Abstract
Pancreas divisum (PD) represents a prevalent congenital pancreatic variant, typically arising from the failure of fusion between the ventral and dorsal pancreatic ducts. This condition is frequently associated with recurrent pancreatitis. We herein present a case involving an incomplete PD diagnosis following the identification of a refractory postoperative pancreatic fistula (POPF) after laparoscopic distal pancreatectomy (DP) for pancreatic cancer. A 74-year-old female patient, who had undergone laparoscopic DP for pancreatic cancer, developed a POPF accompanied by intraabdominal bleeding, necessitating urgent intervention radiology to avert life-threatening complications. Following this, intraabdominal drainage was performed through an intraoperative drainage root. Subsequent fistulography and endoscopic retrograde pancreatography unveiled the presence of an incomplete PD for the first time. Consequently, a stent was placed in the Santorini duct. However, the volume of pancreatic juice from the intraabdominal drainage tube exhibited no reduction. Despite repeated attempts to access the pancreatic duct via a guidewire through the drainage tube, these endeavors proved futile. Paradoxically, the removal of the external drainage tube led to a recurrence of intraabdominal abscess formation. Consequently, reinsertion of the drainage tube became imperative. Consideration was given to draining the abscess under endoscopic ultrasonography and performing pancreatic duct drainage. However, due to the diminution of the abscess cavity through the external fistula drainage procedure, coupled with the absence of pancreatic duct dilation and its tortuous course, it was deemed a formidable challenge. the patient necessitated a lifestyle adaptation with a permanently placed percutaneous drainage tube.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreas divisum (PD) stands as the most prevalent congenital variation in pancreatic anatomy, correlating with episodes of acute or chronic pancreatitis [1, 2]. PD results from the incomplete fusion of the embryological ventral and dorsal pancreatic ducts, categorized into three types: Type 1 involves complete separation of the ventral pancreatic duct and dorsal pancreatic duct, Type 2 lacks the ventral pancreatic duct, and Type 3 exhibits a functionally inadequate, narrow communication branch between the ventral and dorsal pancreatic duct [3] (Fig. 1). Although a relatively rare pathology, constituting approximately 1% of cases in Japan [1], its prevalence in Western countries is reported to be around 10% [4]. Typically, in PD, the accessory ducts and the accessory papilla serve as the primary drainage route for the larger dorsal pancreas. However, owing to the narrower opening of the accessory papilla compared to the main papilla, functional insufficiency in pancreatic juice outflow is more likely to occur [3].
Pancreas divisum is categorized into three types based on the degree of ductal connection. Type 1, representing 70% of cases, is characterized by the complete absence of fusion between the ventral and dorsal pancreatic ducts (a). Type 2, accounting for 25% of cases, is marked by the absence of the ventral duct, leading to the convergence of the pancreatic and bile ducts at the minor papilla (b). Type 3, less common at 5%, involves incomplete fusion between the dorsal and ventral ducts (c)
Conversely, distal pancreatectomy (DP), a frequently employed surgical intervention for tumors located in the pancreatic tail, carries the potential complication of postoperative pancreatic fistula (POPF) [5]. While POPF often ameliorates with suitable decompression and drainage measures, our case exhibited persistent symptoms despite the insertion of a pancreatic duct stent and an intraperitoneal drainage tube. The presence of incomplete PD was postulated as a contributing factor to the refractory nature of POPF in this particular case.
In this report, we present a case of PD with refractory POPF following laparoscopic DP. The condition was successfully managed by combining endoscopic accessory papillotomy with transpapillary and percutaneous drainage, although the removal of the external fistula tube was not achieved. Our findings are discussed in the context of existing literature.
Case report
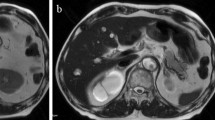
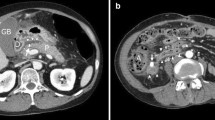
A 74-year-old woman with pancreatic body cancer was referred from a previous hospital because the patient is considered high-risk due to coronary artery calcification, the use of steroids for rheumatoid arthritis, and the presence of diabetes. Although the previous doctor performed MRCP, we could not unfortunately diagnose PD at that time (Fig. 2). The preoperative considered risk factors for POPF were diabetes (HbA1c 7.1%) and long-term use of steroids (5 mg/day, over 30 years). In contrast, Obesity (BMI 19.6) and malnutrition (Albumin 3.9 g/dL) were not observed. She had no history of pancreatitis. Thus, the patient has been scheduled for laparoscopic distal pancreatectomy (DP) for resectable pancreatic body cancer. Based on intraoperative observations, the pancreatic tissue, however, exhibited a hardened consistency, accompanied by inflammation-induced adhesions and tissue sclerosis in the surrounding area. Consequently, the dissection of the pancreas from the retroperitoneum proved challenging. The patient, who was currently undergoing steroid therapy for rheumatoid arthritis, exhibited no overt symptoms; however, there is speculation that chronic pancreatitis may have been occurring. On the other hand, the pancreas was dissected with a Reinforced black stapler (Medtronic) just above the portal vein. However, in the intraoperative rapid diagnosis, the margin was diagnosed as positive, leading to an additional 1 cm resection. Under these circumstances, the surgery lasted for 554 min with a blood loss of 50 g. On a postoperative day (POD) 7, the patient was diagnosed with a POPF through drain amylase level. In addition, intraabdominal bleeding manifested on POD 11. Contrast-enhanced CT and interventional radiology confirmed intraabdominal bleeding from the common hepatic artery. Thus, embolization due to coiling was performed. On POD 18, an ERP and fistulography were conducted for stent placement, revealing an incomplete PD and intraabdominal abscess cavity (Fig. 3). Despite the placement of a pancreatic duct stent from the minor papilla, there was no observed reduction in drainage output (Fig. 4). Subsequently, despite regular exchanges and cleaning of the external fistula tube, along with attempts to insert a guidewire through the tube to access the pancreatic duct, no success was achieved within a period of two months. Additionally, we considered the possibility of the pancreatic duct being occluded by the pancreatic duct stent and proceeded to remove the pancreatic stent. Furthermore, the percutaneous drainage tube was temporarily removed, considering the continuous drainage of pancreatic fluid from the percutaneous fistula tube. This decision took into account the possibility that pancreatic fluid was preferentially being discharged through the percutaneous fistula tube due to pressure differences. However, these procedures led to the recurrence of an intraabdominal abscess, necessitating the reinsertion of the external drainage tube. (Fig. 5). Even three months postoperatively, with no improvement in POPF, attempts were made to reintroduce an endoscopic pancreatic duct stent. However, due to stenosis of the duodenum caused by an ulcer in the duodenal bulb, the scope could not reach the papilla, and the procedure was abandoned. Ultimately, following thorough consultation with the patient, the decision was made to undergo permanent drainage with a stoma pouch through percutaneous drainage to maintain the quality of life. This choice has persisted to the present moment. Finally, as a point of reflection, we should have initially opted for transgastric drainage when we observed the reformation of the abscess after removing the drain.
ERP findings in pancreas divisum: type 3 diagnosis and associated complications. a examination through ERP and fistulography revealed a communication branch between the Santorini duct and Wirsung duct, leading to a diagnosis of type 3. b The fistulography showed the intraabdominal abscess cavity and pancreatic duct
Discussion
The pancreas is formed by the clockwise rotation and fusion of the ventral pancreatic bud (Wirsung duct) and dorsal pancreatic bud (Santorini duct) between the 6th and 7th weeks of fetal development. It is suggested that abnormalities during this process can lead to pancreatic ductal fusion anomalies [4]. Pathologically, each duct independently opens into the duodenum. Although the dorsal pancreatic parenchyma drains a substantial amount of pancreatic fluid through the dorsal pancreatic duct, the opening of the accessory papilla is generally small, making it prone to functional insufficiency in pancreatic fluid outflow and relative stenosis of the accessory papilla [1]. Hence, it is postulated that the increased intraductal pressure, resulting in pancreatitis, is due to the predominant discharge of pancreatic juice through the dorsal pancreatic duct to the accessory papilla. Traditionally, ERP and magnetic resonance cholangiopancreatography (MRCP) have been deemed the gold standard for PD diagnosis and are commonly employed as the conventional diagnostic approach. Especially, Kawaguchi, et al. suggested that the diagnostic ability of endoscopic pancreatic juice cytology in patients with PD was technically feasible and relatively effective [6].
DP is commonly selected as the standard surgical procedure for tumors arising in the tail of the pancreas. However, a significant complication following DP is the development of POPF, with reported rates ranging from 14 to 33% [7]. For the treatment of POPF, effective drainage becomes paramount. Ordinarily, POPF following DP tends to resolve successfully with proper drainage, given the established outlet for pancreatic juice on the papillary side. However, considering PD, where pancreatic fluid drainage from the dorsal pancreas occurs via the accessory papilla, there is a conjecture that POPF following DP may become challenging to manage due to compromised pancreatic fluid drainage, leading to an elevation in intraductal pressure. In this case, despite the appropriate placement of a pancreatic duct stent, drainage from the external fistula tube did not decrease. Therefore, in this case, the potential causes of refractory POPF include ① the possibility of complete rupture of the pancreatic stump and ② the narrowness of the pancreatic duct leading to the minor papilla, resulting in potentially elevated intraductal pressure on the duodenal side. These factors are speculated to interact in a complex manner, contributing to the likelihood of the persisting POPF in the abdominal cavity with lower intraabdominal pressure.
While there is no unified consensus on the association between PD abnormalities and pancreatic cancer, a report suggests that approximately 10% of cases with PD may also have concomitant pancreatic cancer [8, 9]. The possibility that PD could be a risk factor in the development of pancreatic cancer cannot be dismissed. Nevertheless, careful consideration of the risk of POPF in cases involving PD is deemed crucial for perioperative management during DP. As evidenced by a case report, endoscopic papilla sphincterotomy was conducted prior to LDP for a patient with PD to reduce pancreatic duct pressure [10]. However, the universal applicability of this approach remains a matter of debate. Therefore, diagnosing PD before DP is deemed crucial for predicting POPF. In this case, the assessment of pancreatic duct anomalies, including PD, through preoperative MRCP or ERP, was considered crucial. If non-fusion had been identified preoperatively, measures such as placing a pancreatic duct stent to decompress the dorsal pancreatic duct could have been implemented. The treatment for recurrent pancreatitis due to PD has been reported to achieve a success rate of 67.5% with endoscopic pancreatic duct stent placement [11].
In conclusion, this case serves as an important reminder to consider preoperative imaging modalities for detecting PD before DP to prevent refractory POPF.
References
Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004;39:605–15.
Shen Z, Munker S, Zhou B, et al. The accuracies of diagnosing pancreas divisum by magnetic resonance cholangiopancreatography and endoscopic ultrasound: a systematic review and meta-analysis. Sci Rep. 2016;6:35389.
Warshaw AL, Simeone JF, Schapiro RH, et al. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg. 1990;159:59–66.
Agha FP, Williams KD. Pancreas divisum: incidence, detection, and clinical significance. Am J Gastroenterol. 1987;82:315–20.
Funamizu N, Sogabe K, Shine M, et al. Association between the preoperative C-reactive protein-to-albumin rastio and the risk for postoperative pancreatic fistula following distal pancreatectomy for pancreatic cancer. Nutrients. 2022;14:5277.
Kawaguchi S, Satoh T, Terada S, et al. Diagnostic ability of pancreatic juice cytology via the minor papilla in patients with pancreas divisum. DEN Open. 2021;2:e62.
Tieftrunk E, Demir IE, Schorn S, et al. Pancreatic stump closure techniques and pancreatic fistula formation after distal pancreatectomy: meta-analysis and single-center experience. PLoS ONE. 2018;13:e0197553.
Nishino T, Toki F, Oi I, et al. Prevalence of pancreatic and biliary tract tumors in pancreas divisum. J Gastroenterol. 2006;41:1088–93.
Kamisawa T, Yoshiike M, Egawa N, et al. Pancreatic tumor associated with pancreas divisum. J Gastroenterol Hepatol. 2005;20:915–8.
Nagai K, Masui T, Anazawa T, et al. Preoperative endoscopic minor papilla sphincterotomy for pancreas divisum in a patient with pancreatic cancer who underwent laparoscopic distal pancreatectomy. Ann Surg Oncol. 2023;30:7778–9.
Michailidis L, Aslam B, Grigorian A, et al. The efficacy of endoscopic therapy for pancreas divisum: a meta-analysis. Ann Gastroenterol. 2017;30:550–8.
Acknowledgements
The authors would like to thank Editage (www.editage.com) for English language editing. No specific grants from any funding agency in the public, commercial, or not-for-profit sectors were received for this research.
Funding
None.
Author information
Authors and Affiliations
Contributions
NF and YT were the attending surgeons for this patient and wrote the article. YN, MK, and YI performed an endoscopic retrograde pancreatography and stenting to the pancreatic duct. NF, MU, and KO performed the surgery. YI and YT assisted in reporting the case. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
The informed consent was obtained from the patients to the publication of details of this case.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Funamizu, N., Uraoka, M., Numata, Y. et al. Refractory postoperative pancreatic fistula following laparoscopic distal pancreatectomy for pancreatic cancer caused by incomplete pancreas divisum: a case report. Clin J Gastroenterol 17, 587–591 (2024). https://doi.org/10.1007/s12328-024-01942-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-024-01942-x