Abstract
Haploids and doubled haploids are very important tools which shorten the breeding periods and 100% homozygous lines. Especially in cross-pollinated species, it is known that it takes a long time to obtain homozygosity, and these breeding periods can be reduced by haploids considerably. In this study, appropriate bud stage and the effects of different growth regulators in Murashige and Skoog’s nutrient medium (MS) and pre-treatments on in vitro androgenesis were investigated in Stevia rebaudiana Bertoni which is known as sweet herb. In the first year, callus, bar-shaped embryo, and embryogenic calli were obtained. Embryos were obtained from MS 2.0 mg l−1 α-naphthaleneacetic acid (NAA) + 0.5 mg l−1 6-benzylaminopurine (BAP) medium, MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP, and MS 0.5 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) + 0.5 mg l−1 BAP callus ratios are 13.33%, 10%, and 3.33%, respectively. No differentiation was observed from the obtained callus and embryo. In the second year, high-temperature (+ 35 °C) and cold (+ 4 °C) pre-treatments were applied to the anthers, and no difference was observed between the applications. MS 2.0 mg l−1 NAA + 0.5 mg l−1 Kinetin high-temperature application was observed as the most successful medium with 37.5% embryo ratio obtained from two calli. This medium was followed by MS 2.0 mg l−1 NAA + 0.5 mg l−1 BAP and cold application (MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP) with 7.5% callus formation rates. Only cold application resulted in organogenesis through the callus, but the plants obtained were in diploid structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stevia rebaudiana Bertoni is a herbaceous perennial plant which belongs to Asteraceae family. Stevia has worldwide importance because it contains sweet-tasting ent-kaurene diterpenoid glycosides, in particular, stevioside and rebaudioside A, currently used as nonnutritive high-potency (300–400 times sweeter than sucrose) sweeteners (Kinghorn 2002; Anbazhagan et al. 2010; Ruiz-Ruiz et al. 2015; Ucar et al. 2017). Stevioside and rebaudioside are non-toxic, non-mutagenic, and they have long shelf life. Also, it has been approved for food supplement by FDA (US Food and Drug Administration) in different countries such as Indonesia, Japan, Korea, Canada, Amerika, and the UK (Aman et al. 2013). Stevia is used in the food and pharmaceutical industries for the first time in Japan, and its use is increasing day by day. In 2014, its market value reached up to 336 million dollars and predicted to reach 553.7 million dollars by 2024 (GRV 2018).
Growing market value of Stevia led to a breeding goal which is creating a new variety with sweeter glycoside concentration, but it has some difficulties. Stevia is cross-pollinated, and conventional breeding technique takes more than 9 years. In addition, these are self-incompatible, and its seeds have low germination rate. Stevia seeds are small, and most of them are infertile. Fertile seeds are darker in color than infertile seeds (Singh and Rao 2005; Skaria et al. 2014; Ucar et al. 2016). For these reasons, conventional stevia-breeding technique has not have bright future, and generally DH (doubled-haploid) techniques are the best solution for breeding. It shortens the breeding period, and 100% homozygous lines can be obtained. Haploids (n) have only one set chromosome number (gametophytic chromosome number) which is later doubled with some chemicals or spontaneously (Dunwell 2010; Germanà 2011a). The haploid plant can be obtained by different techniques such as androgenesis (anther and microspore culture), gynogenesis (ovary and ovule), wide hybridization, and parthenogenesis (Asif 2013). The most preferred technique for obtaining haploids is anther culture because it is easier than other techniques. If anther culture is not capable, other techniques can be studied (Bhojwani and Razdan 1996; Kasha and Maluszynski 2003). There are many factors that influence success in anther culture such as genotype, growing condition of donor plants, various stress factors, microspore development stage, and media composition. The most influential factor is genotype. If the genotype is not capable of embryogenesis, other treatments have limited effects. After genotype, the most effective pre-treatment is considered to be temperature shock. Not only degree and duration of temperature but also application type (directly anther or cultured anthers) has effect on success (Germanà 2011b). Cold pre-treatment has been used in many plants for inducing androgenesis, and some of them had positive effect, but some of them did not give a response. The aim of cold treatment is to induce microspore embryogenesis, nursing effect on microspore, help to decrease the degradation of microspores, and preserve the bicellular microspore stage (Duncan and Heberle 1976; Sato et al. 2002; Zheng 2003). On the other hand, using heat pre-treatment, with or without sugar starvations, has been found as an efficient embryogenesis inducer. In conducted research, it is showed that heat pre-treatment increases the frequency of embryo or embryo-like structure (ELS) and green plants on some genotypes, but high pre-treatment applications are more limited than cold application (Raina and Irfan 1998; Pechan and Smykal 2001; Asif 2013).
This study aimed to test the effect of pre-treatments and different growth regulators concentration on androgenic response of stevia. Especially in plants that are difficult to breeding (cross-pollinated, infertile seeds, self-incompatibility), DH application is very important. To the best of our knowledge, this study is the first attempt to extensive anther culture on stevia, and it will be a guide to future work.
Materials and Methods
Plant Material
S. rebaudiana seedling were taken from Grow Fide (Antalya, Turkey). Plants were grown in 10-cm-diameter pots in a peat–perlite mixture (4:1). Plants were fertilized every 2 weeks with nitrogen (N):phosphore (P):potassium (K) (15:15:15). Plants were grown in greenhouse, and temperature was fluctuated between 20 and 35 °C in growing season. All plants grew healthily, and no disease was seen in experiment area.
Flower buds of S. rebaudiana were collected September of 2014 and 2015. Plant flower buds showed different developmental stages for 1 month after flowering.
Pollen development stage was determined with 0.1% ethidium bromide (EtBr). This solution was diluted 0, 10, and 50 times. EtBr solution was also mixed by adding two drops of 0.1% Triton-100 solution to each 50 ml. One anther per bud size was placed on a microscope slide. Anther was squashed and microspores were liberated in 1–2 drops EtBr solutions. Pollen development stage was determined by fluorescence microscope (Fig. 1). Flower bud size containing the highest percentage of uninucleate microspores was associated with flower buds opening (Fig. 2).
Surface Disinfestation of Buds
Collected buds were washed with distilled water three times to remove dust, sand, and soil on the surface. The pre-washed buds were kept in 70% ethyl alcohol for 1 min followed by 10 min in 20% commercial sodium hypochlorite with 1–2 drops Tween 20 solution to complete surface disinfestation. Surface-disinfestationed buds were rinsed three times with sterile distilled water.
Anther Culture Technique
Buds were collected in the early morning. Since the stevia anthers were too small to be seen with eyes, a stereomicroscope was placed into the laminar flow cabinet, and the anthers were dissectioned with the help of a stereomicroscope. Anthers were carefully dissected, and filament was cut to prevent callus formation from somatic cells and placed on polystyrene sterile petri dishes (150 mm × 15 mm) containing 20 ml of solid medium. Fifteen anthers were placed into each petri dish. Petri dishes were sealed with plastic paraffin film (parafilm) for protecting contamination and incubated at 24 ± 1, °C 4000 lx, 16/8 h photoperiods in Panasonic MLR-352-PE Climate Chamber.
Culture Media
MS (Duchefa-Murashige and Skoog medium including vitamins) basal medium was used. 30 g l−1 sucrose (Duchefa-S0809) was used as a carbon source and solidified with 0.4% gelrite (Duchefa- GELRITE G1101). Plant growth regulators were added to culture medium, and pH of the medium was adjusted to 5.7 with 1 normality (N) sodium hydroxide (NaOH) or 1 N hydrochloric acid (HCl) before autoclaving. Sterilization was carried out for 20 min in an autoclave having 1 atmosphere (atm) pressures at 121 °C.
Ethylene is released from cultured plants, tissues, and organs, and all kinds of cells and the amount of secretion can be changed when stress conditions are increased (Garcia and Einset 1983). It is known that silver ions control the ethylene mechanism in plants, but at the same time, it can prevent copper uptake (Beyer 1976a, b). For this purpose, 5 mg l−1 silver nitrate (AgNO3) was initially used as a silver ion source in the media to control the amount of ethylene released, and this rate was reduced to 2 mg l−1 in the second year.
First-Year Experiment
Cultured anthers were kept in the dark for 1 week in first-year experiments and then incubated at 25 °C, 4000 lx light intensity, and 16/8 h photoperiod. The first year experiment was started with 11 media with different growth regulators (Table 1). AgNO3 which is known to have a positive effect on the viability of the anthers was added at 5 mg l−1. The first-year experiment aimed to promote embryo formation both directly from the anthers and through callus formation.
Second-Year Experiment
AgNO3 was optimized to be 2 mg l−1 on medium. In order to initiate androgenesis, high-temperature (35 °C) and cold (+ 4 °C) shocks have also been applied to encourage embryogenesis directly or indirectly from anthers. Pre-treatments were applied directly to the anthers in culture condition. All pre-treatments were applied in dark condition with 2 days later anthers stayed on 25 °C ± 1 5 days dark, and finally anthers were incubated in normal 25 °C ± 1, 4000 lx light intensity, and 16/8-h photoperiod. Culture medium and replicates of all experiments are given in Table 1.
Results and Discussion
Anther culture is regarded as a potential technique for production of 100% homozygous lines. However, the underlying mechanisms of the androgenesis have not been fully exploited, and there are no preliminary studies on stevia. One of the most important factors in pollen embryogenesis, which is under the influence of many factors, is the genotype. In studies conducted within the same species, one genotype gives response to androgenesis and the other genotype does not, and some species are known to be recalcitrant (Heberle-Bors 1982; Bajaj 1990; Chupeau et al. 1998; Pechan and Smykal 2001; Germanà 2007). Our study suggests that stevia is a recalcitrant species on the basis of anther culture in the present study and further studies are required to confirm the results.
Optimum temperature and duration of application in anther culture vary from species to species, and application times vary according to the source of the explant (all flowers, only buds or isolated anthers) to be used at the same time (Dunwell 1981). In our preliminary studies, buds were subjected to cold pre-treatment at different times (2, 4, 6, 8 days) but were found to have no effect on androgenesis. Therefore, in this study, pre-treatment was applied directly on anthers in culture medium. It is aimed that all microspores stay in the same stage and shift the development of microspores from the gametophyte direction to the sporophyte direction. Indeed, the underlying reason for the hot pre-treatment is to direct the development of unresponsive anthers.
In the first year experiments, callus was obtained from three different mediums, and no differentiation from anthers occurred in other culture media. The best response at the end of the first year was obtained with an embryo rate of 13.33% from the MS 2.0 mg l−1 NAA + 0.5 mg l−1 BAP medium, followed by MS 0.5 mg l−1 2,4-D + 0.5 mg l−1 BAP and MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP with callus formation rates of 10% and 3.33%, respectively (Table 2). Both the embryogenic calli and the non-embryogenic calli in the yellow watery crisp structure did not come to a differentiation afterward, and these structures slowly turned brown and lost their vitality.
In the results of the second year, although there was no significant difference between the applications, organogenesis occurred through the callus from the cold pre-treatment, and a large number of bar-shaped embryos were obtained from the hot pre-treatment. Embryogenic callus was obtained in 7.5% each of MS 0.5 mg l−1 NAA + 0.5 mg l−1 BAP and MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP media that were not applied to pre-treatment. In hot pre-treatment, MS 1.0 mg l−1 NAA + 0.5 mg l−1 Kinetin media produced 2.5% embryogenic callus and MS 2.0 mg l−1 NAA + 0.5 mg l−1 Kinetin media produced 37.5% bar-shaped embryo. In the cold pre-treatment, MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP medium had organogenesis through callus at a rate of 7.5% (Table 3).
In this study, the anthers were cultured in combination with NAA, 2,4-D, BAP, Kinetin, and their different doses in order to find optimum conditions in the stevia. Maximal callus was taken from medium containing MS 1.0 mg l−1 and 2.0 mg l−1 NAA + 0.5 mg l−1 BAP. Plant growth regulators, especially auxins, are widely used to initiate androgenesis from plants, and necessary growth regulators concentrations vary from plant to plant (Kasha et al. 1990). Stress treatments (cold pre-treatment, heat pre-treatment, starvation) applied to plants are known to stimulate androgenesis in different species and to increase the rate of androgenic callus obtained (Touraev et al. 1997; Immonen and Robinson 2000; Pechan and Smykal 2001). Particularly, cold and hot pre-treatment are known to direct plant growth when applied in the first stage of the microsporal development and affects positively but the applications to be made and the duration of these applications vary in every species and there are also differences in the plant organ to be treated (isolated anther, whole plant or just bud) (Ferrie et al. 1995). Two days of temperature shocks (+ 4 °C and 35 °C) were tried because there was no study made in stevia. According to Badigannavar (1996) and Saji and Sujatha (1998), cold pre-treatment positively affects androgenesis in sunflower, but in our study, it was found that temperature shocks did not affect callus formation. On the contrary, organogenesis via callus has come to the end of the cold pre-treatment. It can be said that cold pre-treatment promotes organogenesis from callus while not affecting callus formation. On the other hand, while kinetin hormones application increases the formation of bar-shaped embryos, unfortunately, the transformation from embryos to plantlets is not achieved.
The whole progress started from the inoculation of anther to obtained plantlet which is shown in Fig. 3. The plantlet obtained from cold pre-treatment (MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP) was understood in the light of observations that they are diploid structure. One of the widespread problems in anther culture is the plant development origin from somatic tissues (anther wall). In further studies, microspore cultures can be performed to prevent plant growth from somatic tissue. On the other hand, the anthers of stevia are too small to see in naked eyes, and all operations are performed under a stereomicroscope, which makes anther culture very difficult in stevia. Also, anthers are too fragile. DH technique is very important on cross-pollinated species for shortening the breeding period. This study showed that stevia anther culture performance is promising, but more extensive studies with different stress factors are needed to obtain haploid plants.
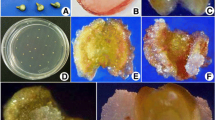
aStevia rebaudiana Bertoni plant, whose filaments are cut and cultured on MS, b callus with yellow-green and dark yellow areas obtained from MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP medium, c embryogenic callus containing dark green-colored bar-shaped embryo and anthocyanin structures developed in MS 2.0 mg l−1 NAA + 0.5 mg l−1 BAP medium, d green embryogenic callus and hairy anthocyanin structures observed in MS 2.0 mg l−1 NAA + 0.5 mg l−1 BAP medium, e embryogenic callus obtained in MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP medium, f embryogenic callus developing three different anthers in the MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP media g a single piece of callus obtained by warm application from a large number of bar-shaped embryo formation in MS 2.0 mg l−1 NAA + 0.5 mg l−1 KINETIN, h organogenesis via callus by cold application of MS 1.0 NAA + 0.5 BAP medium, i clonal propagation using axillary buds from sufficiently developed plants, j as a result of the cold application, the plantlets from the MS 1.0 mg l−1 NAA + 0.5 mg l−1 BAP medium were transferred to the soil (15 plants were clonally propagated in vitro conditions)
References
Aman, N., F. Hadi, S.A. Khalil, R. Zamir, and N. Ahmad. 2013. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). Comptes Rendus - Biologies 336: 486–492. https://doi.org/10.1016/j.crvi.2013.10.002.
Anbazhagan, M., M. Kalpana, and R. Rajendran. 2010. In vitro production of Stevia rebaudiana Bertoni. Emirates Journal of Food and Agriculture 22(3): 216–222.
Asif, M. 2013. Androgenesis: A fascinating doubled haploid production process. In Progress and opportunities of doubled haploid production, 7–44. Heidelberg: Springer.
Badigannavar, A.M., and M.S. Kuruvinashetti. 1996. Bractas an Explant for callus induction and shoot bud formation in sunflower (Helianthus annus L.). Agricultural Sciences 19: 38–38.
Bajaj, Y.P.S. 1990. In vitro production of haploids and their use in cell genetics and plant breeding, 3–44. https://doi.org/10.1007/978-3-642-61499-6_1.
Beyer, E.M. 1976a. A potent inhibitor of ethylene action in plants. Plant Physiology 58: 268–271. https://doi.org/10.1104/pp.58.3.268.
Beyer, E.M. 1976b. Silver ion: A potent antiethylene agent in cucumber and tomato. HortScience 11(3): 195–196.
Bhojwani, S.S., and M.K. Razdan. 1996. Plant tissue culture: Theory and practice, a revised edition. Amsterdam: Elsevier Science Publishers.
Chupeau, Y., M. Caboche, and Y. Henry. 1998. Androgenesis and haploid plants. Berlin: Springer.
Duncan, E.J., and E. Heberle. 1976. Effect of temperature shock on nuclear phenomena in microspores of Nicotiana tabacum and consequently on plantlet production. Protoplasma 90: 173–177. https://doi.org/10.1007/BF01276486.
Dunwell, J.M. 1981. Stimulation of pollen embryo induction in tobacco by pretreatment of excised anthers in a water-saturated atmosphere. Plant Science Letters 21(1): 9–13. https://doi.org/10.1016/0304-4211(81)90063-8.
Dunwell, J.M. 2010. Haploids in flowering plants: Origins and exploitation. Plant Biotechnology Journal 8(4): 377–424. https://doi.org/10.1111/j.1467-7652.2009.00498.x.
Ferrie, A.M.R., C.E. Palmer, and W.A. Keller. 1995. Haploid embryogenesis. In In vitro embryogenesis in plants, ed. T.A. Thorpe, 309–344. Dordrecht: Springer. https://doi.org/10.1007/978-94-011-0485-2_9.
Garcia, G.F., and J.W. Einset. 1983. Ethylene and ethane production in 2,4-D treated and salt treated tobacco tissue cultures. Annals of Botany 51(3): 287–295. https://doi.org/10.1093/oxfordjournals.aob.a086469.
Germanà, M.A. 2007. Haploidy. In Citrus genetics, breeding and biotechnology, ed. I.A. Khan, 167–196. Wallingford: CABI. https://doi.org/10.1079/9780851990194.0167.
Germanà, M.A. 2011a. Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Reports 30(5): 839–857. https://doi.org/10.1007/s00299-011-1061-7.
Germanà, M.A. 2011b. Anther culture for haploid and doubled haploid production. Plant Cell, Tissue and Organ Culture 104(3): 283–300. https://doi.org/10.1007/s11240-010-9852-z.
GRV. 2018. Grand view research report on Stevia Market Size, Share & Trends Analysis report by application (beverages, food, pharmaceutical, tabletop sweeteners), by region (North America, APAC, Europe, MEA, Latin America), and segment forecasts, 2018–2024. https://www.grandviewresearch.com/press-release/global-stevia-market. Accessed 11 Jan 2019.
Heberle-Bors, E. 1982. In vitro pollen embryogenesis in Nicotiana tabacum L. and its relation to pollen sterility, sex balance, and floral induction of the pollen donor plants. Planta 156(5): 396–401. https://doi.org/10.1007/BF00393309.
Immonen, S., and J. Robinson. 2000. Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Science 150(1): 77–84. https://doi.org/10.1016/S0168-9452(99)00169-7.
Kasha, K.J., and M. Maluszynski. 2003. Production of doubled haploids in crop plants. An introduction. In Doubled haploid production in crop plants, 1–4. Dordrecht: Springer. https://doi.org/10.1007/978-94-017-1293-4_1.
Kasha, K.J., A. Ziauddin, and U.H. Cho. 1990. Haploids in cereal improvement: Anther and microspore culture. In Gene manipulation in plant improvement II, ed. J.P. Gustafson, 213–235. Boston: Springer. https://doi.org/10.1007/978-1-4684-7047-5_11.
Kinghorn, A.D. 2002. Stevia. London: CRC Press.
Pechan, P.M., and P. Smykal. 2001. Androgenesis: Affecting the fate of the male gametophyte. Physiologia Plantarum 111(1): 1–8. https://doi.org/10.1034/j.1399-3054.2001.1110101.x.
Raina, S.K., and S.T. Irfan. 1998. High-frequency embryogenesis and plantlet regeneration from isolated microspores of indica rice. Plant Cell Reports 17(12): 957–962. https://doi.org/10.1007/s002990050517.
Ruiz-Ruiz, J.C., Y.B. Moguel-Ordonez, A.J. Matus-Basto, and M.R. Segura-Campos. 2015. Antidiabetic and antioxidant of Stevia rebaudiana extracts (var. Morita) and their incorporation into a potential functional bread. Journal of Food Science and Technology 52(12): 7894–7903.
Saji, K.V., and M. Sujatha. 1998. Embryogenesis and plant regeneration in anther culture of sunflower (Helianthus annuus L.). Euphytica 103(1): 1–7.
Sato, S., N. Katoh, S. Iwai, and M. Hagimori. 2002. Effect of low temperature pretreatment of buds or inflorescence on isolated microspore culture in Brassica rapa (syn. B. campestris). Breeding Science 52(1): 23–26. https://doi.org/10.1270/jsbbs.52.23.
Singh, S.D., and G.P. Rao. 2005. Stevia: The herbal sugar of 21st century. Sugar Tech 7(1): 17–24. https://doi.org/10.1007/BF02942413.
Skaria, B.P., R. Joseph, G. Mathew, S. Malhew, and P.P. Joy. 2014. Stevia: A sweet herb. Indian Journal of Arecanut Spices and Medicinal Plants 6: 24–27.
Touraev, A., O. Vicente, and E. Heberle-Bors. 1997. Initiation of microspore embryogenesis by stress. Trends in Plant Science 2(8): 297–302. https://doi.org/10.1016/S1360-1385(97)89951-7.
Ucar, E., Y. Ozyigit, A. Demirbas, D.Y. Guven, and K. Turgut. 2017. Effect of different nitrogen doses on dry matter ratio, chlorophyll and macro/micro nutrient content in sweet herb (Stevia rebaudiana Bertoni). Communications in Soil Science and Plant Analysis 48(10): 1231–1239. https://doi.org/10.1080/00103624.2017.1341917.
Ucar, E., Y. Özyiğit, and K. Turgut. 2016. The effects of light and temperature on germination of Stevia (Stevia rebaudiana BERT) seeds. Türkiye Tarımsal Araştırmalar Dergisi 3(1): 37–40. https://doi.org/10.19159/tutad.76528.
Zheng, M.Y. 2003. Microspore culture in wheat (Triticum aestivum)—doubled haploid production via induced embryogenesis. Plant Cell, Tissue and Organ Culture 73(3): 213–230. https://doi.org/10.1023/A:1023076213639.
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (Project No. 113O938).
Author information
Authors and Affiliations
Contributions
KT conceived and designed research. TU conducted laboratory work and handled the writing of the article, and DA helped laboratory work.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uskutoğlu, T., Uskutoğlu, D. & Turgut, K. Effects on Pre-treatment and Different Tissue Culture Media for Androgenesis in Stevia rebaudiana Bertoni. Sugar Tech 21, 1016–1023 (2019). https://doi.org/10.1007/s12355-019-00722-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00722-z