Abstract
This study aimed to investigate the plant growth-promoting ability of the four most abundant AMF species isolated from the sugarcane rhizosphere, Acaulospora denticulata (ACD), Racocetra fulgida (RAF), Rhizophagus clarus (RHC) and Glomus sp.7 (GLS), on the cultivation of sugarcane under pot conditions. The results showed that GLS and RHC significantly increased root colonization, root morphology (root length, root surface area, root volume), P uptake, plant height and diameter, leaf area, relative water content, photosynthetic rate, stomatal conductance, transpiration rate and total biomass. In contrast, ACD and RAF only affected leaf area, plant height and root qualities. This is the first report of identification of ACD and RAF as the most abundant AMF species in sugarcane rhizosphere soils. Additionally, we are the first to show that these 2 AMF could affect some physiologies of sugarcane. Furthermore, Pearson's correlation analysis suggested that AMF root colonization was the most crucial factor affecting the plant growth parameters of sugarcane. This finding confirmed that inoculation with a specific AMF species could better enhance the growth of sugarcane. The results suggested that GLS and RHC could be used as effective biofertilizers for improving the growth of sugarcane under pot conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum species hybrids) is one of the most economically valuable crops and is used as a resource for sugar and energy production in many countries in tropical and subtropical regions. Thailand is the fifth-largest sugar producer and the second-largest sugar exporter in the world (Shahbandeh 2021a; b). Global sugar production for the marketing year of 2021/22 is forecasted to rise to 186 million tonnes (USDA 2021), resulting in a high demand for sugarcane cultivation. Therefore, it is important to find effective ways to scale up sugarcane production.
Chemical fertilizers have been commonly applied to increase the yield and productivity of sugarcane due to their ease of use and efficiency. Nitrogen and potassium are required in large amounts, and their uptake corresponds to the pattern of biomass accumulation. Phosphorus (P) is also essential for tillering and root and shoot growth (Kingston 2014). The use of mineral phosphorus fertilizer is also relatively inefficient because of the strong sorption to and fixation by metal oxides in the soil matrix as insoluble, immobilized, and/or precipitated forms. Moreover, phosphate rock, the source of mineral P fertilizers, is a finite natural resource that is expected to last for only a few hundred years based on the current rate of consumption (Cordell et al. 2009; Gilbert 2009; Walan et al. 2014; Baker et al. 2015). Due to the limited rate of P mobility around the root zone, the circulation of available P has become a serious concern for agricultural purposes. Therefore, one of the alternative methods for P soil amendments is the use of microorganisms capable of P utilization, such as arbuscular mycorrhizal fungi (AMF). In this respect, interest has increased in the role of AMF as an alternative way to promote sugarcane production. AMF play an important role in agroecosystems by increasing the availability and translocation of various nutrients, particularly P, by extending extramatrical hyphae through rhizospheric zones (Smith and Read 2008; Rouphael et al. 2015). Moreover, AMF improve the safeguarding of plants from fungal pathogens and several abiotic stress factors (Jung et al. 2012; Sun et al. 2018). Furthermore, AMF may affect atmospheric CO2 fixation by facilitating photosynthetic rates in host plants by increasing the C sink and the movement of photoassimilates from the aerial parts to the roots (Gavito et al. 2019).
Several species of AMF have symbiotic associations with sugarcane (Kumalawati et al. 2014; Pontes et al. 2017; Rokni and Goltapeh 2011). A previous study by our group reported that the application of AMF (Funneliformis mosseae) and mineral P fertilizer significantly increased sugarcane productivity and soil fertility under field conditions compared to those of noninoculated plants (Juntahum et al. 2020). Therefore, the aim of this work was to isolate and select AMF species to enhance the growth of sugarcane and help plants take up P under greenhouse conditions.
Materials and Methods
Soil Sampling
Sugarcane rhizosphere soils were collected from four sugarcane fields where sugarcane has been semiorganically and organically cultivated as a monoculture for longer than 3 years. The sugarcane fields were located in northeastern Thailand with latitudes and longitudes of 15°40′45.6″N and 102°12′14.0″E, 15°38′23.2″N and 102°12′23.9″E, 15°36′43.5″N and 102°12′41.5″E and 15°43′16.3″N and 102°17′08.4″E. Soil samples (500–700 g per sampling point) were randomly collected from 10 points per area at a depth of 0–20 cm. Each soil sample was kept in a plastic bag and then air-dried for the isolation and quantification of AMF spores.
Isolation of Native AMF
AM fungal spores were isolated from 100 g soil samples. The spores were extracted using a wet sieving and decanting technique (Gerdemann and Nicolson 1963). Suspension of soil in tap water was decanted through a series of sieves with the following apertures: 250 µm, 125 µm, 90 µm and 63 µm, stacked from top to bottom, respectively. The AM fungal spores were collected from each sieve in Petri dishes and then visualized under a stereomicroscope for identification and multiplication of spores.
Identification of Native AM Fungi
The isolated AMF spores were separated based on morphology differences to count the number of spores of each morphotype, in which the dominant AMF species were then determined. After that, identification of dominant AM fungal species was carried out based on spore morphological characteristics following the instruction manual for the identification of VA mycorrhizal fungi (Schenck and Pérez, 1990) and the Glomeromycota species list (amf-phylogeny 2018). The native AM fungal spores of the highly abundant species from each sugarcane monoculture field at coordinates 15°40′45.6″N and 102°12′14.0″E, 15°38′23.2″N and 102°12′23.9″E, 15°36′43.5″N and 102°12′41.5″E and 15°43′16.3″N and 102°17′08.4″E were found to be Acaulospora denticulata, Racocetra fulgida, Rhizophagus clarus and Glomus sp.7, respectively.
Production of AMF Inocula from Native AMF Spores
The dominant AM fungal species from each field were used for multiplication by the pot culture technique, with maize as a host plant (Boonlue et al. 2012). Maize seeds were surface sterilized by soaking in 10% (v/v) NaClO solution for 10 min. The sterile seeds were placed in sterilized glass plates containing moist tissue paper to allow germination for 5 days. Each AM fungal species was surface-sterilized by washing with 2% (w/v) chloramine-T solution for 3 min. Five healthy maize seedlings were transplanted in a pot containing 2.5 kg of twice-sterilized soil. The seedlings were put into a premade hole in the soil, and then, a spore suspension containing 10 viable spores was inoculated onto the roots of the seedlings and covered with soil. Plants were watered with filtered tap water once a day without additional fertilizer applied and grown for 90 days under a semi-open-sided greenhouse, a mobile shelter with 3-sided walls. The surface of the soil was covered with plastic sheets. The plants were exposed to natural photoperiods at 22–31 °C from October to December 2018.
After that, plant shoots were cut, and then, the soil was air-dried and crushed. For the determination of spore abundance, AM fungal spores in the soil were isolated by sucrose centrifugation according to the method of Daniels and Skipper (1982). Five grams of soil sample were mixed with 20 mL of tap water and then centrifuged at 4000 rpm for 5 min, and the supernatant was discarded. Spores were resuspended in 20 mL of the 50% sucrose solution and lysed after centrifugation at 3000 rpm for 1 min. The supernatant was poured onto sieves with the smallest pore size and then carefully rinsed with tap water. The spores were filtered on Whatman paper No. 4 and counted under a stereomicroscope. Soil inoculums containing pure spores that showed successful spore multiplications were selected for use in further experiments. Roots were stained according to the method of Koske and Gemma (1989). The percentage of AMF colonization was determined according to the method described by Trouvelot et al. (1986). AMF propagules in the form of soil media consisting of AMF spores, hyphal fungal fragments in dry soil and root fragments were used as AMF inocula for further experiments.
Experimental Design and Sugarcane Plantation
The experiment was carried out using a completely randomized design (CRD) including 5 treatments with 3 replications, in which there were 3 sub-replications for each replication. The experiment consisted of 4 treatments of inoculation with different AM fungal isolates, including Acaulospora denticulata (ACD), Racocetra fulgida (RAF), Rhizophagus clarus (RHC) and Glomus sp.7 (GLS). A treatment without AM fungal inoculation was set up as a control.
A pot experiment was conducted from March to June 2019 in a semi-open-sided greenhouse, a mobile shelter with 3-sided walls covering plantations from natural disturbances such as rain and wind. The ground was covered with plastic sheets to avoid such disturbances. The temperature in the shelter ranged from 22 to 37 °C. The cultivation site was located at Khon Kaen University’s agronomic farm in Khon Kaen, Thailand (16°28′13.3″N + 102°48′35.5″E, 200 m above mean sea level). The soil used in this experiment was collected from an agricultural field (16°32′18.2″N + 102°46′39.8″E) with the following physicochemical properties: sandy loam type, pH 6.8; 2.7 g soil organic matter kg−1; 140 mg total N kg−1; 50 mg total P kg−1; 280 mg total K kg−1; 15 mg available P kg−1; 36 mg exchangeable K kg−1; 104 mg exchangeable Ca kg−1; and 56 mg exchangeable Na kg−1. The 2.5 kg soil was sterilized twice by autoclaving prior to use in the experiment.
Commercial sugarcane cultivar KK3 was used in this experiment. Healthy 8-month-old cane stalks were cut into pieces with a single budded chip each. Sugarcane-budded chips were germinated in plastic seedling bags (0.2 × 0.6 m) containing twice-sterilized soils mixed with rice husk charcoal at a ratio of 1:1 (v/v) for 30 days. After that, uniform and healthy sugarcane seedlings were selected for transplanting into pots containing 15 kg of twice-sterilized soil. In the AM fungal treatment, 4–5 g of soil inoculum (20–25 spores g−1 soil inoculum), which accounted for a total of 100 spores per plant, was inoculated onto the plant’s root ball adjacent to the roots of sugarcane seedlings. Plants were watered (300 mL) once a day. One hundred millilitres of a half-strength modified Hoagland’s nutrient solution without P (Hoagland and Arnon 1950) were applied to each pot once a month. The plants were grown for 120 days before carrying out the plant growth performance analysis.
Plant Growth Performance Analysis
Plant growth performance was assessed 120 days after transplanting. Plant height was measured from the ground to the insertion of the top visible dewlap (TVD) leaf blade (Brandes 1952) using a metre ruler. Stalk diameter was measured at the bottom, middle and top of the stalk using a Vernier calliper.
The physiological parameters of sugarcane were also determined. The leaf below the insertion of the TVD was used for the measurement of the SPAD chlorophyll metre reading (SCMR), photosynthesis and relative water content (RWC). First, the chlorophyll content was measured at the base, middle and tip of the leaf using a SPAD metre (SPAD-502, Konica Minolta, Japan) from 9 to 10 a.m. (Jangpromma et al. 2010). Second, photosynthesis parameters, including net photosynthetic rate (PR), stomatal conductance (SC) and transpiration rate (TR), were measured in the middle part of the leaf from 10 to 11 a.m. using a LI-6400 portable open gas-exchange system (LI-6400, LI-COR, Lincoln, NE, the USA). The parameters were measured under the following processing conditions: photosynthetically active radiation of 1,000 µmol m−2 s−1; CO2 concentration of 350 µmol mol−1; leaf temperature of 25 °C; leaf humidity of 35–50%; and air flow rate of 0.5 dm3 min−1. Finally, the leaf was cut into fragments 3–5 cm in length without leaf margin and leaf midrib. The leaf fragments were kept in a glass-covered container from 9 to 11 a.m. Their fresh weight (FW) was immediately measured. After that, the leaf fragments were soaked in deionized water at room temperature for 24 h under dark conditions to measure the turgid weight (TW). Then, the leaf fragments were dried at 70 °C for 72 h to measure the dry weight (DW). RWC was calculated according to Matin et al. (1989) using the formula below:
To harvest plants from the pot, plants were cut at a position just above the soil surface. Plant shoots (stems, dead and fresh leaves, and TVD) were weighed. Leaf area was measured from fresh leaves using a leaf area metre (Li-3100C Area Meter, USA). Roots of sugarcane plants were carefully washed with tap water, and excess water was removed using tissue paper before measuring the FW. Then, plant roots were scanned with an Epson scanner V700 PHOTO. Plant root characteristics, including root length (RL), root surface area (RS), root volume (RV) and root diameter (RD), were analysed using WINRHIZO Pro2004a software (REGENT Instruments Inc., QC, Canada). AMF colonization in plant roots was also determined. To measure P uptake in plants, plant shoots were crushed into fine powders, and then, the amount of P was determined by a wet oxidation method using a spectrophotometer (U-5100 spectrophotometer Hitachi, Japan).
Statistical Analysis
Sugarcane growth parameter values were reported as the means ± standard errors (SE) of triplicate data. The data were subjected to analysis of variance (ANOVA) for CRD, and then, the mean values of all treatments were compared based on the least significant difference (LSD) test at a significance level of p ≤ 0.05. Pearson’s correlation coefficient was applied to assess relationships among plant growth parameters at a significance level of p ≤ 0.05. All statistical analyses were conducted using the Statistic programme version 10.0.
Results
Growth and Physiology of Sugarcane
The growth parameters of sugarcane plants inoculated with four different AMF species were investigated in comparison to those of the noninoculated plants. Plant growth parameters, including plant height, diameter, leaf area, SCMR, RWC, PR, SC, and TR, are shown in Table 1. All plant growth parameters of plants inoculated with AMF were significantly higher than those of the noninoculated plants (the control). This suggested that all 4 AMF species could enhance the growth of sugarcane. The highest values of plant height, diameter, leaf area, RWC, PR, SC and TR were found in plants inoculated with Glomus sp.7 (GLS). Similarly, plants inoculated with Rhizophagus clarus (RHC) had significant increases in plant height, diameter, PR, SC and TR compared to the control. In contrast, Acaulospora denticulata (ACD) and Racocetra fulgida (RAF) could significantly increase only leaf area and plant height, respectively. Additionally, SCMR values were not significantly different among treatments, which suggested that GLS and RHC were more effective plant growth promoters than ACD and RAF.
Plant Root Parameters
AMF structures, including vesicles, arbuscules and hyphae, were observed in the roots of inoculated plants, with %root colonization ranging from 31.50 to 58.33% (Table 2). The highest %root colonization was found in plants inoculated with GLS. The longest roots of ~ 57–59 m were found in plants inoculated with GLS and RHC, which were significantly longer than the control plant roots (~ 43 m). Likewise, GLS and RHC were able to significantly increase root surface area up to ~ 2,480–2,545 cm2, while the area of the noninoculated plant roots was only ~ 1,667 cm2. The root volume also increased from ~ 10.3 cm3 to ~ 16.7–18.2 cm3 when plants were inoculated with GLS, RHC and RAF. In contrast, the root diameter of plants inoculated with AMF was not significantly different from that of the control plants. Moreover, ACD did not have a significant effect on any root characteristics of sugarcane plants. All these results suggested that GLS, RHC and RAF, but not ACD, played an important role in enhancing sugarcane root qualities.
Plant Biomass
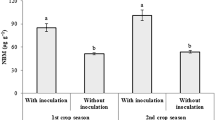
Figure 1 shows plant biomass results, including root and shoot dry weights (Fig. 1A) and P uptake (Fig. 1B), in sugarcane plants inoculated with AMF compared to the noninoculated plants. Similar to the other results, GLS was the most efficient AMF in this study, in which it significantly enhanced root dry weight (root DW), shoot dry weight (shoot DW) and P uptake. Moreover, RHC increased root DW and shoot DW compared to those of the control plants. In contrast, the root DW, shoot DW and P uptake of plants inoculated with RAF and ACD were not significantly different from those of the noninoculated controls. These results suggested that GLS and RHC were effective plant growth promoters for sugarcane.
Root and shoot dry weight (DW) (A) and P uptake (B) of sugarcane plants 120 days after transplantation. All treatments were as follows: control, noninoculated plants; ACD, plants inoculated with Acaulospora denticulata; RAF, plants inoculated with Racocetra fulgida; RHC, plants inoculated with Rhizophagus clarus; and GLS, plants inoculated with Glomus sp.7 Each value is the means ± SE of triplicate data. Different letters indicate significant differences among values within each parameter when analysed using LSD at p ≤ 0.05
To investigate the relationship among factors affecting plant growth parameters, Pearson’s correlation analysis was performed, and the results are shown in Table 3. The analysis results indicated that root characteristics, especially AMF root colonization and root volume, were the factors that most affected the plant growth parameters of sugarcane. Several plant growth parameters, including root volume (RV), leaf area, photosynthetic rate (PR), stomatal conductance (SC), transpiration rate (TR), P uptake, and plant biomass (shoot, root and total DW), were positively correlated with AMF root colonization. In this regard, root DW had the greatest effect on the total DW of plants. Likewise, RV had significantly positive correlations with plant height, leaf area, TR, P uptake, shoot DW and total DW. Additionally, the analysis suggested that the characteristics of the aboveground parts of plants, especially leaf area, PR, SC, TR, and shoot DW, had significant effects on plant growth parameters. The results showed that leaf area was positively correlated with relative water content (RWC), PR, SC, TR, and shoot DW. PR, SC, TR, and shoot DW had significantly positive correlations with plant total DW, while only TR and shoot DW had positive correlations with shoot DW and root DW, respectively. Moreover, PR and SC were significantly correlated with TR, while only PR had a positive correlation with SC.
Discussion
In this study, 4 AMF species, Acaulospora denticulata (ACD), Racocetra fulgida (RAF), Rhizophagus clarus (RHC) and Glomus sp.7 (GLS), were the most abundant AMF species isolated from rhizospheric soils collected from sugarcane fields. The effects of these AMF species on enhancing the growth and yield of sugarcane under pot conditions were investigated. The results showed that most of these AMF, especially GLS and RHC, could improve sugarcane growth and biomass when applied under pot conditions. In contrast, ACD and RAF did not greatly affect the growth and biomass of sugarcane. The effects of Glomus sp.7 on plant growth in this study were commonly found among other species of Glomus. For example, the application of Glomus spores increased the formation of the rooting system and lengthened the roots of sugarcane seedlings grown on tissue culture media (Muniyamma et al. 2000). Similarly, the growth of cacao seedlings was promoted by the inoculation of AMF spores of the native species of Glomus sp. (Ramírez et al. 2016). Such an effect on the growth of seedlings was also found in the case of cucumber and tomato plants (Yildiz 2010). Moreover, we found that Rhizophagus clarus in this study played an important role in enhancing the root characteristics of sugarcane plants. This agreed with other previous reports. For example, the inoculation of Rhizophagus clarus could improve the specific root length and root density of Tectona grandis (Alexandre et al. 2021). Another report by Goetten et al. 2016 also found that this AMF species could increase the plant height and stem diameter of Luehea divaricata, Centrolobium robustum and Cedrela fissilis. Different results, however, were found in the case of sugarcane plants, in which inoculation with Rhizophagus clarus alone had little effect on sugarcane growth. The effects were pronounced when an inoculation of AMF was used in combination with compost (30 t ha−1), especially in terms of an increase in root dry mass (Mattos Abreu et al. 2021). This finding contradicted the results in our work that RHC could effectively enhance sugarcane growth. This suggested that the plant growth-promoting effects of Rhizophagus clarus depended upon the type of plant and other environmental factors during cultivation. Accordingly, a study showed that the inoculation of Rhizophagus clarus increased the leaf area and P content of sugarcane variety SP81-3250 grown in soil with high P levels (9.35 mg kg−1) (Fors et al. 2020). This work indicated that the level of nutrient contents in soils also influenced the functions of AMF.
Variation in the plant growth-promoting ability of AMF was also found in the species Acaulospora sp. and Racocetra fulgida. In our work, Acaulospora denticulata and Racocetra fulgida had fewer effects on promoting the growth and yield of sugarcane than other native AMF species. These results were in agreement with some previous studies. For example, root colonization in cacao seedlings of Acaulospora sp. was lower than that of Glomus sp.7 Additionally, the inoculation with Acaulospora sp. could not greatly improve plant dry weight, while its effect on increasing the P content in the plants was more significant than that in the noninoculated plants (Ramírez et al. 2016). Similar results were found in the case of chilli, in which Acaulospora denticulata had the least effect on promoting the growth of chilli when compared to other AMF species, including Gigaspora albida, Glomus geosporum, Scutellospora corolloidea and Scutellospora scutata (Nimasow and Singh 2020). Likewise, limited effects on plant growth promotion of Racocetra fulgida were still observed even though the culture was coinoculated with other efficient AMF species (Middleton et al. 2015). However, we are the first to investigate the plant growth-promoting effects of Acaulospora denticulata and Racocetra fulgida in sugarcane plants. Although these 2 AMF species showed the least effects on enhancing the growth of sugarcane, they could significantly increase the leaf area and root volume of the inoculated plants.
Furthermore, we found that the level of root colonization was a main factor affecting the efficiency of AMF to increase plant growth parameters in sugarcane. This was evidenced in the case of GLS, which had the highest root colonization and thus was the most effective plant growth promoter among the other 3 tested AMF species. This finding agreed with a study on the effects of Rhizophagus irregularis BEG 72 on leek plant growth, which showed that this AMF had high root colonization and could stimulate shoot development of leek plants grown both in vivo and in in vitro culture (Calvet et al. 2013). Moreover, it is known that AMF species belonging to the Glomeraceae possess a better ability to colonize new plant roots via extension of their mycelium into root fragments (Tommerup and Abbott 1981) than the AMF species in the families Acaulosporaceae and Gigasporaceae (Hart and Reader 2002). An improvement in root qualities in AMF-inoculated plants is a result of carbon allocation from soils to the rooting system (Lambers et al. 2009; Wu et al., 2015). Thus, AMF-inoculated plants have higher photosynthesis rates (PR), respiration rates and plant biomass (Miller et al. 2002; Mishra et al., 2009). Our correlation analysis (Table 3) agreed with this claim, in which root colonization was the factor that positively correlated with almost all plant growth parameters, such as root qualities (i.e. root volume, root dry weight), PR, stomatal conductance (SC), transpiration rate (TR), relative water content (RWC), total plant biomass and P uptake. Similarly, Sulistiono et al. 2020 also found a correlation between root colonization and nutrient uptake by plants. This result was similar to a previous report indicating that Rhizophagus irregularis BGC BJ109, which had high root colonization, had a positive effect on RWC in black locust (Chen et al. 2017). This is probably because AMF hyphal networks allow higher hydraulic conductivity, resulting in enhanced plant water uptake and subsequently enhanced SC and TR (Kapoor et al. 2008; Sheng et al. 2008). Under optimal conditions, increases in SC can allow plants to increase CO2 and water uptake and subsequently enhance photosynthesis (Kusumi et al. 2012). The combined effects of nutrient uptake and photosynthesis were important factors for the increase in plant biomass (Usuda 2002; Tong et al. 2019), which was also found in our study.
Finally, all of the results found in our study suggested that the plant growth parameters of sugarcane seedlings were significantly improved by the inoculation of our AMF species, especially Glomus sp.7 (GLS) and Rhizophagus clarus (RHC). The efficiency of plant growth promotion by these 2 AMF species was pronounced even without the addition of any fertilizer. This clearly confirmed that GLS and RHC had the potential to be applied as mycorrhizal inoculants to promote the growth of the sugarcane cultivar KK-3, which is the most popular cultivar of sugarcane in Thailand.
In conclusion, 4 AMF species, Acaulospora denticulata (ACD), Racocetra fulgida (RAF), Rhizophagus clarus (RHC) and Glomus sp.7 (GLS), isolated from rhizosphere soils obtained from sugarcane fields were selected for use as biofertilizers in pot experiments to enhance the growth of sugarcane. Correlation analysis suggested that AMF root colonization was the most important factor affecting the plant growth parameters of sugarcane. The whole root morphology was significantly improved in the plants with effective mycorrhizal colonization. Meanwhile, a significantly greater P uptake in the plants with effective mycorrhizal colonization might be derived from a better root morphology. It was likely that mycorrhizal effects on root morphology and nutrient uptake resulted in enhanced photosynthesis. Therefore, increases in plant growth and biomass were obtained, explaining why the growth and yield of sugarcane could be enhanced by the inoculation of a specific AMF species. Among 4 AMF species in this work, we found that GLS and RHC were the most efficient plant growth promoters and could be suitable for further development in applications as biofertilizers for sugarcane growers in Thailand.
References
Alexandre, F.S., L.V.D. Flora, I.G. Henrique, D.C. da Silva, A.P. Mercedes, A.C. Silva, A.S. de Oliveira, M.P.B. da Silva, B.P.F. Ronning, D.R. Dreher, B.G. Cano, M.F.L. Andreata, J.B. Filho, E.R. Santos, F.H. Takisawa, R.F. Alfenas, G. Andrade, and M.V.T. Cely. 2021. Arbuscular mycorrhizal fungi (Rhizophagus clarus) and rhizobacteria (Bacillus subtilis) can improve the clonal propagation and development of teak for commercial plantings. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2021.628769.
amf-phylogeny.com. 2018. Glomeromycota SPECIES LIST. http://www.amf-phylogeny.com/. Accessed October 1, 2018.
Baker, A., S.A. Ceasar, A.J. Palmer, J.B. Paterson, W. Qi, S.P. Muench, and S.A. Baldwin. 2015. Replace, reuse, recycle: Improving the sustainable use of phosphorus by plants. Journal of Experimental Botany 66 (12): 3523–3540.
Boonlue, S., W. Surapat, C. Pukahuta, P. Suwanarit, A. Suwanarit, and T. Morinaga. 2012. Diversity and efficiency of arbuscular mycorrhizal fungi in soils from organic chili (Capsicum frutescens) farms. Mycoscience 53 (1): 10–16.
Brandes, E.W. 1952. Botany of Sugarcane. C. van Dillewijn. Waltham, Mass.: Chronica Botanica; New York: Stechert-Hafner, 1952. 371 pp $6.00. Science 116 (3013): 333–333.
Calvet, C., A. Camprubi, A. Pérez-Hernández, and P. Lovato. 2013. Plant growth stimulation and root colonization potential of in vivo versus in vitro arbuscular mycorrhizal inocula. HortScience 48 (7): 897–901.
Chen, J., H. Zhang, X. Zhang, and M. Tang. 2017. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Frontiers in Plant Science 8:1739. https://doi.org/10.3389/fpls.2017.01739.
Cordell, D., J.O. Drangert, and S. White. 2009. The story of phosphorus: Global food security and food for thought. Global Environmental Change 19 (2): 292–305.
Daniels, B.A., and H.D. Skipper. 1982. Method for the recovery and quantitative estimation of propagules from soil. In Method and principle of micorrhizal research, ed. N.C. Schenck, 29–36. Minessotta: American Phytopathological Society.
Fors, R.O., O.J.S. Júnior, M.A.C. Carneiro, and R.L.L. Berbara. 2020. Selection of arbuscular mycorrhizal fungi for sugarcane in four soils with the presence of darkseptate endophytes. Acta Scientiarum: Agronomy 42(1): e42477. https://doi.org/10.4025/actasciagron.v42i1.42477.
Gavito, M.E., I. Jakobsen, T.N. Mikkelsen, and F. Mora. 2019. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytologist 223: 896–907.
Gerdemann, J.W., and T.H. Nicolson. 1963. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society 46 (2): 235–244.
Gilbert, N. 2009. Environment: The disappearing nutrient. Nature 461: 716–718.
Goetten, L.C., G. Moretto, and S.L. Stürmer. 2016. Influence of arbuscular mycorrhizal fungi inoculum produced on-farm and phosphorus on growth and nutrition of native woody plant species from Brazil. Acta Botanica Brasilica 30 (1): 9–16.
Hart, M., and R. Reader. 2002. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytologist 153 (2): 335–344.
Hoagland, D.R. and D.I. Arnon. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station, Circular-347.
Jangpromma, N., P. Songsri, S. Thammasirirak, and P. Jaisil. 2010. Rapid assessment of chlorophyll content in sugarcane using a SPAD chlorophyll meter across different water stress conditions. Asian Journal of Plant Sciences 9: 368–374.
Jung, S.C., A. Martinez-Medina, J.A. Lopez-Raez, and M.J. Pozo. 2012. Mycorrhiza-induced resistance and priming of plant defenses. Journal of Chemical Ecology 38 (6): 651–664.
Juntahum, S., N. Jongrungklang, W. Kaewpradit, S. Lumyong, and S. Boonlue. 2020. Impact of arbuscular mycorrhizal fungi on growth and productivity of sugarcane under field conditions. Sugar Tech 22 (3): 451–459.
Kapoor, R., D. Sharma, and A.K. Bhatnagar. 2008. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Scientia Horticulturae 116 (3): 227–239.
Kingston, G. 2014. Mineral nutrition of sugarcane. In Sugarcane: Physiology, biochemistry, and functional biology, ed. P.H. Moore and F.C. Botha, 85–120. Oxford: John Wiley & Sons.
Koske, R.E., and J.N. Gemma. 1989. A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92: 486–488.
Kumalawati, Z., Y. Musa, N. Amin, L. Asrul, and I. Ridwan. 2014. Exploration of arbuscular mycorrhizal fungi from sugarcane rhizosphere in south Sulawesi. International Journal of Scientific & Technology Research 3 (1): 201–203.
Kusumi, K., S. Hirotsuka, T. Kumamaru, and K. Iba. 2012. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. Journal of Experimental Botany 63 (15): 5635–5644.
Lambers, H., C. Mougel, B. Jaillard, and P. Hinsinger. 2009. Plant-microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant and Soil 321: 83–115.
Matin, M.A., J.H. Brown, and H. Fergunson. 1989. Leaf water potential, relative water content, and diffusive resistance as screening techniques for drought resistance in barley. Agronomy Journal 81: 100–105.
Middleton, E.L., S. Richardson, L. Koziol, C.E. Palmer, Z. Yermakov, J.A. Henning, P.A. Schultz, and J.D. Bever. 2015. Locally adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere. https://doi.org/10.1890/ES15-00152.1.
Miller, R.M., S.P. Miller, J.D. Jastrow, and C.B. Rivetta. 2002. Mycorrhizal mediated feedbacks influence net carbon gain and nutrient uptake in Andropogon gerardii. New Phytologist 155: 149–162.
Mishra, B.S., M. Singh, and A. Laxmi. 2009. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4(2): e4502. https://doi.org/10.1371/journal.pone.0004502.
Muniyamma, M., B.K. Barti, and C.N. Reddy. 2000. Effect of VAM on root induction in vitro sugarcane (Saccharum officinarum L.) seedlings-a new technologue. Mycorrhiza News 12 (1): 13–16.
Nimasow, O.D., and R.K. Singh. 2020. Effect of arbuscular mycorrhizal inoculation on growth of chili plant (Capsicum frutescens L.) in nitrogens amended soil. Plant Cell Biotechnology and Molecular Biology 21 (41–42): 35–50.
Pontes, J.S., F. Oehl, F. Marinho, D. Coyne, and D.K.A.da Silva, A.M. Yano-Melo, and L.C. Maia,. 2017. Diversity of arbuscular mycorrhizal fungi in Brazil’s Caatinga and experimental agroecosystems. Biotropica 49 (3): 413–427.
Ramírez, J.G., L. Osorno, and N.W. Osorio. 2016. Presence of mycorrhizal fungi and a fluorescent Pseudomonas sp. in the rhizosphere of cacao in two agroecosystems and their effects on cacao seedling growth. Agronomía Colombiana 34 (3): 385–392.
Rokni, N., and E.M. Goltapeh. 2011. Diversity of arbuscular mycorrhizal fungi associated with common sugarcane varieties in Iran. International Journal of Agricultural Technology 7 (4): 1017–1022.
Rouphael, Y., P. Franken, C. Schneider, D. Schwarz, M. Giovannetti, and M. Agnolucci. 2015. Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Scientia Horticulturae 196: 91–108.
Schenck, N.C., and Y. Pérez. 1990. Manual for the identification of VA mycorrhizal fungi. Florida, USA: Gainesville.
Shahbandeh M. 2021a. Global sugar production by leading country 2020/2021a. https://www.statista.com/statistics/495973/sugar-production-worldwide/. Accessed October 1, 2021a.
Shahbandeh M. 2021b. Sugar: exports of major countries 2020/2021b. https://www.statista.com/statistics/273437/exported-amount-of-sugar-in-leading-countries/. Accessed October 1, 2021b.
Sheng, M., M. Tang, H. Chan, B. Yang, F. Zhang, and Y. Huang. 2008. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18: 287–296.
Smith, S.E., and D.J. Read. 2008. Mycorrhizal symbiosis, 3rd ed. London: Academic Press.
Sulistiono, W., B. Brahmantyo, S. Hartanto, H.B. Aji, and H.K. Bima. 2020. Effect of arbuscular mycorrhizal fungi and NPK fertilizer on roots growth and nitrate reductase activity of coconut. Journal of Agronomy 19: 46–53.
Sun, Z., J. Song, X. Xin, X. Xie, and B. Zhao. 2018. Arbuscular mycorrhizal fungal proteins 14–3-3- are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Frontiers in Microbiology 9: 91. https://doi.org/10.3389/fmicb.2018.00091.
Tommerup, I.C., and L.K. Abbott. 1981. Prolonged survival and viability of VA mycorrhizal hyphae after root death. Soil Biology and Biochemistry 13: 431–433.
Tong, Z., G. Quan, L. Wan, F. He, and X. Li. 2019. The effect of fertilizers on biomass and biodiversity on a semi-arid grassland of Northern China. Sustainability 11(10): 2854. https://doi.org/10.3390/su11102854.
Trouvelot, A., J. Kough, and V. Gianinazzi-Pearson. 1986. Mesure d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and genetical aspects of mycorrhizae, ed. V. Gianinazzi-Pearson and S. Gianinazzi, 217–221. Paris: INRA Press.
USDA. 2021. Sugar: World markets and trade. Usda, 1–8. Retrieved from https://www.fas.usda.gov/data/sugar-world-markets-and-trade. Accessed October 1, 2021.
Usuda, H. 2002. Evaluation of the effect of photosynthesis on biomass production with simultaneous analysis of growth and continuous monitoring of CO2 exchange in the whole plants of Radish, cv Kosena under ambient and elevated CO2. Plant Production Science 7 (4): 386–396.
Walan, P., S. Davidsson, S. Johansson, and M. Höök. 2014. Phosphate rock prodution and depletion: Regional disaggregated modeling and global implications. Resources, Conservation and Recycling 93: 178–187.
Wu, Q.S., A.K. Srivastava, and Y. Li. 2015. Effects of mycorrhizal symbiosis on growth behavior and carbohydrate metabolism of trifoliate orange under different substrate P levels. Journal of Plant Growth Regulation 34 (3): 495–508.
Yildiz, A. 2010. A native Glomus sp. from fields in Aydın province and effects of native and commercial mycorrhizal fungi inoculants on the growth of some vegetables. Turkish Journal of Biology 34: 447–452.
Acknowledgements
This research was financially supported by the Research and Researcher for Industry (RRI) with project code PHD60I0056, under funding agency of Thailand Science Research and Innovation (TSRI). We also thank the Northeast Thailand Cane and Sugar Research Center, Salt-tolerant Rice Research Group Khon Kaen University, Thailand, and Centre of Excellence on Biodiversity (BDC), Office of Higher Education Commission under project code BDC-PG1-163002 for partial support of financial, implements and instruments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Juntahum, S., Ekprasert, J. & Boonlue, S. Efficiency of Arbuscular Mycorrhizal Fungi for the Growth Promotion of Sugarcane Under Pot Conditions. Sugar Tech 24, 1738–1747 (2022). https://doi.org/10.1007/s12355-022-01129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01129-z