Abstract
Pulverized sugarcane bagasse consists of 37 ± 0.29 % cellulose, 28 ± 0.26 % hemicellulose, and 21 ± 0.28 % lignin on dry solid basis. About 11.58 % xylose, 6.39 % glucose, and 4.56 % fructose were liberated in hydrolysate when bagasse was treated with 1 % HNO3, under steam explosion. Simultaneous saccharification and fermentation of pretreated bagasse by cellulase and Pachysolen tannophilus MTCC 1077 were investigated in the present study. Important process variables for ethanol production from pretreated bagasse were optimized using response surface methodology based on central composite design (CCD) experiments. A three-level CCD experiments with central and axial points was used to develop a statistical model for the optimization of process variables. Data obtained from RSM on ethanol production were further subjected to the analysis of variance, and contour plots were used to study the interactions among three relevant variables (incubation temperature, inoculum concentration, and nutrient factors) of the fermentation process. Maximum ethanol concentration 9.15 g/l was obtained after 72-h incubation with P. tannophilus MTCC 1077 at the optimized process conditions in anaerobic batch fermentation when optimum values for temperature (34 °C), inoculum level (6 %), and fermentation medium (ammonium sulfate, KH2PO4, peptone, and yeast extract) were applied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world is developing not only industrially but also technologically. Along with these developments, the concern has also grown for an economy and environment particularly with the issues like global warming and depleting oil reserves. These issues are driving worldwide interest in exploring for renewably sourced materials that can be used as feedstock for biofuel production. When a fuel is obtained from organic materials such as energy crops, residues of crop, or waste biomass, it is termed as biofuel.
Ethanol can be a best option to mitigate the problems associated with the rising energy demands across the world. An advantage of biomass-based biofuels with respect to fossil fuels is that it significantly reduces the green house gas emission to an extent of 85 % (Perlack et al. 2005). Ethanol is not only an oxygenated fuel with high octane value but also provides superior performance because it is known to run combustion engines at higher compression ratios (Wheals et al. 1999). The blending of ethanol into petroleum-based automobile fuels can significantly decrease petroleum consumption. Methyl tertiary butyl ether (MTBE) is a known toxic contaminant in groundwater. Ethanol can be a safer substitute to this common additive in gasoline (Wang and Sheu 2000).
First-generation process of cellulosic ethanol production from sugarcane and starch-rich feed stocks will require more cultivable land, and this will lead to hike in food prices (Mitchell 2008). Use of lignocellulosic material for cellulosic ethanol production is a second-generation process and is one of the best approaches because of its widespread abundance, easy procurement, and affordable cost. Agro-industrial wastes such as husk, shell, peel, straw, stem, stalk, and bagasse are generated from sugarcane or sweet sorghum milling, during their industrial processing (Basavaraj et al. 2013; Ray et al. 2012). These wastes are generated in abundance, and available throughout the year. One of the major lignocellulosic materials to be considered in tropical countries is sugarcane bagasse, the fibrous residue obtained after extracting the juice from sugarcane in the sugar production process. It is the most abundant agricultural residue in Brazil, India, and China (Chandel et al. 2012) and has tremendous potential for the production of second-generation ethanol (Betancur and Pereira 2010). About 317–380 × 106 tons of bagasse is produced per year globally (Carmen 2009). India produces 179 metric tons/year sugarcane bagasse on an average, second after Brazil (Kapoor et al. 2006). Sugarcane bagasse is accumulated in large quantities at cane-to-sugar processing plants and consists approximately of 50 % cellulose, 25 % hemicellulose, and 25 % lignin (Haagensen and Ahring 2002). These are compounds of industrial engrossment. Due to this richness in composition, one can find a large scope both from economic and from environmental aspects if this waste is reutilized. The economical aspect is based on the fact that such waste may be used as low-cost raw material for the production of cellulosic ethanol, finally reduces the production costs. Most of the agro-industrial wastes also contain phenolic compounds of toxic nature, which may effect the environment.
Taking all the above aspects into consideration, the aim of the study is to develop an economical and environment-friendly bioprocess to produce cellulosic ethanol from bagasse. To achieve the above aim, experiments were scientifically designed using response surface methodology (RSM) for developing, improving and optimizing the ethanol yield.
Materials and Methods
Materials
Sugarcane bagasse sample was obtained from sugarcane juice vendors of Gwalior, India. The bagasse sample was made into 100-mesh (0.15-mm) fine powder by use of laboratory blender at 3000 rpm. Sample was preserved in a sealed plastic bag at 4 °C to prevent any possible degradation or spoilage.
Analysis of Chemical Composition of Bagasse
The cellulose, lignin, and hemicellulose fractions of powdered bagasse were determined according to Technical Association of the Pulp and Paper Institute (TAPPI), test methods (1992).
Delignification and Detoxification
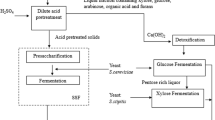
Various pretreatment techniques such as steam autoclaving in an autoclave at 15 psi (121 °C) for 60 min; dilute sulfuric acid (1 % v/v), concentrated sulfuric acid (10 % v/v), sodium hydroxide (1 % v/v), nitric acid (1 % v/v), and calcium hydroxide (1 % v/v) with steam autoclaving at 15 psi (121 °C) for about 20 min, were adopted separately for the pretreatment of bagasse. The pretreated bagasse was collected and filtered in crucibles followed by a wash with distilled water under suction. Finally, it was dried at room temperature before enzymatic hydrolysis (Szczodrak and Fiedurek 1996; Kaar et al. 1998).
Detoxification was followed the standard methods of overliming (calcium oxide), neutralization (HCl), and treatment with activated charcoal (Carvalheiro et al. 2005). These detoxifying agents were used directly without any preparation. After neutralization, the mixture was left for 30 min under moderate mixing followed by vacuum filtration to remove precipitates and other salts. In total, 2.5 % activated charcoal was then added and continued to mixing for 30 min in orbital shaker. The reaction mixture was filtered twice to remove charcoal, and pH was cross-checked. The pH was adjusted to 6.0–6.5 (Chandel et al. 2013a, b).
Hydrolytic Enzymes Production
Production of crude cellulase was done from Trichoderma reesei NCIM 1052. The medium for crude cellulase enzyme extraction was prepared by adding (per liter) 45 g wheat bran, 15 g yeast extract, 10 g glucose, 2.5 g NH4Cl, 0.5 g thiamine hydrochloride, 2.0 g K2HPO4, 0.5 g MgSO4·7H2O, 0.1 g CaCl2, and 0.5 g KCl. This medium was inoculated with actively growing T. reesei NCIM 1052. The flasks were incubated for 10 days on a rotary shaker. After 10 days of incubation, the culture broth was centrifuged at 10,000 rpm for 20 min to remove mycelia and spores. The supernatant was collected and used as the source of enzyme, which was stored at 4 °C till use (Abdulla et al. 2000).
Enzyme Assay
One milliliter of 0.05 M sodium citrate having pH 4.8 was added to a test tube. To this 0.5 ml of enzyme was added. One strip of Whatman No. 1 filter paper (weighing 50 mg) was put into test tube (the filter paper strip has to be pushed down whenever it winds up the test tube). The tube along with blank was kept in a water bath at 50 °C for 60 min. After 60 min, the tubes were taken out, and dinitrosalicylic acid (DNSA) method of Miller (1959) was followed further to account for the amount of sugars released by the cellulase (Singhania et al. 2006). One unit (U) of each enzyme activity is defined as the amount of enzyme, which produces 1 µmol reducing sugar as glucose in the reaction mixture per minute under the above-specified conditions.
Enzymatic Hydrolysis
Enzymatic hydrolysis of 1 % HNO3 delignified bagasse (300 g) was carried out in a 5-l round-bottom glass vessel (equipped with agitator for stirring and outer jacket for water circulation to maintain the required temperature) containing 3-l citrate buffer (pH 5.0 ± 0.2, 50 mM, 50 ± 0.5 °C) at 100 rpm. The cellulosic substrate was soaked in the citrate buffer for 2 h before adding the enzymes. Sodium azide was also added at a concentration of 0.005 % to restrict any microbial growth during the course of enzymatic hydrolysis. The substrate soaked in citrate buffer was supplemented with cellulase 5 FPU/g at substrate to enzyme ratio of 1:5 (Singh et al. 1990). Samples were withdrawn after 48 h, centrifuged, and supernatant analyzed for total reducing sugars released. The amount of reducing sugars was estimated by DNSA method as described by Miller (1959). The extent of hydrolysis was calculated as:
Microorganism and Maintenance
The wild-type strain of Pachysolen tannophilus MTCC 1077 was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India, and was used in the present investigation. The wild strain of T. reesei NCIM 1052 was procured from National Collection of Industrial Microorganisms, National Chemical Laboratory, Pune, India. Yeast culture was maintained on malt yeast agar medium with following composition (g/l)—malt extract, 3; yeast extract, 3; peptone, 5; glucose, 10; agar, 20, pH 7.0 ± 0.2. The strain of T. reesei NCIM 1052 was maintained on PDA slants consisted of (g/l): potato, 200; dextrose, 20; agar, 25, pH 4.8 ± 0.2. Stock cultures were stored at 4 °C. The liquid medium for the growth of inoculum for yeast was YEPD medium consisted of (g/l): yeast extract, 10; peptone, 20; dextrose, 20, pH 5.00 ± 0.2 for 48 h at 28 ± 0.5 °C (Pasha et al. 2007). Inocula was grown aerobically in 250-ml Erlenmeyer flasks containing the above-mentioned medium at 28 °C in an environmental shaker (Remi Scientific) at 200 rpm for 24 h. Active cells were centrifuged in a clinical centrifuge (1200 rpm), washed with sterile water, and were used as inoculum.
Immobilization of Yeast and Simultaneous Saccharification and Fermentation
Immobilization of yeast was done by sodium alginate method (Smidsrod and Skjak-Braek 1990). Batch experiment was conducted as per the central composite experimental design for ethanol production in a 500-ml capacity of Erlenmeyer flask. Parameters, like temperature, inoculum concentration, and nutrient factor, were chosen as the most significant ones, considering the experimental design. The process was conducted at the initial substrate concentration of 20 g/l pretreated substrate (i.e., bagasse) and 200-ml citrate buffer (pH 5.0 ± 0.2, 50 mM) followed by sterilization for 15 min, at 15 psi (121 °C). The substrate soaked in citrate buffer was supplemented with cellulase, at substrate to enzyme ratio of 1:5 (20-g pretreated substrate: 100-ml crude cellulase) or 5 FPU of cellulase was used for hydrolyzing per gram of substrate. The saccharification was done for 24 h at 50 °C after which simultaneous fermentation was conducted in the same vessel by addition of 50 ml of sterilized detoxified hydrolysate (obtained after pretreatment) and different nutrients (nutrient parameter 1/2/3, as shown in Table 1) at lower temperatures (30/32/34 °C). Immobilized yeast cells were used as inoculum at different concentrations viz. 2, 4, or 6 %. MgSO4, 0.5 g/l; KCl, 0.5 g/l, and FeSO4 0.01 g/l were used as common nutrients in all fermentation experiments other than different nutrient parameters mentioned above. Fermentation was carried out for 72 h after which samples were withdrawn and centrifuged in a laboratory centrifuge at 1200 rpm, and the supernatants were analyzed for ethanol concentration (Chandel et al. 2009).
Analytical Methods
Biochemical Composition Analysis
Total reducing sugars were estimated by dinitrosalicylic acid method of Miller (1959).
The quantitative and qualitative analysis of sugars in the hydrolysates after pretreatment were analyzed using high-performance liquid chromatography (HPLC, Waters) with Sugar-Pak column (Waters USA) and a system composed of a 510 pump, a refraction index differential detector (RI 2414 USA) and a data processor with register (Waters, USA). The samples were filtered through membrane filters 0.45 µm (Millipore) before injection. The temperature of the column was maintained at 70 °C by column oven (Dyna, Mumbai) with injection valve of 20 µl. The RI detector was operated at 30 °C, and the solvent systems used were water as mobile phase at flow rate of 0.2 ml/min. Calculations and analysis were performed using Empower 2 software Build 2154 (Waters).
The supernatants of SSF were analyzed for ethanol concentration using gas liquid chromatography with the following specifications—AGELIENT 7890 was the G. C system with DB1624 (30*0.5) 3 Um column. Water was used for solubility. In total, 10 µl of ethanol was taken as standard and dissolved in 10 ml water. A total of 2.7 g of sample was dissolved in 5 ml of water.
All experiments were carried out in duplicate, and all the reported results are the mean values. The average standard deviation of the achieved results was <4 %.
Experimental Design and Statistical Analysis
In the central composite design (CCD), the total number of experimental combinations was 2K + 2K + n 0, where K is the number of independent variables, and n 0 is the number of repetitions of the experiments at the central point, which indicated that 20 experiments were required for this procedure. The dependent variable selected for this study was ethanol concentration, Y (g/l). The independent variables chosen were incubation temperature (30, 32 and 34 °C) X1, inoculum level (2, 4 and 6 %) X2 and nutrients (1/2/3) X3. A mathematical model, describing the relationships between the process-dependent variable and the independent variables in a second-order equation, was developed (Giovanni 1983). Design-based experimental data were matched according to the following second-order polynomial equation (1).
where i, j are linear, quadratic coefficients, respectively, while ‘b’ is regression coefficient, k the number of factors studied and optimized in the experiment, and ‘e’ is random error. The quality of fit of the second-order equation was expressed by the coefficient of determination R 2, and its statistical significance was determined by F test. The significance of each coefficient was determined using Student’s t test. The Student t test was used to determine the significance of the parameters regression coefficients. The P values (probability value) were used as a tool to check the significance of the interaction effects, which in turn may indicate the patterns of the interactions among the variables. In general, larger magnitudes of t and smaller of P indicate that the corresponding coefficient term is significant. The response surface equation was optimized for maximum yield in the range of process variables using Design Expert software version 9.0.2. Analysis of variance (ANOVA) for the final predictive equation was performed using the same software package. Iso-response contour plots were obtained based on the effect of the levels of three parameters and their interactions on the yield of ethanol by keeping the other parameters at their optimal concentrations. From these contour plots, the interaction of one parameter with another parameter was studied. The optimum concentration of each parameter was identified based on the hump in the contour plots (Sasikumar and Viruthagiri 2010).
Optimized Simultaneous Saccharification and Fermentation (SSF) in a Modular Fermenter
Batch experiment was conducted as per the central composite experimental design for ethanol production in a fermenter (BioFlo®/CelliGen® 115), with 2-l capacity, equipped with flat-blade impeller, oxygen and pH electrodes, and temperature and dO2 (dissolved oxygen) probe. The equipment also monitored temperature, agitation speed, gas purging flow rate, pumping rates, antifoam addition, and the vessel level. All processing parameters were online monitored, with the aid of BioXpert Lite 1.00 software. Other parameters, like temperature, inoculum concentration, and nutrient factor, were chosen as the most significant ones, considering the experimental design. The process was conducted at the initial substrate concentration of 50 g/l (pretreated bagasse) with the addition of citrate buffer (pH 5.0 ± 0.2, 50 mM) followed by sterilization for 15 min, at 15 psi (121 °C). The substrate soaked in citrate buffer was supplemented with cellulase, at substrate to enzyme ratio of 1:5 (i.e., 50 g pretreated substrate: 250-ml crude cellulase), i.e., 5 FPU/g substrate. The saccharification was done for 24 h at 50 °C after which simultaneous fermentation was conducted in same vessel by adding equal volume of sterilized detoxified bagasse hydrolysate (obtained after pretreatment) and components of nutrient factor 1. Immobilized P. tannophilus was used as inoculum at 6 % concentration. Fermentation was carried out anaerobically for 72 h at lower temperature of 30 °C after which samples were withdrawn and centrifuged in a laboratory centrifuge at 1200 rpm, and fermented broth was distilled at 74–75 °C to recover ethanol.
Results and Discussion
Chemical Composition of Bagasse
Bagasse was initially characterized with regard to its chemical composition. Pulverized bagasse consists of 37 ± 0.29 % cellulose, 28 ± 0.26 % hemicellulose, and 21 ± 0.28 % lignin on dry solid (DS) basis. The holocellulosic (hemicellulose + cellulose) content of bagasse was found to be 65 %. The presence of cellulose and hemicellulose together makes the total carbohydrate content (TCC) of the substrate (65.0 %). It can be fairly compared with the extensively explored lignocelluloses (corn stover, 58.29 %; wheat straw, 54 %; poplar, 58.2 %; birch 73 %; spruce, 63.2 %) for ethanol production (Chandel et al. 2013a, b).
Delignification and Enzymatic Hydrolysis
Among all the chemicals, steam explosion pretreatment of bagasse with HNO3 (1 % v/v) resulted in maximum hydrolysis (Table 2). The hydrolysate obtained after this pretreatment consisted of 11.58 % xylose, 6.39 % glucose, and 4.56 % fructose. HPLC chromatogram reflecting these sugars can be seen in Fig. 1. A comprehensive account of the above result indicates that 52.15 % saccharification (maximum) was obtained when bagasse was steam exploded with 1 % HNO3.
The decrease in sugar content in acid treated samples with increasing of acid concentration (10 % H2SO4) may be because of degradation of monomeric sugars (xylose, glucose) to furfural and hydroxymethyl furfural. These substances are toxic for yeast and can inhibit the yeast growth (Chandel et al. 2013a, b).
The presence of lignin in cellulosic substrates and the crystalline nature of cellulose make it inaccessible to cellulase and their coordinated action (Berlin et al. 2007). It is required to remove lignin for altering the structure of cellulosic biomass aiding the amenability of cellulolytic enzymes which in turn release the fermentable sugars (Tu et al. 2007; Rezende et al. 2011); therefore, lignocellulosic biomass is pretreated with either chemically or biologically prior to enzymatic hydrolysis. Pretreatment of lignocellulose with acids primarily reduces lignin content along with some fractions of hemicellulose while decrystallizing cellulose. Thus, it affects both micro- and macro-accessibility of the cellulases to the cellulose (Kim et al. 2008).
The maximum sugar liberated in the filtrate is a hemicelluloses sugar, i.e., xylose. It is because of amorphous nature of hemicelluloses that they are easily degraded. The cellulose has a majority crystalline character, and so its structure is not so easily broken by a mineral acid, especially when it is at low concentration, as in this work. 1 % HNO3 steam explosion pretreatment has been interpreted as representing the hydrolysis of the easy to hydrolysis fraction of the xylan. Peaks and area covered by different sugars representing the above percentages are shown in Fig. 1. One aspect that should be emphasized is that high concentration achieved, especially for xylose, was due to the scale used. The average volume of hydrolysate was 10.0 ml, making the sugar stay more concentrated. At larger scale, the concentration value would be lower because the volume of hydrolysate would be greater.
Enzymatic hydrolysis of 1 % HNO3 pretreated bagasse was carried out for depolymerization of cell wall carbohydrate fraction into fermentable sugars. Culture supernatant from T. reesei was used for enzymatic saccharification. Enzyme activity (U/ml) of crude cellulase produced by T. reesei NCIM 1052 was 311.1 µmol/ml/min and was loaded @5 FPU/g of substrate and then enzymatic hydrolysis was done at physical parameters (50 ± 0.5 °C, 100 rpm). During the course of enzymatic hydrolysis, a regular increase in released sugars was observed till 50 h and remained constant thereafter (data not shown). Enzymatic saccharification of 1 % HNO3 pretreated bagasse yielded a maximum of 340.10 ± 0.38 mg/g (3.4 ± 0.40 g/l) glucose with a hydrolysis efficiency of 70 ± 0.34 % after 48 h of treatment.
Enzyme loading of 5 FPU/g substrate was found sufficient to hydrolyze the cellulose present in pretreated bagasse. Results indicate that hemicellulose removal and the possible relocalization of lignin moieties during pretreatment could yield the desired amount of sugar toward the goal of developing an intensified and simplified process for cellulose saccharification.
Substrate concentration is one of the main factors that affects the yield and initial rate of enzymatic hydrolysis of cellulose. At low substrate levels, an increase in substrate concentration normally results in an increase in the yield and reaction rate of the hydrolysis (Cheung and Anderson 1997). However, high substrate concentration can cause substrate inhibition, which substantially lowers the rate of the hydrolysis, and the extent of substrate inhibition depends on the ratio of total substrate to total enzyme (Huang and Penner 1991; Penner and Liaw 1994). Huang and Penner (1991) found that the substrate inhibition occurred when the ratio of the microcrystalline substrate Avicel pH 10.1 to the cellulase from T. reesei [grams of cellulase FPU (filter paper unit, defined as a micromole of reducing sugar as glucose produced by 1 ml of enzyme per minute) of enzyme] was >5. Penner and Liaw (1994) reported that the optimum substrate to enzyme ratio was 1.25 g of the microcrystalline substrate Avicel pH 10.5 per FPU of the cellulase from T. reesei.
The amounts of enzymes required for hydrolysis of pretreated raw material depend upon the pretreatment applied to the substrate and the availability of carbohydrate content in the substrate (Saha et al. 2005). Zheng et al. (2009) observed that high enzyme loadings did not alter saccharification and yields. Rezende et al. (2011) reported 72 % cellulose conversion from consecutive acid–base-pretreated bagasse. A 65 % cellulose conversion was obtained after the enzymatic hydrolysis (1.91 % w/w pretreated bagasse, 20 FPU/g enzyme loading, 0.05 g/g surfactant) of bagasse pretreated with dilute sulfuric acid (1.75 % w/w bagasse content, 1.7 % w/w HNO3 loading, 150 °C, and 30-min pretreatment time) (Santos et al. 2011). Kristensen et al. (2008) also observed similar effect on wheat straw cell walls after hydrothermal pretreatment. It is possible that the acid pretreatment makes the lignin more receptive to cellulase adsorption through a change of surface properties, e.g., increased hydrophobicity or hydrogen-bonding capacity. It is also possible that the treatment dissolves hemicelluloses associated with or covering lignin, thereby increasing the accessibility of lignin and hence the adsorption (Kristensen et al. 2007).
Optimization of Process Variables in Ethanol Fermentation
Important process variables for ethanol production from pretreated substrates were optimized using response surface methodology (RSM) based on central composite design (CCD) experiments. The experimental results associated to the processing setup of each independent variable are listed in Table 3.
Three-level central composite design matrix and the experimental responses of the dependent variable (ethanol conc.) are listed in Table 4.
Besides the linear effect of the ethanol concentration, Y g/l, the response surface method also gives an insight into the parameters quadratic and combined effects. The analyses were done by using both Fisher’s F test and Student’s t test statistical tools. The regression coefficient, t and P values for all the linear, quadratic, and combined effects with a 95 % significance level are given in the Table 5.
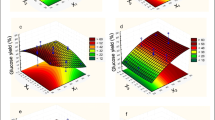
Figures 2, 3, and 4 show the iso-response contour plots of the interactive effect of incubation temperature, inoculum concentration, and nutrient factor on ethanol production. The response values for the variables can be predicted from these plots. The effect of inoculum concentration and nutrient on ethanol production, while other variable (temperature) was fixed at central level, is shown in Fig. 2. The effect of inoculum concentration and temperature on ethanol production, while other variable (nutrient) was fixed at central level, is shown in Fig. 3. The effect of nutrient and temperature on ethanol production, while other variable (inoculum concentration) was fixed at central level, is shown in Fig. 4. The results are as follows.
The Model F value of 8441.02 implies the model is significant (Table 5). There is only a 0.01 % chance that an F value this large could occur due to noise. Values of “Prob > F” <0.0500 indicate model terms are significant. In this case A, B, C, BC, A^2, B^2, C^2 are significant model terms. Values >0.1000 indicate the model terms are not significant. ANOVA of the regression model for ethanol yield demonstrated that the model was significant due to a very high F value and a very low probability value.
The regression equation obtained from the ANOVA shows that the R 2 (coefficient of determination) was 0.9999 (a value >0.75 indicates fitness of the model). This is an estimate of the fraction of overall variation in the data accounted by the model, and thus the model is capable of explaining 99.9 % of the variation in the response. The ‘adjusted R 2’ is 0.9997, which indicates that the model is good (for a good statistical model, the R 2 value should be in the range of 0–1.0, and the nearer to 1.0 the value is, the more fit the model is deemed to be). The “Predicted R 2” of 0.9990 is in reasonable agreement with the “Adjusted R 2” of 0.9997; i.e., the difference is <0.2. “Adequate Precision” measures the signal-to-noise ratio. A ratio >4 is desirable. Ratio of 393.995 indicates an adequate signal. Hence, this model can be used to navigate the design space.
The significant interaction between nutrient and temperature was apparent not only from the elliptical nature of the contour plot (Fig. 4), but also from the low probability value of 0.0009 (Table 5). The contours around the stationary point were elliptical, and it became elongated more and more along the inoculum concentration axis and temperature axis, which meant that a small change of the response value would require a small move along the inoculum concentration axis and temperature axis. It was evident that the ethanol concentration steadily increased with increasing inoculum concentration up to 6 % and temperature up to 34 °C. So a higher inoculum concentration and higher temperature enhances the ethanol yield. The other pair of the independent variables inoculum concentration and nutrient shows a less interactive effect (Fig. 2) while keeping the third independent variable, temperature at 34 °C. From Fig. 2, it was evident that the interactive effects between the test variables were less significant not only from the circular nature of the contour plot but also from the high probability value (P = 1.000). The other pair of the independent variables inoculum concentration and temperature also shows a less interactive effect (Fig. 3) while keeping the third independent variable, nutrient at 1. From Fig. 3, it was evident that the interactive effects between the test variables were less significant not only from the circular nature of the contour plot and also from the high probability value (P = 1.000).
The response surfaces can be used to predict the optimum range for different values of the test variables and the major interactions between the test variables can be identified from the circular or elliptical nature of the contours. The circular nature of the contours signify that the interactive effects between the test variables are not significant and optimum values of the test variables can be easily obtained.
From equations derived by differentiating equation 1, the optimum values for the independent variables obtained were incubation temperature 34 °C, inoculum concentration 6 % and nutrient factor 1. Based on the model, the optimal working conditions were obtained to attain high ethanol yield. Response analysis revealed the maximum ethanol concentration (10.1986 g/l) by P. tannophilus could be achieved at the optimum process conditions (Figs. 2, 3, 4).
Among yeasts, P. tannophilus resulted very interesting for their capacity to ferment xylose. Yeasts metabolize xylose by means of the xylose reductase (XR) that converts xylose to xylitol and xylitol dehydrogenase (XDH) that convert xylitol to xylulose. After phosphorylation, xylulose is metabolized through the pentose phosphate pathway (PPP) (Zaldivar et al. 2001). Bhatia and Paliwal (2011) also found ammonium sulfate and yeast extract best suited for growth of P. tannophilus.
Optimized SSF in a Modular Fermenter and Product Recovery
A single study at modular fermentor level was performed for ethanol production from bagasse. Response analysis revealed the maximum ethanol concentration (10.1986 g/l) by P. tannophilus could be achieved at the optimum process conditions from bagasse. For fermentation the optimum values for the independent variables obtained after RSM were incubation temperature 34 °C, inoculum concentration 6 % and nutrient factor 1. Based on the model, the optimal working conditions were set to attain high ethanol yield. These optimized parameters were set for ethanol production in modular fermentor for 72 h. Gas Liquid Chromatography (GLC) results reveled that fermented broth of modular fermentor had 9.15 g/l ethanol concentration after 72 h.
Conclusion
Bagasse is a potential, renewable and low-cost biomass for the production of ethanol by fermentation. 1 % HNO3 pretreated bagasse showed the maximum saccharification. It can therefore be concluded that cellulosic ethanol can be extracted through simultaneous saccharification and fermentation from the bagasse using the yeast P. tannophilus MTCC 1077 at the optimized process conditions in anaerobic batch fermentation.
Further research are required on other combinations and refinement of the different aspects of this methodology for higher cellulosic ethanol yield, as well as the feasibility examinations and about the economic returns.
References
Abdulla, E., T. Feanov, S. Costa, M.K. Robra, C.A. Paulo, and M.G. Gubitz. 2000. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsute. Applied and Environmental Microbiology 66: 3357–3362.
Basavaraj, G., P.P. Rao, K. Basu, R. Reddy, A.A. Kumar, P.S. Rao, and B.V.S. Reddy. 2013. Assessing viability of bio-ethanol production from sweet sorghum in India. Energy Policy 56:501–508. doi:10.1016/j.enpol.2013.01.012.
Berlin, A., V. Maximenko, N. Gilkes, and J.N. Saddler. 2007. Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnology Bioengineering 97: 287–296.
Betancur, G.J.V., and N. Pereira Jr. 2010. Sugarcane bagasse as feedstock for second generation ethanol production. Part I: Diluted acid pretreatment optimization. Electronic Journal of Biotechnology. doi:10.2225/vol13-issue3-fulltext-3.
Bhatia, L., and S. Paliwal. 2011. Ethanol producing potential of Pachysolen tannophilus from sugarcane bagasse. International Journal of Biotechnology and Bioengineering Research 2(2): 271–276.
Carmen, S. 2009. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances 27: 185–194.
Carvalheiro, F., L.C. Duarte, S. Lopes, J.C. Parajo, H. Pereira, and F.M. Girio. 2005. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochemistry 40: 1215–1223.
Chandel, A.K., F.A. Antunes, and V. Anjos. 2013a. Ultra-structural mapping of sugarcane bagasse after oxalic acid fiber expansion (OAFEX) and ethanol production by Candida shehatae and Saccharomyces cerevisiae. Biotechnology for Biofuels 6: 4. doi:10.1186/1754-6834-6-4.
Chandel, A.K., S.S. da Silva, and O.V. Singh. 2013b. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering towards white biotechnology. Bioengineering Research 6: 388–401.
Chandel, A.K., M.L. Narasu, G. Chandrasekhar, A. Manikyam, and L.V. Rao. 2009. Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresource technology 100: 2404–2410.
Chandel, A.K., S.S. Silva, W. Carvalho, and O.V. Singh. 2012. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. Journal of Chemical Technology and Biotechnology 87: 1–20.
Cheung, S.W., and B.C. Anderson. 1997. Laboratory investigation of ethanol production from municipal primary wastewater. Bioresource technology 59: 81–96.
Giovanni, M. 1983. Response surface methodology and product optimization. Journal of Food Technology 37: 41–45.
Haagensen, F., and B.K. Ahring. 2002. Enzymatic hydrolysis and glucose fermentation of wet oxidized sugarcane bagasse and rice straw for cellulosic ethanol production. Riso-R-1517 (EN) 1: 184–195.
Huang, X.L., and M.H. Penner. 1991. Apparent substrate inhibition of the Trichoderma reesei cellulase system. Journal of Agriculture Food Chemistry 39: 2096–2100.
Kaar, W.E., C.V. Gutierrez, and C.M. Kinoshita. 1998. Steam explosion of sugarcane bagasse as a pretreatment for conversion to ethanol. Biomass and Bioengineering 14(3): 277–287.
Kapoor, R.K., A.K. Chandel, S. Kuhar, R. Gupta, and R.C. Kuhad. 2006. Cellulosic ethanol from crop residues, production forecasting and economics: An Indian perspective. In Lignocellulose biotechnology: Current and future prospects, ed. R.C. Kuhad, and A. Singh, 32–45. New Delhi: I.K. International.
Kim, T.H., F. Taylor, and K. Hicks. 2008. Cellulosic ethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresource technology 99: 5694–5702.
Kristensen, J.B., J. Borjesson, M.H. Bruun, F. Tjerneld, and H. Jorgensen. 2007. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocelluloses. Enzyme and Microbial Technology 40: 888–895.
Kristensen, J.B., L.G. Thygesen, C. Felby, H. Jorgensen, and T. Elder. 2008. Cell-wall structural changes in wheat straw pretreated for cellulosic ethanol production. Biotechnology Biofuels 1(5): 1–9.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426–428.
Mitchell, D. 2008. In: A note of rising food prices. Policy research working paper 4682. Development Prospects Group. The World Bank, Washington DC pp. 1–21.
Pasha, C., N. Valli, and L.V. Rao. 2007. Lantana camara for fuel ethanol production using thermotolerant yeast. Letter of Applied Microbiology 44: 666–672.
Penner, M.H., and E.T. Liaw. 1994. Kinetic consequences of high ratios of substrate to enzyme saccharification systems based on Trichoderma cellulase. In Enzymatic conversion of biomass for fuels production, ed. M.E. Himmel, J.O. Baker, and R.P. Overend, 363–371. Washington, DC: American Chemical Society.
Perlack, R.D., L.L. Wright, A.F. Turhollow, R.L. Graham, B.J. Sotcks, and D.C. Erbach. 2005. In: Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply. DOE/GO-10 2005-2135, April. US. Department of Energy and US. Department of Agriculture. Oak Ridge National laboratory. USA, pp. 1–78.
Ray, S., A. Goldar, and S. Miglani. 2012. The ethanol blending policy in India. Economic and Political Weekly 1: 23–25.
Rezende, C.A., M.A. de Lima, P. Maziero, E.R. deAzevedo, W. Garcia, and I. Polikarpov. 2011. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnology for Biofuels 4: 54. doi:10.1186/1754-6834-4-54.
Saha, B.C., L.B. Iten, M.A. Cotta, and Y.V. Wu. 2005. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochemistry 40: 3693–3700.
Santos, V.T.O., P.J. Esteves, A.M.F. Milagres, and W. Carvalho. 2011. Characterization of commercial cellulases and their use in the saccharification of a sugarcane bagasse sample pretreated with dilute sulfuric acid. Journal of Indian Microbiology and Biotechnology 38: 1089–1098.
Sasikumar, E., and T. Viruthagiri. 2010. Simultaneous saccharification and fermentation (SSF) of sugarcane bagasse: Kinetics and modeling. International Journal of Chemical and Biological Engineering 3(2): 57–64.
Singh, A., A.B. Abidi, N.S. Darmwal, and A.K. Agarwal. 1990. Saccharification of cellulosic substrates by Aspergillus niger cellulase. World Journal of Microbiology & Biotechnology 6: 333–336.
Singhania, R.R., R.K. Skumaran, A. Pillai, P. Prema, G. Szakacs, and A. Pandey. 2006. Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian Journal of Biotechnology 5: 332–336.
Smidsrod, O., and G. Skjak-Braek. 1990. Alginate as an immobilization matrix for cells. Trends in Biotechnology 8(3): 71–78.
Szczodrak, J., and J. Fiedurek. 1996. Technology for conversion of lignocellulosic biomass to ethanol. Biomass and Bioengineering 10: 367–375.
Tappi Test Methods. 1992. Technical Association of the Pulp and Paper Institute (TAPPI), Test Methods 1992–1993. Atlanta: TAPPI Press.
Tu, M., R. Chandra, and J.N. Saddler. 2007. Evaluating the distribution of cellulases and recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnology Progress 23: 398–406.
Wang, F.S., and J.W. Sheu. 2000. Multiobjective parameter estimation problems of fermentation processes using a high ethanol tolerance yeast. Chemical Engineering Sciences 55: 3685–3695.
Wheals, A.E., L.C. Basso, M. Denise, G. Alves, and H. Amorim. 1999. Fuel ethanol after 25 years. Trend in Biotechnology 17: 482–487.
Zaldivar, J., J. Nielsen, and L. Olsson. 2001. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Applied Microbiology and Biotechnology 56: 17–34.
Zheng, Y., Z. Pan, R. Zhang, and D. Wang. 2009. Enzymatic saccharification of dilute acid pretreated saline crops for fermentable sugar production. Applied Energy 86: 2459–2467.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, L., Johri, S. Optimization of Simultaneous Saccharification and Fermentation Parameters for Sustainable Ethanol Production from Sugarcane Bagasse by Pachysolen tannophilus MTCC 1077. Sugar Tech 18, 457–467 (2016). https://doi.org/10.1007/s12355-015-0418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-015-0418-6