Abstract
This study evaluates the effect of the soil application of potassium (K) on the physiological, productive, and economic variables of the first sugarcane ratoon grown in the Brazilian Central region. The experiment was arranged in a randomized complete block design, with five replicates. Treatments consisted of five doses of K (0; 41.5; 83.0; 124.5; and 166.0 kg ha−1). K contents in the soil, biometric variables, relative chlorophyll index, gas exchange parameters, and stem and sugar yield were evaluated. Soil application of K increased the K content in the soil with quadratic adjustment at depths from 0.0 to 0.2 m, with the maximum content of 0.38 cmolc dm−3 when 164.3 kg K ha−1 was applied. The significant adjustment was linear at the depths from 0.2–0.4 and 0.6–0.8 m, reaching 0.27 and 0.11 cmolc dm−3, respectively, for the highest dose applied. The application of K did not affect plant height in any of the periods evaluated, although affecting the number of tillers and leaf area at 120 DAB, reaching higher values at the dose of 166.0 kg K ha−1. Stomatal conductance, transpiration, photosynthesis, and internal CO2 concentration were affected by the increased application of K, with the dose of approximately 83.0 kg ha−1 resulting in the highest values. The dose of 166.0 kg K ha−1 obtained the best stem and recoverable sugar yields, 135.4 t ha−1 and 18.6 t ha−1, respectively, also obtaining the highest differential profit, US$ 425.8, in relation to the non-application of K fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum spp) stands out as the most important species for sugar and bioenergy production in the world, with Brazil being the largest producer, followed by India and China (Wu et al. 2020). For the 2021/22 crop season, Brazilian production is estimated to be 628.1 million tons. The State of Goiás stands out in this scenario as the second largest national producer (960.9 thousand hectares of harvested area, production of approximately 73.4 million tons, and average yield of 76.4 t ha−1) (Conab 2021).
Sugarcane is a tall perennial grass of the genus Saccharum, belonging to the family Poaceae (Clark et al. 1995), with C4 plant metabolism and high efficiency to store solar energy and convert it (Srivastava and Rai 2012), having high biomass production (Sage et al. 2014). The crop is grown in tropical and subtropical areas, being totally influenced by the edaphoclimatic characteristics of each region (Caetano and Casaroli 2017; Paixão et al. 2020; Vasconcelos et al. 2020). In order to maximize sugarcane production, among other cultivation practices, soil fertility management plays a major role in determining stem yields (Rhodes et al. 2018), combined with the study of climatological aspects of the region. In addition, sugarcane is very demanding regarding soil nutrient reserves, as large quantities are extracted during the growth period and exported at harvest (Mthimkhulu et al. 2018).
Potassium is the most required nutrient for sugarcane cultivation (Malavolta 1982; Otto et al. 2010), with the amount extracted ranging from 0.83 to 2.08 kg K for each ton of stem produced (Gopalasundaram et al. 2012). This behavior occurs in plants that accumulate sucrose, such as sugarcane, and K is essential for the synthesis and accumulation of this carbohydrate. K acts in the conversion of reducing sugars into total recoverable sugars (TRS), also eliminating tissue moisture, which favors sugarcane maturation (Hunsigi 2011). Furthermore, K activates more than 60 enzymes in the plant, acts on osmotic regulation, improves N absorption, helps in carbon assimilation and photosynthesis, and also provides resistance for sugarcane against pests and diseases (Srivastava and Rai 2012; Marenco and Lopes 2013; Prado 2021; Sardans and Peñuelas 2021).
However, soils from tropical regions naturally have low levels of exchangeable K (< 0.15 cmolc dm−3) mainly due to the high degree of weathering of the primary minerals that originated these soils. Thus, supplementation with potassium fertilizers is required to achieve high production rates in sugarcane (Otto et al. 2010). The fertilization of the sugarcane ratoon is performed on the surface and without incorporation into the soil, evidencing the need to know the dynamics of the vertical mobility of nutrients in the soil, with K among them (Werle et al. 2008). There are several factors that regulate the vertical mobility of K in the soil profile, among which the granulometric texture, the cation exchange capacity (CTC), and the soil moisture, besides the type (solubility) and amount of fertilizer applied stand out (Duarte et al. 2013; Rosolem and Steiner 2017).
Several studies have evaluated the stem yield of sugarcane as a function of application of K fertilizer under the most diverse conditions of climate, soil, cultivars, and management, reaching the consensus that proper management of K is fundamental for crop yield (Oliveira et al. 2010a; Otto et al. 2010; Floreset al. 2014a; Flores et al. 2014b, 2020; Almeida et al. 2015; Korndorfer et al. 2018). However, few studies have evaluated the effect of K on the physiological parameters of the crop, with the most recent being that by Jaiswal et al. (2020) in the conditions of subtropical India.
In this context, due to the relevance of K for sugarcane, it is believed that the soil application of K improves the biometric and physiological variables of sugarcane, affecting the stem and sugar yield and increasing the differential profit obtained in the crop production system. Thus, this study evaluates the effect of the soil application of K on the biometric, physiological, productive, and economic variables of the first sugarcane ratoon grown under edaphoclimatic conditions in the Brazilian Central region.
Material and Methods
Characterization of the Experimental Area
The experiment was conducted after the first ratoon budding, variety IAC 91-1099, in the period between June 2017 and May 2018, in a commercial area of cultivation located in the municipality of Goiatuba, State of Goiás, Brazil (18º 2′ 39.55″ S; 49º 30′ 28.5″ W; 619 m asl).
According to the Köppen Climate Classification system, the region climate is Aw (tropical savanna), presenting a well-defined rainfall regime with dry winters (May–September) and rainy summers (October–April) (Alvares et al. 2013). The soil of the experimental area was classified as an Oxisol (i.e., Latossolo Vermelho distrófico—LVd), according to the Brazilian Soil Classification System) (Santos et al. 2018), with clayey texture (clay 440 g kg−1; silt 130 g kg−1; sand 430 g kg−1). Before starting the experiment, samples were collected at the depths of 0.0–0.2, 0.2–0.4, 0.4–0.6, and 0.6–0.8 m for the chemical characterization of the soil profile. The analysis of chemical attributes was performed according to the methodology by Teixeira et al. (2017), and the results are shown in Table 1.
Climate Assessments
The climatic variables—air temperature (maximum, minimum, and daily average), relative humidity, and rainfall, were obtained from an automatic weather station, located 5.0 km distant from the study area. From the climatic variables, the sugarcane water balance was calculated on a daily basis according to the methodology described by Thornthwaite and Mather (1955). Therefore, the available water capacity (AWC, mm) was determined by Eq. (1):
where FC is the soil humidity at field capacity; PWP is the soil humidity at permanent wilting point; and Ze is the effective root zone depth given by the average exploitation of 80% of sugarcane roots, in which the value of 600 mm was adopted (Farias et al. 2008; Flores et al. 2020).
The potential evapotranspiration (ET0, mm day−1) was estimated using the method by Hargreaves and Samani (1985), according to Eq. (2):
where Q0 is the extraterrestrial solar irradiance (mm day−1); NDP is the number of days in the period (daily scale); and Tmax, Tmin, and Tmean are the maximum, minimum, and average temperatures, respectively.
The crop evapotranspiration (ETc, mm day−1) represents the product between ET0 and the crop coefficient (Kc), for which different values were used for each stage of sugarcane development, as described by Doorenbos and Kassam (1994). From ETc and ETr (real evapotranspiration) values of water balance, the water requirement satisfaction index was obtained (WRSI = ETr/ETc) (Casaroli et al. 2019). The three following WRSI classes were established for the sugarcane crop: (a) WRSI ≥ 0.50, for situations where sugarcane is exposed to low climate risk (favorable); (b) 0.40 < WRSI < 0.50, for situations where sugarcane is exposed to medium climate risk (intermediate); and (c) WRSI ≤ 0.40, for situations where sugarcane is exposed to high climatic risk (unfavorable) (Mapa 2016).
Treatments and Experimental Design
The treatments consisted of the following five doses of K: 0.0 (control), 41.5, 83.0, 124.5, and 166.0 kg of K ha−1, using KCl (potassium chloride—58% K2O or 48% K) as a source. The experiment was arranged in a randomized complete block design with five replicates, totaling 25 experimental plots. Each plot comprised five rows of 10 m in length and 1.5 m between rows (75 m2 per plot), with evaluations performed on the three center rows of each area (usable area of 40.5 m2), excluding 0.5 m in length from each extremity.
The treatments were implemented in June 2017, 30 days after the sugarcane harvest. The doses of K fertilizer were sidedressed, without incorporation, as performed by Almeida et al. (2015). Similarly, together with K fertilization, 100 kg ha−1 N and 30 kg ha−1 P2O5 were applied in the form of ammonium nitrate (NH4NO3—33% N) and MAP (monoammonium phosphate—48% P2O5), respectively, according to the initial analysis of the soil and recommendations by Sousa and Lobato (2004).
K Content in the Soil
At 180 days after budding (DAB), soil samples were collected in the same place where K fertilization was applied (fertilization range), according to recommendations by Flores et al. (2012), Flores et al. (2014a) and Cavalcante et al. (2016), at the depths of 0.0–0.2, 0.2–0.4, 0.4–0.6, and 0.6–0.8 m at five random points within each experimental plot. For the determination of exchangeable K contents in the soil (KS), the methodology by Teixeira et al. (2017) was used.
Plant Analysis
The biometric evaluations were performed at 120 and 240 DAB, assessing: number of tillers per plant in a linear meter (measured in the three central rows of each plot); stem diameter (measured at the stem base, immediately after the first node insertion, using a digital caliper); and average plant height (measured using a measuring tape, from the base of the plant until insertion of the first fully visible sheath, leaf + 1), which were measured by selecting 10 random plants from the usable rows of each experimental plot, according to the methodology by Marafon (2012). Leaf area (LA) was estimated according to Hermann and Câmara (1999), Eq. (3):
where L is leaf length + 3; W is the leaf width + 3; 0.75 is the correction factor for the crop leaf area; N is the number of open leaves, with at least 20% of green area; and 2 is the weighting factor for leaves that are not yet fully expanded.
Relative chlorophyll index (RCI) was obtained by the aid of a chlorophyll meter (model Falker®, ClorofiLOG CFL 1030), and readings on the center of the leaf blade of leaf + 1, according to the methodology described by Amaral and Molin (2011). Stomatal conductance, transpiration, net photosynthetic rate, and internal CO2 concentration were performed by the aid of a photosynthesis meter, IRGA (Infrared Gas Analyzer), model LCpro-SD/iFL Portable, with a 6.25 cm2 leaf chamber. Readings occurred between 9:00 a.m. and 3:00 p.m. in fully expanded leaves with good phytosanitary aspect (Flores et al. 2018). Such evaluations occurred at 120 and 240 DAB of the sugarcane ratoon.
Harvesting was performed manually at 330 DAB from two linear meters within the usable area of each experimental plot, separating stems from leaves (dry or green). Afterward, stems were weighed to determine the yield of experimental plot, which was extrapolated to tons per hectare. In each experimental plot, a bundle containing ten stems was collected to determine TRS, expressed in kg of sugar per ton of crushed stems, in accordance with the methods described by Consecana (2006). Through multiplying the value obtained for TRS by the real yield, the value of TRS in ton per hectare was obtained.
Economic Analysis
The economic analysis was performed using the partial budget technique (Noronha 1987). The differential profit was calculated using the budget costs and the differential income, using the control treatment as the reference. The yield (TRS) was used to calculate the yield increase in (rates of K) treatments related to the control (PG). The production value (PV) was obtained by Eq. (4):
where P is the product price in Brazil. The average price deflated of US$ t−1 148.21 (Udop 2020) was used. This value was calculated using price data from the last 12 years (2008 up to 2020).
The differential profit was obtained by Eq. (5):
where Pd is the differential profit; Id is the differential Income (Iti – It0); Cd is the differential cost (Cti – Ct0); ti is the treatment I, and t0 is the control.
The costs of the doses of K fertilizer were obtained using the price per ton of potassium chloride. The current price of US$ ton−1 343.87 (US$ = R$ 5.38) was adopted, once verified the historical rise of fertilizer prices in Brazil (Saab and Paula 2008).
Statistical Analysis
The results obtained were submitted to the analysis of variance and to the F test using the statistical software AgroEstat (Barbosa and Maldonado Júnior 2015). In order to study the effect of the doses of Kon the variables analyzed, polynomial regression analysis was used. The choice of the regression model was based on the significance of the regression coefficients, on the most significant p-value, and subsequently the highest coefficient of determination (R2).
Results
Climate Assessments
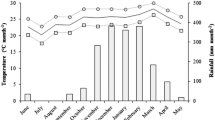
Accumulated rainfall during the cycle of sugarcane was 1766 mm, with 93% of the precipitated volume concentrated between the months of October to March, with average temperature of 24.0 ± 5.0 °C. Maximum temperatures in the period ranged from 28.1 to 30.0 ºC. The crop water balance during the ratoon cycle presented water deficit values at the budding stage (0–30 DAB) and the tillering stage (30–120 DAB) of 1.79 mm and 68.06 mm, respectively. At the growing stage (120–290 DAB), water surplus of 1,223.05 mm and a deficit equal to 111.13 mm were recorded. At the maturation stage (290–330 DAB), a water deficit of 28.67 mm was recorded (Fig. 1).
The WRSI in Fig. 2, considering the average values of each crop development stage, showed relative water consumption of 0.82, 0.52, 0.86, and 0.54 for the budding, tillering, growth, and maturation stages, respectively. These indexes ranged between 0.77–0.87; 0.19–1.00; 0.36–1.00; and 0.41–1.00, respectively. Figure 2 systematizes this behavior demonstrating that WRSIs were favorable to most stages. Increased risk of yield loss by water deficit was only evidenced during tillering.
K Content in Soil
Soil application of K for the production of sugarcane ratoon influenced the K content in the soil at 180 DAB, except at the depth of 0.4–0.6 m, which had an average K+ content of 0.13 cmolc dm−3 (Fig. 3). Differences in the mobility of K+ were observed in the soil profile. At the 0.0–0.2 m depth, the K+ content was significantly adjusted to the second-order polynomial regression. In addition, an inflection at 164.3 kg K ha−1 was observed at the depth of 0.0–0.2 m, after which K+ contents in the soil presented a reduction.
Significant linear increases were observed at the depths of 0.2–0.4 and 0.6–0.8 m and K contents in the soil increased up to 0.27 and 0.11 cmolc dm−3, respectively, with the use of the dose of 166.0 kg K ha−1, increase of 160% and 30%, respectively, in relation to the control.
Although data suggested a decreasing gradient of distribution of K+ contents in the soil profile up to 0.4 m (Fig. 3), the zone of soil resistance at 0.6 m (9.67 kPa), found by Andrade et al. (2021) for the same soil, demonstrates the physical effect of the soil on the distribution of K in the system.
Biometric Variables
The application of K fertilizer did not significantly influence plant height in any of the evaluated periods, presenting an average height of 113.9 and 242.1 cm in the 180 and 240 DAB evaluations, respectively (Table 2). In the evaluation at 240 DAB, an increasing trend in plant height with increasing levels of K was observed, although the differences were not statistically significant. Stem diameter was not affected by K fertilization when evaluated at 180 DAB, with average diameter of 30.9 mm. However, increases were observed when the evaluation was performed at 240 DAB, although with a difference of only 4% between the largest stem diameter (36.1 mm) and the smallest (34.6 mm).
The doses of K applied promoted significant increases in the number of tillers and in the leaf area of sugarcane, with linear increases in the evaluation at 120 DAB (number of tillers: Y = 0.0119x + 13.344, R2 = 0.82, F = 12.942**; leaf area: Y = 1.1913x + 1,763, R2 = 0.84, F = 28.96**), reaching 15 tillers m−1 and 1946.6 cm−2 of leaf area in the application of 166.0 kg K ha−1, i.e., values 16% and 10% higher than the control, respectively (Table 2). The results suggests that plants fertilized with K showed accelerated initial growth, which was not observed in the evaluations at 240 DAB, presenting average values of 17.5 tillers m−1 and 5837.7 cm2 of leaf area.
As for RCC, the K application in the soil led to a linear increase in RCC in the evaluation at 240 DAB (Y = 0.0627x + 54.319, R2 = 0.93**, F = 93.59**), with a maximum content of 65.2 μg cm−2 at the dose of 166.0 kg K ha−1, being 22% higher than the RCC obtained in the control (53.4 μg cm−2) (Table 2). For the evaluation performed at 120 DAB, the average RCC of 37.6 μg cm−2 was observed regardless of the dose of K.
Physiological Variables
The physiological variables of sugarcane were affected by increased doses of K fertilizer in the soil application. Net photosynthesis (Fig. 4a) and transpiration (Fig. 4b) presented a similar behavior, with quadratic adjustment for both periods, and increases in the value of these variables being observed up to the dose of: 108.5 kg K ha−1 (17.07 µmol m−2 s−1) and 100.3 kg K ha−1 (23.76 µmol m−2 s−1) for photosynthesis, and 110.3 kg K ha−1 (6.00 mol m−2 s−1) and 68.5 kg K ha−1 (5.89 mol m−2 s−1) for transpiration, at 120 and 240 DAB, respectively.
Stomatal conductance (Fig. 4c) presented linear response in the evaluation at 120 DAB, reaching 0.25 mol m−2 s−1, an increase of 25% when compared to the control. In the evaluation at 240 DAB, the increase was explained by a second-order polynomial equation, with the highest results (0.42 mol m−2 s−1) obtained using 68.3 kg K ha−1. The internal CO2 concentration (Fig. 4d) also presented quadratic behavior at 240 DAB, in which the dose of 81.1 kg K ha−1 provided the highest internal CO2 concentration (231 µmol mol−1). At 120 DAB, no effect of the treatments was observed for internal CO2 concentration, with an average value of 260 µmol mol−1.
Yield, Technological Quality, and Differential Profit
The application of K fertilizer increased stem and TRS production similarly with linear increases, reaching 135.4 t ha−1 of stems and 18.6 t ha−1 of TRS with the application of 166.0 kg K ha−1(Fig. 5). Such results corresponded to an increase of 22 and 25% in relation to the control, respectively.
All doses of K applied to the soil produced positive results for TRS production at the industry level regarding the differential profit compared to the control treatment (Fig. 6). The results indicated that all doses were economically viable when compared to the treatment without K fertilizer application. However, comparing the analyzed prices, the dose of 166.0 kg K ha−1 was the most efficient, providing a differential profit of US$ 425.8 ha−1 compared to the treatment without K fertilizer application.
Discussion
Climate Assessments
Sugarcane is a crop grown throughout the Brazilian territory for its adaptability to wide temperature ranges (25.0 ± 7.0 °C) and for having a water demand above 1000 mm year−1 (Paixão et al. 2020). Figure 1 confirms that the climatic conditions met the physiological needs for much of the crop development in the study region. The intense growth stage of sugarcane (November to March—Figs. 1 and 2) is considered one of the crop most critical phases regarding the water deficit (Machado et al. 2009). Approximately 70 to 80% of all the biomass accumulated during the crop cycle is produced in this stage (Oliveira et al. 2010b). According to Caetano and Casaroli (2017), in the climatic conditions of Central Brazil, the agrometeorological models estimate potential yield between 160 and 240 t ha−1. However, when this maximum yield is penalized due to the water deficit in the region, it decreases to 128 t ha−1. In the present study, this same response was observed (Fig. 5a) and yield projections may have been related to the relative decrease in the WRSI (Fig. 2). In addition, almost the entire tillering stage and the beginning of the growth stage presented a water requirement satisfaction index below the ideal for the plant, coinciding with the first evaluation of physiological factors (120 DAB).
K Content in the Soil
The application of K in the soil led to higher K contents at the depths of 0.0–0.2 m and 0.2–0.4 m, with minimal leaching below this depth due to the water deficit observed up to 120 DAB. Since it was a clayey soil (440 g kg−1of clay) with high CEC at the 0.0–0.2 m depth (10.3 cmolc dm−3) (Table 1), studies suggested that K+ losses by leaching become quite reduced under these conditions (Mendes et al. 2016; Flores et al. 2020). In addition, the study region is characterized by a predominant lithology of basalt from the Serra Geral formation, and the predominant relief ranges from smooth wavy to wavy (Santos and Sparovek 2011; Sá et al. 2016). Basalt consists of minerals such as feldspars, feldspathoids, and micas (Crmp 2015), which tend to form kaolinite (Manning 2010). The point of zero charge (PZC) of kaolinite is approximately 4.74 (Sousa et al. 2016), at which the total concentration of anionic and cationic surface sites are equal (Bleam 2012). Therefore, the evidence presented in Table 1 shows the ability of K+ to remain fixed at the depths up to 0.4 m. This behavior can be explained by the fact that the pH is higher than the PZC and presents net charges on the surface of clay minerals.
Biometric Variables
Effect in the number of tillers at 240 DAB was not observed probably due to intraspecific competition among plants for natural sources and stimulating a vegetative condition of the plants and due to the interruption of the tillering process (Marin et al. 2009). Generally, sugarcane can develop its maximum tillering capacity until 120 DAB, as demonstrated in this present study (Table 2). This is one of the reasons nutrients must be available to plants, so that stems and leaves can achieve their adequate nutrient uptake, as indicated by Oliveira et al. (2011).
Despite evidences, some studies are reported a compensatory effect of sugarcane in water stress situations (Ellis and Lankford 1990; Robertson et al. 1999). Reinforcing our findings, Otto et al. (2010) proved significant effect for the number of tillers, with a decrease in response from the dose of 107.9 kg K ha−1 applied to the furrow.
Apparently, K has the ability to stimulate some physiological mechanism that affects the leaf area in the tillering stage. However, the present study illustrated how K application can reduce the effects of stress, increasing the leaf area during the tillering stage (120 DAB) (Table 2). In this case, K application can be used as strategy in dry regions or seasons, as observed in several places worldwide. The same behavior was observed by Smit and Singels (2006).
Despite the effects of K fertilization in stem diameter, plant height was not statistically significant. Furthermore, the opposite was observed in stem diameter, which leads to the hypothesis that K application in the system stimulated an anticipation of the increase in stem diameter without necessarily entering a state of senescence.
In the literature, several researchers reported a positive response to the application of K for the following variables of growth and development of sugarcane: number of tillers (Uchôa et al. 2009; Otto et al. 2010), plant height (Otto et al. 2010; Flores et al. 2012; Almeida et al. 2015), and stem diameter (Uchôa et al. 2009; Flores et al. 2020). These studies had the low initial content of K in the soil in common with the present research.
According to Rocha et al. (2019), the biometric variables of sugarcane that correlate the most with productivity are the number of tillers, counted in the initial months of the crop cycle, and plant height. However, small increases in each of these categories are not always differentiated by statistical analysis (Flores et al. 2012), as observed for plant height at 240 DAB, in which the height increase as the doses of K increased was notorious, although not being statistically they significant. When these small differences are estimated to a population of plants per hectare, it can lead to increased productivity.
Physiological Variables
The N metabolism in plants requires adequate contents of K in the cytoplasm, which can increase K absorption and consequently allow a rapid assimilation of the NH4+ absorbed, maintaining its content low in the plant and preventing toxicity (Xu et al. 1992; 2002; Viana and Kiehl 2010). This relationship between K and N may have contributed to an increase in the RCC of sugarcane with increasing doses of K fertilizer. The absence of a significant effect on RCC at 120 DAB can be attributed to the water deficit observed in the period (Fig. 1), although an increase in leaf area and tillers was observed (Table 2). According to Begum et al. (2012), a significant decrease in chlorophyll content was observed under conditions similar to those observed in the present study.
Studies performed on different species, including sugarcane, under controlled conditions of water stress and moderate deficit of K observed the inhibition of the stomatal closure in plants (Singh et al. 1998; Benlloch-González et al. 2008). The present study suggests that similar phenomena have occurred at 120 DAB, when a water deficit of 68.06 mm was observed and sugarcane presented a linear increase in stomatal conductance. According to Benlloch-González et al. (2008), these results suggested that this physiological phenomenon was widespread among plants and may be the main cause of tissue dehydration in many non-irrigated crops with low K content.
K acts directly on stomatal adjustment and can affect photosynthetic rates (Galon et al. 2013; Izquierdo-Hernández et al. 2016). Furthermore, it influences the turgor of guard cells, increasing the osmotic potential and resulting in the absorption of water from adjacent cells (Marenco and Lopes 2013; Prado 2021). On the other hand, the exit of ions from guard cells causes water loss and, as a result, stomatal closure (Marenco and Lopes 2013). Thus, plants well supplied with K+ have increased water use efficiency (Prado 2021), reinforcing the hypothesis of K being a strategic nutrient for arid regions or with low water availability.
Transpiration and photosynthesis are metabolic processes that are directly related (Boehringer, 2010). The results presented in Figs. 4a and b show similar behavior for these variables. According to Prado (2021), the participation of K+ in controlling the stomatal opening/closing is also important for the photosynthetic rate, as stomatal opening does not occur regularly in K-deficient plants, reducing the entry of CO2. Thus, the application of K fertilizer can increase CO2 assimilation by the leaves. These behaviors were well understood since the application of K can increase CO2 diffusion in mesophilic cells and also activates RuBisCO carboxylase (ribulose-1,5-bisphosphate carboxylase oxygenate), contributing to increase photosynthetic activity (Marenco and Lopes 2013; Prado 2021).
Yield, Technological Quality, and Differential Profit
This study proved the beneficial effect of K on sugarcane yield, which was also reported by Flores et al. (2014b) and (2020), who observed the effects on the stem production of first and second sugarcane ratoons after the application of K fertilizer in the soil. Similarly, Pancelli et al. (2015) observed a yield increase of 29% with the application of K fertilizer in the soil. Cavalcante et al. (2016) concluded that the application of K in the soil had a positive effect on the stem production in the first ratoon. Thus, the present study, besides shedding light on the evidence already found, also proved the secondary effects that the application of K can offer to this agroecosystem. The hypothesis proved by Flores et al. (2014a) is that when managing sugarcane ratoons, responses caused by K fertilization are observed in the permanence of plant roots after cutting the cane plant, as it stimulates absorption in the first days of budding by intensifying and altering metabolic flows. As K is the most extracted nutrient by sugarcane, especially by ratoons (Korndorfer et al. 2018), this crop is extremely demanding regarding K nutrition, justifying the linear growth of stem yield observed in the present study (Fig. 5a).
K improves the functioning of plant metabolism at proper contents, making the tillers vigorous and contributing to higher stem and sugar yields, increasing crop yield (Jaiswal et al. 2020). In this context, TRS are the main indicators of sugarcane quality in Brazil, as it is a parameter used to pay sugarcane producers regarding the quality of their production (Cardozo et al. 2014, 2015), hence the importance of adequate fertilization management to improve the quality of sugarcane juice. In a study on the management of K fertilization for sugarcane cultivation, Otto et al. (2010) also observed significant effect of doses of K fertilizer on the production of TRS, in which the authors observed maximum sugar production (25.6 t ha−1) at the dose of 103.8 kg K ha−1. According to Tu et al. (2017), K fertilization decreases abscisic acid content, which is inversely proportional to the sucrose content in the plant, thereby increasing the sugar content in the vegetable. In addition, K is responsible for the translocation of carbohydrates from photosynthesis, mainly from leaves, to the stems (Marschner 2012), as the data from the present study also suggested. Moreover, soil application of K frequently increases the percentage of TRS in the sugarcane juice (Hunsigi 2011; Gopalasundaram et al. 2012; Zeng et al. 2015), as also observed in the present study.
Sucrose formation is positively related to chlorophyll content and photosynthetic rate (Begum et al. 2012). The present study demonstrated that doses of K increased relative chlorophyll content, which may have contributed to a higher production of TRS. In addition, it was confirmed that the application of 166.0 kg K ha−1 via potassium chloride is feasible for the sugarcane production system (Fig. 6). Feasibility-related factors are directly involved with yield, which is recognized as common for large-scale systems, as commented by Reis (2007) and Cardozo et al. (2020). According to Mellis and Quaggio (2009), the financial feasibility found in the present study is compatible with the intended application of K. From the industry perspective, this behavior is promising for the advantages of K applications, especially in dry conditions, making potassium one of the most important inputs in the sugarcane complex.
References
Almeida, H.J., F.J.R. Cruz, M.A. Pancelli, R.A. Flores, R.L. Vasconcelos, and R.M. Prado. 2015. Decreased potassium fertilization in sugarcane ratoons grown under straw in different soils. Australian Journal of Crop Science 9: 596–604.
Alvares, C.A., J.L. Stape, P.C. Sentelhas, J.L.M. Gonçalves, and G. Sparovek. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711–728.
Amaral, L.R., and J.P. Molin. 2011. Optical sensor to support nitrogen fertilization recommendation for sugarcane crops. Pesquisa Agropecuaria Brasileira 46: 1633–1642.
Andrade, A.F., R.A. Flores, D. Casaroli, A.M. Bueno, M.A. Pessoa-de-Souza, F.S.R. Lima, and E.P. Marques. 2021. K dynamics in the soil–plant system for sugarcane crops: A current field experiment under tropical conditions. Sugar Tech. https://doi.org/10.1007/s12355-021-00985-5.
Barbosa, J.C., and W. Maldonado Júnior. 2015. Experimentação agronômica & Agroestat - Sistema para análises de ensaios agronômicos. 1 th. Jaboticabal: Funep.
Begum, M.K., M.R. Alam, M.S. Islam, and M.S. Arefin. 2012. Effect of water stress on physiological characters and juice quality of sugarcane. Sugar Tech 14: 161–167.
Benlloch-González, M., O. Arquero, J.M. Fournier, D. Barranco, and M. Benlloch. 2008. K+ starvation inhibits water-stress-induced stomatal closure. Journal of Plant Physiology 165: 623–630.
Bleam, W.F. 2012. Clay mineralogy and clay chemistry. Soil and Environmental Chemistry: 85–116.
Caetano, J.M., and D. Casaroli. 2017. Sugarcane yield estimation for climatic conditions in the center of state of Goiás. Revista Ceres 64: 298–306.
Cardozo, N.P., P.C. Sentelhas, A.R. Panosso, and A.S. Ferraudo. 2014. Multivariate analysis of the temporal variability of sugarcane ripening in south-eastern Brazil. Crop and Pasture Science 65: 300–310.
Cardozo, N.P., P.C. Sentelhas, A.R. Panosso, A.L. Palhares, and B.Y. Ide. 2015. Modeling sugarcane ripening as a function of accumulated rainfall in Southern Brazil. International Journal of Biometeorology 59: 1913–1925.
Cardozo, N.P., R.O. Bordonal, A.R. Panosso, and C.A.C. Crusciol. 2020. A Multivariate approach to determine the economic profitability of sugarcane production under diverse climatic conditions in Brazil. Sugar Tech 22: 954–966.
Casaroli, D., J. Alves Júnior, and A.W.P. Evangelista. 2019. Quantitative and qualitative analysis of sugarcane productivity in function of air temperature and water stress. Comunicata Scientiae 10: 203–212.
Cavalcante, V.S., R.M. Prado, H.J. Almeida, T.M.R. Silva, R.A. Flores, and M.A. Pancelli. 2016. Potassium nutrition in sugarcane ratoons cultured in red latosol with a conservationist system. Journal of Plant Nutrition 39: 315–322.
Clark, L.G., W. Zhang, and J.F. Wendel. 1995. A phylogeny of the grass family (Poaceae) based on ndhF sequence data. Systematic Botany 20: 436–460.
Conab - Compainha Naciobal de Abastecimento. 2021. Acompanhamento da Safra Brasileira de cana-de-açúcar - safra 2021/22, first survey, Brasília: Conab.
Consecana. 2006. Manual de Instruções. 5 th. Piracicaba, SP: Consecana-SP.
Crmp. 2015. Serviço Geológico do Brasil. http://www.cprm.gov.br/publique/Redes-Institucionais/Rede-de-Bibliotecas---Rede-Ametista/Rochas-1107.html. Accessed 03 may 2021.
Doorenbos, J., and A.H. Kassam. 1994. Efeito da água no rendimento das culturas. Campina Grande, PB: UFPB.
Duarte, I.N., H.S. Pereira, and G.H. Korndörfer. 2013. Lixiviação de potássio proveniente do termopotássio. Pesquisa Agropecuaria Tropical 43: 195–200.
Ellis, R.D., and B.A. Lankford. 1990. The tolerance of sugarcane to water stress during its main development phases. Agricultural Water Management 17: 117–128.
Farias, C.H.A., P.D. Fernandes, H.M. Azevedo, and J. Dantas Neto. 2008. Growth indices of irrigated and non-irrigated sugarcane in Paraiba, Brazil. Revista Brasileira De Engenharia Agricola e Ambiental 12: 356–362.
Flores, R.A., R.M. Prado, L.S. Politi, and T.B.F. Almeida. 2012. Potassium in the early development stage of unburned sugarcane rattoon. Pesquisa Agropecuária Tropical 42: 106–111.
Flores, R.A., R.M. Prado, H.J. Almeida, M.A. Pancelli, J.P. Souza Junior, and A.A.V.L. Soares. 2014a. Potassium nutrition in ratoon sugarcane under a conservation cropping system. Ciencia Del Suelo 32: 53–62.
Flores, R.A., R.M. Prado, M.A. Pancelli, H.J. Almeida, L.R. Moda, B.M.M.N. Borges, and J.P. Souza Junior. 2014b. Potassium nutrition in the first and second ratoon sugarcane grown in an oxisol by a conservationist system. Chilean Journal of Agricultural Research 74: 83–88.
Flores, R.A., E.M. Arruda, V. Damin, J.P. Souza Junior, D.D.C. Maranhão, M.A.R. Correira, and R.M. Prado. 2018. Physiological quality and dry mass production of Sorgum bicolor following silicon (Si) foliar appliication. Australian Journal of Crop Science 12: 631–638.
Flores, R.A., A.F. Andrade, D. Casaroli, D.T. Quirino, K.O. Abdala, C. Martins, A.M. Bueno, J. Alves Júnior, and A.W.P. Evangelista. 2020. Potassium fertilization in sugarcane ratoon yield grown in a tropical region. Communications in Soil Science and Plant Analysis 51: 896–910.
Galon, G., G. Concenço, E.A. Ferreira, I. Aspiazu, A.F. Silva, C.L. Giacobbo, and A. Andres. 2013. Influence of biotic and abiotic stress factors on physiological traits of sugarcane varieties. In Photosynthesis, ed. Zvy Dubinsky, 38. Intech Open. https://doi.org/10.1016/j.colsurfa.2011.12.014.
Gopalasundaram, P., A. Bhaskaran, and P. Rakkiyappan. 2012. Integrated nutrient management in sugarcane. Sugar Tech 14: 3–20.
Hargreaves, G.H., and Z.A. Samani. 1985. Reference crop evapotranspiration from temperature. Applied Engineering in Agriculture 1: 96–99.
Hermann, E.R., and G.M.S. Câmara. 1999. Um método simples para estimar a área foliar da cana-de-açúcar. Revista Da Sociedade Dos Técnicos Açucareiros e Alcooliros Do Brasil (STAB) 17: 32–34.
Hunsigi, G. 2011. Potassium management strategies to realize high yield and quality of sugarcane. Karnataka Journal of Agricultural Sciences 24: 45–47.
Izquierdo-Hernández, J., S. Salgado-García, L. Luz, D.J. Palma-López, H. Ortiz-Laurel, S. Cordova-Sánchez, and M. Castelán-Estrada. 2016. Nutritional and physiological response of sugarcane varieties to nitrogen fertilization in a Haplic Cambisol. Sugar Tech 18: 493–499.
Jaiswal, V.P., S.K. Shukla, L. Sharma, I. Singh, A.D. Pathak, M. Nagargade, A. Ghosh, C. Gupta, A. Gaur, S.K. Awasthi, R. Tiwari, A. Srivastava, and E. Masto. 2020. Potassium influencing physiological parameters, photosynthesis and sugarcane yield in Subtropical India. Sugar Tech. https://doi.org/10.1007/s12355-020-00905-z.
Korndorfer, G.H., H.S. Pereira, N. Duarte, L. Rocha, N. Pinto, and L.F. Fonseca. 2018. Sugarcane cultivation with source potassium low water-soluble. Acta Scientiarum. Agronomy 40. https://doi.org/10.4025/actasciagron.v40i1.36399.
Machado, R.S., R.V. Ribeiro, P.E.R. Marchiori, D.F.S.P. Machado, E.C. Machado, and M.G.A. Landell. 2009. Respostas biométricas e fisiológicas ao deficit hídrico em cana-de-açúcar em diferentes fases fenológicas. Pesquisa Agropecuária Brasileira 44: 1575–1582.
Malavolta, E. 1982. Potássio e enxofre nos solos e culturas brasileiras. Piracicaba: Potafos.
Manning, D.A.C. 2010. Mineral sources of potassium for plant nutrition. A Review. Agronomy for Sustainable Development 30: 281–294.
Mapa. 2016. Parâmetros de risco climático. https://www.gov.br/agricultura/pt-br/assuntos/riscos-seguro/programa-nacional-de-zoneamento-agricola-de-risco-climatico/documentos/Parametrosderiscoclimatico.pdf. Accessed 03 may 2021.
Marafon, A.C. 2012. Análise Quantitativa de crescimento em cana-de-açúcar: uma introdução ao procedimento prático. 1 th. Aracaju, SE: Embrapa Tabuleiros Costeiros.
Marenco, R.A, and N.F. Lopes. 2013. Fisiologia Vegetal: fotossíntese, respiração, relações hídricas e nutrição mineral. 3 th. Viçosa: Editora UFV.
Marin, F.R., G.Q. Pellegrino, E.D. Assad, H.S. Pinto, and J Zullo Junior. 2009. Cana-de-açúcar. In Agrometeorologia dos Cultivos: O fator meteorológico na produção agrícola, ed. J.E.B.A. Monteiro. 1 th. Brasília, DF: INMET.
Marschner. 2012. Mineral nutrition of higher plants. Edited by Petra Marschner. 3rd ed. Elsevier Ltd.
Mellis, E.V., and J.A Quaggio. 2009. Micronutrientes em cana-de-açúcar: a fome oculta dos canaviais.
Mendes, W.C., J. Alves Júnior, P.C.R. Cunha, A.R. Silva, A.W.P. Evangelista, and D. Casaroli. 2016. Potassium leaching in different soils as a function of irrigation depths. Revista Brasileira De Engenharia Agricola e Ambiental 20: 972–977.
Mthimkhulu, S., N. Miles, R.V. Antwerpen, and D.E. Elephant. 2018. Effect of residue and fertiliser management on soil fertility in a long-term sugarcane trial in South Africa. South African Journal of Plant and Soil 35: 307–315.
Noronha, J.F. 1987. Projetos agropecuários: administração financeira, orçamento e viabilidade econômica. 2 th. São Paulo: Atlas.
Oliveira, E.C.A., F.J. Freire, R.I. Oliveira, M.B.G.S. Freire, D.E. Simões Neto, and S.A.M. Silva. 2010a. Extração e exportação de nutrientes por variedades de cana-de-açúcar cultivadas sob irrigação plena. Revista Brasileira De Ciencia Do Solo 34: 1343–1352.
Oliveira, E.C.A., R.I. Oliveira, B.M.T. Andrade, F.J. Freire, M.A. Lira Júnior, and P.R. Machado. 2010b. Crescimento e acúmulo de matéria seca em variedades de cana-de-açúcar cultivadas sob irrigação plena. Revista Brasileira De Engenharia Agrícola e Ambiental 14: 951–960.
Oliveira, E.C.A., F.J. Freire, R.I. Oliveira, A.C. Oliveira, and M.B.G.S. Freire. 2011. Acúmulo e alocação de nutrientes em cana-de-açúcar. Revista Ciencia Agronomica 42: 579–588.
Otto, R., G.C. Vitti, and P.H.C. Luz. 2010. Potassium fertilizer management for sugarcane. Revista Brasileira De Ciencia Do Solo 34: 1137–1145.
Paixão, J.S., D. Casaroli, R. Battisti, A.W.P. Evangelistar, J. Alves Júnior, and M. Mesquita. 2020. Characterizing sugarcane production areas using actual yield and edaphoclimatic condition data for the state of Goiás, Brazil. International Journal of Plant Production 14: 511–520.
Pancelli, M.A., R.M. Prado, R.A. Flores, H.J. Almeida, L.R. Moda, and J.P. Souza Junior. 2015. Growth, yield and nutrition of sugarcane ratoon as affected by potassium in a mechanized harvesting system. Australian Journal of Crop Science 9: 915–924.
Teixeira, P.C., G.K. Donagemma, A. Fontana, and W.G. Teixeira, ed. 2017. Manual of soil analysis methods. 3 th. Brasília: Embrapa.
Prado, R.M. 2021. Mineral nutrition of tropical plants. Berna: Springer Nature.
Reis, R.P. 2007. Fundamentos de economia aplicada. Lavras: UFLA/FAEP.
Rhodes, R., N. Miles, and J.C. Hughes. 2018. Interactions between potassium, calcium and magnesium in sugarcane grown on two contrasting soils in South Africa. Field Crops Research 223: 1–11.
Robertson, M.J., N.G. Inman-Bamber, R.C. Muchow, and A.W. Wood. 1999. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crops Research 64: 211–227.
Rocha, M.G., F.M.M. Barros, S.R.M. Oliveira, and L.R. Amaral. 2019. Biometric characteristics and canopy reflectance association for early-stage sugarcane biomass prediction. Scientia Agricola 76: 274–280.
Rosolem, C.A., and F. Steiner. 2017. Effects of soil texture and rates of K input on potassium balance in tropical soil. European Journal of Soil Science 68: 658–666.
Sá, M.A.C., J.D.G. Santos Junior, C.A.B. Franz, and T.A. Rein. 2016. Qualidade física do solo e produtividade da cana-de-açúcar com uso da escarificação entre linhas de plantio. Pesquisa Agropecuaria Brasileira 51: 1610–1622.
Saab, A.A., and R.A. Paula. 2008. O mercado de fertilizantes no brasil diagnóstico e prognóstico. Revista de Política Agrícola: 5–24.
Sage, R.F., M.M. Peixoto, and T.L. Sage. 2014. Photosynthesis in sugarcane. In Sugarcane: Physiology, Biochemistry, and Functional Biology, ed. P.H. Moore, and F. C. Botha. 1 th.. Chichester: John Wiley & Sons Inc.
Santos, D.S., and G. Sparovek. 2011. Sediment retention in an agriculture field by riparian forest in Goiatuba, goiás state (Brazil). Revista Brasileira De Ciencia Do Solo 35: 1811–1818.
Santos, H.G., P.K.T. Jacomine, L.H.C. Anjos, V.A. Oliveira, J.F. Lumbreras, M.R. Coelho, J.A. Almeida, J.B. Araujo Filho, J.C. Oliveira, and T.J.F. Cunha. 2018. Brazilian Soil Classification System. 5 th. Brasília, DF: Embrapa.
Sardans, J., and J. Peñuelas. 2021. Potassium control of plant functions: Ecological and agricultural implications. Plants 10: 1–31.
Singh, S., T.N. Tiwari, R.P. Srivastava, and G.P. Singh. 1998. Effect of potassium on stomatal behaviour, yield and juice quality of sugarcane under moisture stress conditon. Indian Journal of Plant Physiology 3: 303–305.
Smit, M.A., and A. Singels. 2006. The response of sugarcane canopy development to water stress. Field Crops Research 98: 91–97.
Sousa, D.M.G., and E. Lobato. 2004. Cerrado: Correction of soil and fertilization. Embrapa Cerrados. 2 th. Planaltina.
Sousa, D.M.G., R.S. Nunes, T.A. Rein, J.D.G. Santos Junior, and S.A. Oliveira. 2016. Acidez do solo e sua correção na região de cerrado. In Práticas de manejo do solo para adequada nutrição de plantas no Cerrado, ed. R.A. Flores, and P.P. Cunha, 125–190. Goiânia: Gráfica UFG.
Srivastava, A.K., and M.K. Rai. 2012. Sugarcane production: Impact of climate change and its mitigation. Biodiversitas Journal of Biological Diversity 13: 214–227.
Thornthwaite, C.W., and J.R. Mather. 1955. The water balance. New Jersey: Drexel institute of technology.
Tu, B., C. Liu, B. Tian, Q. Zhang, X. Liu, and S.J. Herbert. 2017. Reduced abscisic acid content is responsible for enhanced sucrose accumulation by potassium nutrition in vegetable soybean seeds. Journal of Plant Research 130: 551–558.
Uchôa, S.C.P., H.O. Alves Júnior, J.M.A. Alves, V.F. Melo, and G.B. Ferreira. 2009. Response of six varieties of sugarcane to potassium dosages on savannah (cerrado) ecosystem. Revista Ciencia Agronomica 40: 505–513.
Udop. 2020. União nacional de bioenergia. Valores do ATR praticados a partir da Safra 98/99.
Vasconcelos, R.L., C.P. Cremasco, H.J. de Almeida, A. Garcia, A. Bonini Neto, M. Mauad, and L.R.A. Gabriel Filho. 2020. Multivariate behavior of irrigated sugarcane with phosphate fertilizer and filter cake management: Nutritional state, biometry, and agroindustrial performance. Journal of Soil Science and Plant Nutrition. https://doi.org/10.1007/s42729-020-00234-w.
Viana, E.M., and J.C. Kiehl. 2010. Rates of nitrogen and potassium on growth of wheat plants. Bragantia 69. Instituto Agronômico: 975–982.
Werle, R., R.A. Garcia, and C.A. Rosolem. 2008. Potassium leaching affected by soil texture and potassium availability. Revista Brasileira De Ciencia Do Solo 32: 2297–2305.
Wu, Q., W. Zhou, D. Chen, A. Cai, J. Ao, and Z. Huang. 2020. Optimizing soil and fertilizer phosphorus management according to the yield response and phosphorus use efficiency of sugarcane in southern China. Journal of Soil Science and Plant Nutrition 20: 1655–1664 .https://doi.org/10.1007/s42729-020-00236-8.
Xu, Q.F., C.L. Tsai, and C.Y. Tsai. 1992. Interaction of potassium with the form and amount of nitrogen nutrition on growth and nitrogen uptake of maize. Journal of Plant Nutrition 15: 23–33.
Xu, G., S. Wolf, and U. Kafkafi. 2002. Ammonium on potassium interaction in sweet pepper. Journal of Plant Nutrition 25: 719–734.
Zeng, Q., Q. Ling, L. Fan, Y. Li, F. Hu, J. Chen, Z. Huang, H. Deng, Q. Li, and Y. Qi. 2015. Transcriptome profiling of sugarcane roots in response to low potassium stress. PLoS ONE 10: 1–20.
Acknowledgements
Authors acknowledge the Goiasa Company for providing the area for the study and for the execution of the analysis of the technological quality of sugarcane and the National Council for Scientific and Technological Development (CNPq) for the financial contribution for research development, processes: 408180/2016-1 and 306329/2019-0. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. To the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and the National Council for Scientific and Technological Development (CNPq/Brazil) for granting a master's scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Andrade, A.F., Flores, R.A., Casaroli, D. et al. Biometric and Physiological Relationships and Yield of Sugarcane in Relation to Soil Application of Potassium. Sugar Tech 24, 473–484 (2022). https://doi.org/10.1007/s12355-021-01032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-01032-z