications and times on soil inorganic N, plant yield and N use. The statistical model used was a repeated measure. Multiple comparisons were appraised tested using Duncan’s multiple range test with P < 0.05 as the significance level. Factor analysis, using the principal component method, was performed to determine the pattern of treatments (N rates and inhibitors) in the planted study area.
Results and Discussion
Soil Inorganic N
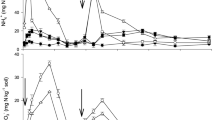
For soil NH4+–N and NO3−–N, main and interaction effects between treatments and sampling time were significant (p < 0.001) for both plots (sugarcane and unplanted) and soil depths of 0–10 cm (topsoil) and 10–30 cm (subsoil), as shown in Table 1. Figures 2 and 3 show the effect of fertilizer rate and inhibitors on NH4+–N and NO3−–N in the topsoil and subsoil for both plots throughout the cropping season (over a year). The sugarcane and unplanted plots had a similar trend for both NH4+–N and NO3−–N at both soil depths, by being greater after fertilizer applied and then reducing with time. The fertilizer rate and inhibitory effects on soil inorganic N were observed in both planted and unplanted plots. The unplanted plot was expected to more clearly express these effects on soil inorganic N, because the applied N fertilizers were not completely utilized by plants and greater remained in the soil. However, these effects were still found in the planted plot as well. This finding indicated the UIs and NIs used were also effective even when small amounts of N fertilizer applied.
Variation of soil NH4+–N on treatment of N fertilizer with UIs and NIs compared to treatment with only N fertilizer under field experiment on a topsoil unplanted plot, b subsoil unplanted plot, c topsoil sugarcane plot and d subsoil sugarcane plot. Bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. Line arrows indicated the timing of fertilizer application
Variation of soil NO3−–N on treatment of N fertilizer with UIs and NIs compared to treatment with only N fertilizer under field experiment on a topsoil unplanted plot, b subsoil unplanted plot, c topsoil sugarcane plot and d subsoil sugarcane plot. Bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. Line arrows indicated the timing of fertilizer application
Effect of N Fertilizer Rate
For the planted and unplanted plots, F had a greater NH4+–N and NO3−–N than S, but inconsistently significant (Figs. 2 and 3). This N rate effect was more pronounced: (1) at the beginning after fertilization and then decreased with time; and (2) during the 2nd or dressing fertilization. This was due to more N being applied in F than S (193.75 and 39.125 kg N ha−1, respectively) or about 5 times greater. At the 2nd fertilization, a much higher N rate was applied in F (143.75 kg N ha−1) than S (20.125 kg N ha−1).
Notably, the increase in soil inorganic N in F and S was not in proportion to the N fertilizer rate applied, suggesting that the more N fertilizer was applied, the greater the N losses to the environment. Once the N fertilizer had been applied in the soil, some of the mineral N was absorbed by plants and the excess N contributed to increasing the soil inorganic N associated with an increase in the N rate (Wang et al. 2022). Greater NO3− leaching was observed when a higher N rate was applied; for example, at applied N rates of 0 and 396 kg N ha−1 year−1, NO3− was leached at 4 and 155 kg N ha−1 year−1, respectively (Cameron et al. 2013).
Effect of UIs and NIs
For the N rate based on soil analysis (S treatment), the UIs and NIs retained N in soil for a higher amount and longer time, and the inhibitory effects seemed to be better in NH4+–N than NO3−–N at both soil depths (Figs. 2 and 3). This observation was perhaps because the inhibitors had a direct effect on NH4+ via urea hydrolysis and NH4+ transformation to NO3−. In addition, NO3− was easily lost from the soil, especially via leaching, due to its negative charge; consequently, it remained relatively lower than the NH4+ form. In addition, the UIs and NIs slowed down the NH4+–to–NO3− transformation of the N fertilizers, resulting in the gradual presence of NO3− in the soil. As a result, soil NO3− was well observed with the inhibitory effects of UIs and NIs in this study (Fig. 3). Another reason on a higher N retained in soil and an observation of the UI and NI effects on soil N was because this studied soil was high in clay (47% or clayey soil) and soil organic matter (2.93%) where NH4+ was highly sorbed on negative charge of soil particles and organic matter, and where N leaching losses were poor compared to sandy soil or light textured soil (Chen et al. 2020).
For the 1st or basal fertilizer application, NH4+ in the inhibitor plots of DMPP, neem and praxelis tended to have higher (but not significantly so) levels and was retained longer in the soil for at least 1 month, compared to the only fertilizer applied or S (Fig. 2). This finding suggested that these NIs of DMPP, neem and praxelis could retard N fertilizer transformations via NH4+–N–to–NO3−–N through the nitrification process and prolong NH4+ in the soil, despite the very low ammonium fertilizer rate (19 kg N ha−1) used in this study. These NIs suppressed nitrifiers (AOB and ammonia oxidizing archaea) by direct binding and interaction with AMO, which is an enzyme that oxidizes NH3 to NH2OH in the first step of the nitrification process and is subsequently converted to NO2− and NO3− (Hooper et al. 1997). Therefore, the longer NH4+ was retained in the soil, the more enhanced the uptake of NH4+–N by plants, with concomitant reduced N losses (such as N2O emissions) and NO3− leaching produced from the next step of the nitrification process (NH2OH to NO3−) (Bremner 1997; Bremner et al. 1980; Signor and Cerri 2013).
The effect of praxelis in the 1st fertilization might have been due to retardation in the nitrification process in a different way from the other NIs used in this study. Yang et al. (2020) investigated phytochemicals in the flowers of praxelis and found 3 chromenes, 3 phenylpropanoids, 3 flavonoids and 3 other compounds. Many researchers have reported that some of the compounds belonging to functional groups have potential to be biological nitrification inhibitors (BNIs), including fatty acids, phenylpropanoids, flavonoids, quinines, diterpenoids, terpenoids, tetranortriterpenoids, pyrazoles, thiadiazoles polyphenol and isothiocyanates (Subbarao et al. 2006, 2013, 2012, 2015; Sadhukhan et al. 2022; Patra et al. 2007; Kiran and Patra 2003; McCarty and Bremner 1989). Their action with the AMO enzymatic pathway results in some of these blocking both AMO and HAO (Sadhukhan et al. 2022). As a result, praxelis might be a potential NI, as was observed in the current study. Further investigation on the side effects of praxelis should be performed to confirm its beneficial use in croplands.
For the 2nd fertilizer application, the UIs of NBPT and garlic and the NIs of DMPP, neem and praxelis were applied with urea fertilizer either solely or in combination. After application, the soil NH4+ in the UI and NI inhibitor plots tended to be higher (but not significantly so) than the only fertilizer applied (S), as shown in Fig. 2. However, these inhibitory effects on soil NH4+ were only observed 3 days after the 2nd fertilizer was applied (day 76) and with greater effect at both soil depths of the sugarcane plot than the unplanted plot. This observation suggested that these UIs, NIs and their combinations could slow down the rate of urea hydrolysis and the nitrification process in the soil, resulting in a higher level of soil NH4+. However, the inhibition of UIs and NIs on soil NH4+ was observed in a short period after the fertilizer was applied, possibly due to the low N rate used in this study (20.175 kg N ha−1). In addition, urea fertilizer was used for dressing and the inhibition of UIs and NIs on urea might be more complicated, resulting in less effects on NH4+ in this study. This could be explained by the urea addition in the form of carbonic diamide being transformed to NH4+ through ammonification, due to urea hydrolysis (Kumar et al. 2021). Adding UIs will delay the urea hydrolysis process by controlling the rate of urea transformations and inhibiting the active site of urease enzyme in the form of H-bonding and/or hydrophobic interactions (Byrne et al. 2020). As a result, UIs will slowly release NH4+ to the soil. However, the remaining urea fertilizer might have greater potential to be lost to the environment, resulting in a decreased amount of urea fertilizer and, subsequently, lower urea conversion to NH4+. The effect of the NIs on urea application was the same as for the 1st fertilization, resulting in an expected higher level of NH4+ in the soil. Therefore, these UIs and combined UIs and NIs had a diverse effect on the soil NH4+.
For both the 1st and 2nd fertilizations, the synthetic and natural inhibitors used in this study had a similar inhibitory efficiency on soil NH4+ and NO3− (Figs. 2 and 3). Notably, during the 2nd fertilization, the sole or combined uses of UIs and NIs produced similar effects on the soil NH4+ and NO3− (Figs. 2 and 3). These observations suggested that both synthetic and natural inhibitors or the sole and combined uses of UIs and NIs were of comparable efficiency on soil inorganic N under the conditions applied in this study. Perhaps further investigation of inhibitory effects on other variables, such as the microbial population and its activity, might provide a better understanding on N fertilization transformation in the soil.
Average Soil Inorganic N
Figure 4 shows the relationship between the average soil NH4+ and NO3− across sampling time with N fertilizer rate. For the only fertilizer application, soil NH4+ and NO3− seemed to increase, with an increase in the N rate for both the topsoil and subsoil in the planted and unplanted plots. This observation regarding the N rate on average soil NH4+ and NO3− suggested that the N fertilizer rate of F was excessive and resulted in a greater loss to the environment compared to S.
Relationship between N application rate and soil NH4+-N under field experiment on a topsoil unplanted plot, b subsoil unplanted plot, c topsoil sugarcane plot and d subsoil sugarcane plot. Each point is mean of 3 and 4 replicates on unplanted and sugarcane plots, respectively. Line plot is for no inhibitor applied. Lowercase letters indicated significantly different (P < 0.05), according to Duncan’s multiple range test. Inset graph shows magnified treatments of UIs and NIs for circled area on main graph
The average soil NH4+ and NO3− in all UIs and NIs seemed to be in a same range as for S, except for soil NH4+ in the planted plot being slightly higher than for S (Figs. 4 and 5). This inhibitory effect was less pronounced for the average soil inorganic N across cropping season, due to the low N rate applied, with the inhibition lasting for a short period after application. In addition, both (1) synthetic and natural inhibitors and (2) sole and combined use of UIs and NIs produced similar inhibitory effects on the average soil inorganic N. This observation suggested that all inhibitors were competitive efficiency on soil inorganic N in this study.
Relationship between N application rate and soil NO3−–N under field experiment on a topsoil unplanted plot, b subsoil unplanted plot, c topsoil sugarcane plot and d subsoil sugarcane plot. Each point is mean of 3 and 4 replicates on unplanted and sugarcane plots, respectively. Line plot is for no inhibitor applied. Lowercase letters indicated significantly different (P < 0.05), according to Duncan’s multiple range test. Inset graph shows magnified treatments of UIs and NIs for circled area on main graph
Crop Yields and N Uptake
The N rate and inhibitor effects on crop yields and N uptake are shown in Fig. 6. For plots where only fertilizer was applied, the cane and sugar yields and N uptake increased with an increasing rate of N applied (C < S < F) and fitted well with quadratic equations (Fig. 6). Fortes et al. (2013) also observed a positive response of sugarcane to N levels, with both linear and quadratic relationships. The cane yields and N uptake were significantly (p < 0.05) higher in the F (102.38 t ha−1 and 112.88 kg ha−1) than S (91.67 t ha−1 and 88.01 kg ha−1), with increases of 12% and 28%, respectively. All treatments with UIs and NIs had a similar or higher yields and N uptake than for F, while all were significantly higher than for S. Notably, the natural inhibitors (neem, garlic and praxelis) seemed to have greater (but inconsistent) cane and sugar yields than the synthetic inhibitors, except for the DM/DM that was the highest for all yields and N uptake among all treatments (p < 0.05) (31% yield increase from the S). This was consistent with another study where the N uptake increased due to increasing the N rate until the optimum rate (80 kg ha−1), above which there were no further increases (Salvagiotti et al. 2009).
Relationship between N application rate and a sugarcane yield, b sugar yield and c N uptake. Each point is mean of 4 replicates with regression from no inhibitor applied. Lowercase letters indicated significantly different (P < 0.05), according to Duncan’s multiple range test. Inset graph shows magnified treatments of UIs and NIs for circled area on main graph
These findings suggested that the N rate based on soil analysis (S) might be insufficient for sugarcane plants, resulting in lower crop yields and N uptake, compared to the N rate applied by farmers (F). These N rate effects were also noticed in soil inorganic N (Figs. 2 and 3). Notably, the applications of the UIs and NIs with the N rate by soil analysis increased yields and N uptake and had comparable yields with the N rate applied by farmers. Possible causes for this result included: (1) the UIs may have reduced urea hydrolysis due to the delayed N release into the soil (Pawlick et al. 2019) and the NI mitigated microbial activity that converted NH4+ to NO3− through nitrification (Norton and Ouyang 2019), resulting in maintaining N in the soil longer and increasing N absorption by plants; (2) the maximum plant uptake was limited to what the crop needed and any excessive rate of N fertilization would limit the yield potential (Sun et al. 2020; Zhao et al. 2019); (3) the increase in NH4+ availability was affected by the inhibitors used that may have promoted nutrient use by the plant; and (4) the soil texture and soil organic matter play an important factor in yield and N uptake (Cambouris et al. 2016). The fine textured soil in this study (clayey soil and high organic matter) induced higher and longer N in soil, resulting in better sorbed NH4+ on surface of soil particles and lower N leaching and loss (Chen et al. 2020). Therefore, in this study, the UI and NI effects in this clayey soil on soil N transformation and crop yields were more pronounced, as evidenced in higher yield response (up to 31% increase). Also notable was that the DM/DM application produced the highest yields and N uptake over all treatment, including F. However, this level of DM/DM did not have such an effect on soil inorganic N, which might require further study to understand this effect.
Principal Component Analysis
Figure 7 shows the plot of the first two components obtained from the factor analysis among soil inorganic N, crop yields and N uptake, which explained 84.5% of the total variance. In the first component (69.6% of the total variance), F and S (with and without inhibitors) were grouped with a positive loading, while C was separated with a highly negative loading. The second component (14.9% of the total variance) was highly positively explained by F, while the others (C, S and inhibitors) were grouped with a lower loading range. The first component was well explained by soil inorganic N and the second component by crop yield and N uptake in this study.
As shown in Fig. 7, all treatments were composed of three groups: Group 1 (F), Group 2 (S with and without inhibitors) and Group 3 (C). In addition of Group 2, all synthetic and natural UIs and NIs were slightly closer than S. This observation was in agreement with the results mentioned in the soil inorganic N and crop yield and N uptake above, where all inhibitors had a similar trend on these variables that somewhat differed for F, S and C. In addition, the result showed the three groups were separated by the influence of the N fertilizer rates applied, indicating that N rate played an important role on soil N and crop yield in this study.
Overall, the current findings suggested that using natural or biological inhibitors might be worthwhile promoting to farmers. Other studies also considered that biological inhibitors are stable, readily available and biodegradable and have a long-term effect, with no side effects and a high benefit-to-cost ratio (Arora and Srivastava 2013; Kiran and Patra 2003; Patra et al. 2007) and thus, show potential for sustainable agriculture (Otaka et al. 2021; Subbarao et al. 2013).
Conclusions
The findings from this study suggested that the N rate applied by farmers seemed to be excessive for sugarcane cultivation, resulting in greater soil inorganic N. The N rate based on soil analysis was relatively low and caused a limiting effect on sugarcane yields and N uptake. This study found that when the applied N rate was based on soil analysis, adding UIs, NIs and their combination was of importance in retarding urea hydrolysis and NH4+ conversion to NO3− via the nitrification process in the soil. These UIs and NIs effects helped to maintain soil NH4+ supplied from both ammonium and urea fertilizers and remained or enhanced sugarcane yields and N uptake, in a comparable to N rate by farmer. As a result, addition of UIs and NIs might be a potential solution to reduce N fertilizer use while maintaining or enhancing yields and N uptake in sugarcane cultivation. In addition, reducing the N rate applied and adding UIs and NIs might possibly decrease N fertilizer losses from the soil, resulting in environmental benefits. In this study, the UIs and NIs or the synthetic and natural inhibitors seemed to have similar levels of efficiency in maintaining soil inorganic N; however, the natural inhibitors might be of interest due to their slight benefits regarding sugarcane yields and N uptake. Further investigation might be required, involving other variables to detect UI and NI effects on N fertilizer transformation or any side effects of praxelis.
References
Arora, Kavita, and Alka Srivastava. 2013. Nitrogen losses due to nitrification: Plant based remedial prospects. International Journal of Bioassays 2: 984–991.
Bhanuvally, M., Y.M. Ramesha, and H. Yogeeshappa. 2017. Nutrient uptake and millable cane yield of sugarcane as influenced by application of slow releasing nitrogen fertilizers. International Journal of Current Microbiology and Applied Sciences 6: 855–862. https://doi.org/10.20546/ijcmas.2017.610.102.
Bremner, J.M. 1990. Problems in the use of urea as a nitrogen fertilizer. Soil Use and Management 6 (2): 70–71. https://doi.org/10.1111/j.1475-2743.1990.tb00804.x.
Bremner, J.M., A.M. Blackmer, and S.A. Waring. 1980. Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biology and Biochemistry 12 (3): 263–269. https://doi.org/10.1016/0038-0717(80)90072-3.
Bremner, John M. 1997. Sources of nitrous oxide in soils. Nutrient Cycling in Agroecosystems 49 (1): 7–16. https://doi.org/10.1023/A:1009798022569.
Byrne, Maria P., John T. Tobin, Patrick J. Forrestal, Martin Danaher, Chikere G. Nkwonta, Karl Richards, Enda Cummins, Sean A. Hogan, and Tom F. O’Callaghan. 2020. Urease and nitrification Inhibitors—as mitigation tools for greenhouse gas emissions in sustainable dairy systems: A review. Sustainability 12 (15): 1–35. https://doi.org/10.3390/su12156018.
Cambouris, Athyna N., Noura Ziadi, Isabelle Perron, Khaled D. Alotaibi, Mervin St, Nicolas Tremblay Luce, and X. Yang. 2016. Corn yield components response to nitrogen fertilizer as a function of soil texture. Canadian Journal of Soil Science 96 (4): 386–399. https://doi.org/10.1139/cjss-2015-0134.
Cameron, K.C., H.J. Di, and J.L. Moir. 2013. Nitrogen losses from the soil/plant system: A review. Annals of Applied Biology 162 (2): 145–173. https://doi.org/10.1111/aab.12014.
Chen, Deli, Helen C. Suter, Arshad Islam, and Robert Edis. 2010. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biology and Biochemistry 42 (4): 660–664. https://doi.org/10.1016/j.soilbio.2009.12.014.
Chen, Long-Fei., Zhi-Bin. He, Wen-Zhi. Zhao, Ji-Liang. Liu, Hai Zhou, Jing Li, Yang-Yang. Meng, and Li-Sha. Wang. 2020. Soil structure and nutrient supply drive changes in soil microbial communities during conversion of virgin desert soil to irrigated cropland. European Journal of Soil Science 71 (4): 768–781. https://doi.org/10.1111/ejss.12901.
Drury, C.F., X. Yang, W.D. Reynolds, W. Calder, T.O. Oloya, and A.L. Woodley. 2017. Combining urease and nitrification inhibitors with incorporation reduces ammonia and nitrous oxide emissions and increases corn yields. Journal of Environmental Quality 46 (5): 939–949. https://doi.org/10.2134/jeq2017.03.0106.
Fortes, Caio, Paulo César Ocheuze. Trivelin, André César. Vitti, Rafael Otto, Henrique Coutinho Junqueira. Franco, and Carlos Eduardo Faroni. 2013. Stalk and sucrose yield in response to nitrogen fertilization of sugarcane under reduced tillage. Pesquisa Agropecuária Brasileira 48 (1): 88–96. https://doi.org/10.1590/S0100-204X2013000100012.
Greenberg, A.E., L.S. Clesceri, and A.D. Eaton. 1992. Standard methods for the examination of water and wastewater. Washington DC: American Public Health Association.
Hirel, Bertrand, Thierry Tétu, Peter J. Lea, and Frédéric. Dubois. 2011. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 3 (9): 1452–1485. https://doi.org/10.3390/su3091452.
Hooper, A.B., T. Vannelli, D.J. Bergmann, and D.M. Arciero. 1997. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Van Leeuwenhoek 71 (1–2): 59–67. https://doi.org/10.1023/A:1000133919203.
Johnston, A.M., and T.W. Bruulsema. 2014. 4R nutrient stewardship for improved nutrient use efficiency. Procedia Engineering 83: 365–370. https://doi.org/10.1016/j.proeng.2014.09.029.
Kiran, Usha, and D.D. Patra. 2003. Medicinal and aromatic plant materials as nitrification inhibitors for augmenting yield and nitrogen uptake of Japanese mint (Mentha arvensis L. Var. piperascens). Bioresource Technology 86 (3): 267–276. https://doi.org/10.1016/S0960-8524(02)00143-8.
Kumar, M., N. Sikri, S. Chahal, J. Sharma, B. Sharma, P. Yadav, M. Bhardwaj, D. Vashishth, P. Kadyan, S.K. Kataria, and S. Dalal. 2021. Urease inhibitory kinetic studies of various extracts and pure compounds from cinnamomum genus. Molecules 26 (13): 1–10. https://doi.org/10.3390/molecules26133803.
Liu, S., J.J. Wang, Z. Tian, X. Wang, and S. Harrison. 2017. Ammonia and greenhouse gas emissions from a subtropical wheat field under different nitrogen fertilization strategies. Journal of Environmental Sciences 57: 196–210. https://doi.org/10.1016/j.jes.2017.02.014.
Mathialagan, Ranitha, Nurlidia Mansor, Beshr Al-Khateeb, Muhammad Helmi Mohamad, and Muhammad Rashid Shamsuddin. 2017. Evaluation of allicin as soil urease inhibitor. Procedia Engineering 184: 449–459. https://doi.org/10.1016/j.proeng.2017.04.116.
McCarty, G.W., and J.M. Bremner. 1989. Inhibition of nitrification in soil by heterocyclic nitrogen compounds. Biology and Fertility of Soils 8 (3): 204–211. https://doi.org/10.1007/BF00266480.
Nguyen, Cuong C., Thanh Q. C. Nguyen, Kenji Kanaori, Tran Duy Binh, Le. Van Vang, and Kaeko Kamei. 2021. Isolation and identification of antibacterial and antifungal compounds from Praxelis clematidea R. M. King & H. Robinson as an effective potential treatment against rice pathogens. Agronomy 11 (11): 2366–2376. https://doi.org/10.3390/agronomy11112366.
Norton, J., and Y. Ouyang. 2019. Controls and adaptive management of nitrification in agricultural soils. Frontiers in Microbiology 10: 1–18. https://doi.org/10.3389/fmicb.2019.01931.
Otaka, Junnosuke, Guntur Venkata Subbarao, Hiroshi Ono, and Tadashi Yoshihashi. 2021. Biological nitrification inhibition in maize—isolation and identification of hydrophobic inhibitors from root exudates. Biology and Fertility of Soils 58 (3): 251–264. https://doi.org/10.1007/s00374-021-01577-x.
Patra, D.D., M. Anwar, S. Chand, U. Kiran, D.K. Rajput, and S. Kumar. 2007. Nimin and Mentha spicata oil as nitrification inhibitors for optimum yield of Japanese mint. Communications in Soil Science and Plant Analysis 33 (3–4): 451–460. https://doi.org/10.1081/CSS-120002756.
Pawlick, Amy A., Claudia Wagner-Riddle, Gary W. Parkin, Aaron A. Berg, and Newton Lupwayi. 2019. Assessment of nitrification and urease inhibitors on nitrate leaching in corn (Zea mays L.). Canadian Journal of Soil Science 99 (1): 80–91. https://doi.org/10.1139/cjss-2018-0110.
Pengthamkeerati, P., and A. Modtad. 2016. Nitrification inhibitor, fertilizer rate, and temperature effects on nitrous oxide emission and nitrogen transformation in loamy sand soil. Communications in Soil Science and Plant Analysis 47: 1–8. https://doi.org/10.1080/00103624.2015.1122795.
Poffenbarger, Hanna, Mark S. Coyne, and Wilbur W. Frye. 2018. Nitrogen in soils/cycle. In Reference module in earth systems and environmental sciences, 1–13. Kentucky: Elsevier Publication.
Ruser, Reiner, and Rudolf Schulz. 2015. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils-a review. Journal of Plant Nutrition Soil Science 178 (2): 171–188. https://doi.org/10.1002/jpln.201400251.
Rütting, T., H. Aronsson, and S. Delin. 2018. Efficient use of nitrogen in agriculture. Nutrient Cycling in Agroecosystems 110 (1): 1–5. https://doi.org/10.1007/s10705-017-9900-8.
Sadhukhan, Rahul, Hanuman Singh Jatav, Suman Sen, Laimayum Devarishi Sharma, Vishnu D. Rajput, Rojeet Thangjam, Anoop Kumar Devedee, Satish Kumar Singh, Andrey Gorovtsov, Sourav Choudhury, and Kiranmay Patra. 2022. Chapter 8–biological nitrification inhibition for sustainable crop production. In Plant perspectives to global climate changes, ed. T. Aftab and A. Roychoudhury, 135–150. Cambridge: Academic Press.
Salvagiotti, Fernando, Julio M. Castellarín, Daniel J. Miralles, and Hugo M. Pedrol. 2009. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Research 113 (2): 170–177. https://doi.org/10.1016/j.fcr.2009.05.003.
Signor, Diana, and Carlos Eduardo Cerri. 2013. Nitrous oxide emissions in agricultural soils: A review. Pesquisa Agropecuária Tropical 43: 322–338. https://doi.org/10.1590/S1983-40632013000300014.
Stein, L.Y., and D.J. Arp. 1998. Loss of ammonia monooxygenase activity in nitrosomonas europaea upon exposure to nitrite. Applied and Environmental Microbiology 64 (10): 4098–4102.
Subbarao, G.V., T. Ishikawa, O. Ito, K. Nakahara, H.Y. Wang, and W.L. Berry. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant and Soil 288 (1–2): 101–112. https://doi.org/10.1007/s11104-006-9094-3.
Subbarao, G.V., K. Nakahara, T. Ishikawa, H. Ono, M. Yoshida, T. Yoshihashi, Y. Zhu, H.A.K.M. Zakir, S.P. Deshpande, C.T. Hash, and K.L. Sahrawat. 2013. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant and Soil 366 (1–2): 243–259.
Subbarao, G.V., K.L. Sahrawat, K. Nakahara, T. Ishikawa, M. Kishii, I.M. Rao, C.T. Hash, T.S. George, P. Srinivasa Rao, P. Nardi, D. Bonnett, W. Berry, K. Suenaga, and J.C. Lata. 2012. Chapter six–Biological nitrification inhibition—a novel strategy to regulate nitrification in agricultural systems. In Advances in agronomy, ed. D.L. Sparks, 249–302. Cambridge: Academic Press.
Subbarao, G.V., T. Yoshihashi, M. Worthington, K. Nakahara, Y. Ando, K.L. Sahrawat, I.M. Rao, J.C. Lata, M. Kishii, and H.J. Braun. 2015. Suppression of soil nitrification by plants. Plant Science 233: 155–164. https://doi.org/10.1016/j.plantsci.2015.01.012.
Sun, Jianbo, Wenbin Li, Chunqiang Li, Wenjun Chang, Shiqing Zhang, Yanbo Zeng, Changying Zeng, and Ming Peng. 2020. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Frontiers in Plant Science 11: 1–11. https://doi.org/10.3389/fpls.2020.613760.
Suter, H.C., H. Sultana, R. Davies, C. Walker, and D. Chen. 2016. Influence of enhanced efficiency fertilisation techniques on nitrous oxide emissions and productivity response from urea in a temperate Australian ryegrass pasture. Soil Research 54 (5): 523–532.
Thepphakhun, Tamonwan, and Suphannika Intanon. 2020. Total phenolics, flavonoids, antioxidant activity, and allelopathic potential of praxelis. Journal of Current Science and Technology 10: 59–65.
Upadhyay, R.K., D.D. Patra, and S.K. Tewari. 2011. Natural nitrification inhibitors for higher nitrogen use efficiency, crop yield, and for curtailing global warming. Journal of Tropical Agriculture 49: 19–24.
Wang, J., S. Hussain, X. Sun, P. Zhang, T. Javed, E.S. Dessoky, X. Ren, and X. Chen. 2022. Effects of nitrogen application rate under straw incorporation on photosynthesis, productivity and nitrogen use efficiency in winter wheat. Frontier in Plant Science 13: 1–13. https://doi.org/10.3389/fpls.2022.862088.
Yang, Jian Ni, Xiu Qiao Zhou, Xu Hua Nong, Jian Cao, Yang Hui, Mian Wen, and Wen Hao Chen. 2020. Phytochemical investigation of the flowers of Praxelis clematidea (Griseb.) R.M. King & H Rob. Natural Product Research 35: 3504–3508. https://doi.org/10.1080/14786419.2019.1709189.
Zhao, Hui, Xuyong Li, and Yan Jiang. 2019. Response of nitrogen losses to excessive nitrogen fertilizer application in intensive greenhouse vegetable production. Sustainability 11 (6): 1513–1527. https://doi.org/10.3390/su11061513.
Acknowledgements
The Thailand Research Fund provided financial support from the Royal Golden Jubilee Ph.D. program [Grant No. PHD/0154/2559] to Miss Phatchariya Welutung and Assoc. Prof. Dr. Patthra Pengthamkeerati. We are grateful for research area support from the Lop Buri Research Station, Faculty of Agriculture, Kasetsart University, Lop Buri province, Thailand. Lastly, we thank Assoc. Prof. Dr. Srunya Vajrodaya, Kasetsart University for technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Welutung, P., Pengthamkeerati, P., Tawornpruek, S. et al. Fertilizer Rate and Urease and Nitrification Inhibitors Effects on Soil Inorganic Nitrogen and Sugarcane Yields in Central Thailand. Sugar Tech 25, 1070–1081 (2023). https://doi.org/10.1007/s12355-023-01282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-023-01282-z