Abstract
We report the case of a 48-year-old male with a history of pulmonary and ocular sarcoidosis. Non-caseating granulomas, identified histologically, are the most characteristic manifestation of sarcoidosis. Hepatic sarcoidosis is difficult to diagnose using radiological imaging. In the patient reported in this study, ultrasound and contrast-enhanced computed tomography scans identified multiple intra-abdominal lymphadenopathies, with evidence of liver and splenic infiltrations. The first liver biopsy revealed non-caseating granulomatous hepatitis consistent with hepatic sarcoidosis. The patient was treated with ursodeoxycholic acid (UDCA), but his laboratory parameters did not improve. Prednisone was initiated at a dose of 30 mg daily and slowly tapered. At a dose of 12.5 mg daily, marked improvements in the fibrotic and sarcoid-like lesions were noted at the second biopsy. A third biopsy was performed, with the patient on a prednisone taper of 5 mg/day showed mild fibrous expansion in the portal tracts and mild parenchymal necro-inflammatory lesions. However, overall, fibrosis marker levels remained stable over the course of treatment. A fourth biopsy was performed after a 5-year course of 5 mg/day prednisone. This revealed minimal lobular inflammation without fibrosis. Thus, treatment of this patient with corticosteroids and UDCA resulted in marked improvements in his biochemical and histological parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a systemic inflammatory disease of unknown origin characterized by non-caseating granulomas in multiple organs. Ocular, pulmonary, and cutaneous involvement are the three areas most commonly affected by sarcoidosis in Japan [1]. Hepatic sarcoidosis represents 5–30% of systemic types [2]. The sex- and age-specific incidence rates display a biphasic pattern, with a peak among 20–34-year-old males and a peak among 50–60-year-old females [3].
Hepatic sarcoidosis is usually asymptomatic and most cases do not lead to life-threatening liver disease. However, hepatic involvement can range from mild liver dysfunction to clinically evident disease with cholestasis or, in advanced instances, cirrhosis; with incidental findings of multiple small hypodense hepatic lesions [4].
Hepatic sarcoidosis is diagnosed based on clinical findings consistent with liver disease, histopathologic identification of non-caseating granulomas, and the exclusion of alternative causes of granulomatous disease. Serum angiotensin-converting enzyme (sACE) is one of the most frequently used biomarkers in sarcoidosis diagnosis. However, its sensitivity varies from 60 to 80% and depends substantially on the organ system involved [5]. Most patients with hepatic sarcoidosis have mild biochemical abnormalities. Elevated levels of alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) are more common than increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, indicating that the infiltrative nature of sarcoidosis is more characteristic of cholestatic disorders than hepatocellular damage. The severity of any elevation in liver enzyme levels is indicative of the extensiveness of the granulomatous disorder. In liver sarcoidosis, repeated assessment of serum fibrosis markers and liver histology can provide diagnostic insights, aid treatment selection, and determine the best approach to disease management. Despite the brevity of the existing randomized-controlled trials of corticosteroid efficacy, it remains a mainstay of extrapulmonary sarcoidosis treatment. Ursodeoxycholic acid (UDCA) is commonly used as an adjunctive treatment for cholestatic liver disease; although, at the time of writing, no randomized clinical trials of UDCA for hepatic sarcoidosis have been performed. Here, we report the case of a hepatic sarcoidosis patient with histopathological improvements following combined treatment with prednisone and UDCA.
Case report
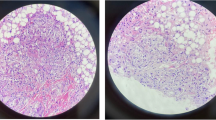
A 48-year-old Japanese man was referred to the Department of Gastroenterology of Nara Medical University with liver dysfunction that had begun two years earlier. His medical history included ocular and pulmonary sarcoidosis with a biopsy-supported diagnosis and compatible uveitis and epithelioid cell granulomas in the bronchial mucosa, respectively. He was initially treated with corticosteroids, but this was discontinued when blurred vision healed. Our initial medical examination revealed the following: serum alanine aminotransferase (ALT) 39 IU/L, aspartate aminotransferase (AST) 49 IU/L, alkaline phosphatase (ALP) 881 U/L, gamma-glutamyl transferase (GGT) 226 U/L, and total bilirubin 0.8 mg/dL (Table 1). Serum levels of these markers (type IV collagen 7S fragments: 4COL7S; 6.8 ng/ml and hyaluronic acid: HA; 60 ng/mL)W and of angiotensin-converting enzyme (ACE) (31.5 U/L) were elevated. The patient tested negative for hepatitis B surface (HBs) antigen, hepatitis C virus genotype 3 (HCV lll), and antinuclear antibodies (ANA). Ultrasonography found heterogeneous parenchymal echo patterns suggestive of an infiltrative liver disease. It also found homogeneous echogenicity with numerous irregular nodule-like hypo-echogenicities of the liver and spleen (Fig. 1). The first liver biopsy found non-caseating granulomas with fibrous expansion of the portal areas, including occasional portal-to-portal bridging (Fig. 2). After a biopsy sample was obtained from a nodule in the liver, ursodeoxycholic acid (UDCA) treatment at a 600 mg daily dose was initiated. As the patient’s hepatic sarcoidosis did not respond sufficiently to the dose, we increased the dosage to 900 mg/day for 7 months. However, liver enzymes including aminotransferase (ALT) and alkaline phosphatase (ALP) were increased. Therefore, the dose of UDCA was reduced back to 600 mg/day, and prednisolone was initiated at a dose of 30 mg/day and slowly tapered over a 4 month period. The second biopsy findings at a dose of 12.5 mg/day showed significant improvements in the portal inflammation and fibrosis (Fig. 3). The hepatic histological findings from the second liver biopsy showed obvious improvements as a result of the combined administration of corticosteroids and UDCA. The third biopsy was performed 3 years after the second biopsy with the patient on a tapered prednisone dose of 5 mg/day. This biopsy found the portal inflammation and fibrosis to be confined to the portal tracts (Fig. 4). The fourth biopsy following a 5-year course of prednisone at 5 mg/day revealed minimal lobular inflammation and no fibrosis (Fig. 5).
First histological examination of a 48-year-old Japanese man with hepatic sarcoidosis. a Azan stain; b hematoxylin–eosin (HE) stain. The first liver biopsy showed advanced fibrosis and non-caseating granulomas. Original magnification × 40 (azan) and × 100 (H&E). c The arrow shows a non-caseating granuloma containing multinucleated Langhans-type giant cells (magnification × 400)
Second histological examination of a 48-year-old Japanese man with hepatic sarcoidosis. a Silver impregnation stain; b Azan stain; c hematoxylin and eosin stain (panels a–c: original magnification × 100). Marked improvement was seen in the fibrous enlargement of the portal area and extending fibrous septa
Decreased serum levels of ALP, ALT, HA, and ACE were demonstrative of improvements in liver histology (Fig. 6). Levels of the serum macrophage galactose-specific lectin-2-binding protein glycosylation isomer M2BPGi fluctuated slightly but remained essentially unchanged over 2 years. UDCA was continued until corticosteroids were discontinued. After initiating systemic corticosteroids, US revealed that the multiple hypointense nodules in the liver and spleen gradually shrank over time (Fig. 1). Postcorticosteroid therapy initiation, multiple hypointense nodules in the liver and spleen gradually shrank and finally disappeared, as shown in the CT scans (Fig. S2A). Bilateral hilar lymphadenopathy was not detected by chest X-ray film pre- or post-corticosteroid treatment (data not shown). A ground-glass opacity in the right lower lung disappeared shortly after initiating corticosteroid therapy (Fig S2B).
Discussion
Sarcoidosis is a systemic inflammatory disease that can affect any organ system, including the liver [6]. Hepatic involvement occurs in 11.5% of patients with sarcoidosis [7]. Corticosteroid therapy at an initial dose of 30–60 mg/day is recommended in patients having hepatic sarcoidosis with severe liver dysfunction, by guide to sarcoidosis clinical practice 2020 [8]. Yet, the efficacy and safety of corticosteroid therapy for hepatic sarcoidosis is undetermined. To the best of our knowledge, this is the first case report to show substantial amelioration of pathological liver histology in a sarcoidosis patient treated with combined UDCA and corticosteroids. The four biopsies over the course of the patient’s treatment provide unique insights into the effects of this treatment option. Liver biopsy is the gold standard for assessing chronic hepatitis but has some limitations [9]. Magnetic resonance elastography and transient elastography (FibroScan) were better than noninvasive biomarkers at assessing liver fibrosis in chronic liver diseases, including hepatic sarcoidosis [10, 11]. However, we did not perform either of these assessments in this case.
Advanced cases of hepatic sarcoidosis are often difficult to treat. Recent research has found that a third of patients with hepatic sarcoidosis present with clinically significant portal hypertension, and 14.5% with cirrhosis [12]. Corticosteroid therapy may improve the systemic manifestations of this disease, but the biochemical response to corticosteroids varies in hepatic sarcoidosis. Corticosteroids cannot reverse the progression of liver fibrosis or prevent the development of portal hypertension, so patients are advised to monitor their signs and symptoms for evidence of clinical deterioration [13]. A previous case report found that corticosteroid treatment worsened the progression of symptomatic hepatic sarcoidosis in a patient with a comorbid rupture of the esophageal varices [14]. The pathophysiology of portal hypertension and cirrhosis in sarcoidosis is not fully understood and multiple mechanisms are thought to contribute. Arteriovenous shunting of granulomas in the liver and spleen can increase portal blood flow. This is followed by elevated intrahepatic vascular resistance due to increased vascular tone in the hepatic microcirculation [15]. Intrahepatic resistance can also be attributed to a large confluence of granulomas caused by fibrosis of the parenchyma healing in a haphazard distribution [16]. Thus, contrary to the results in our case, these findings suggest that improvement in liver function parameters after corticosteroid treatment is not accompanied by improvements in liver histology. Glucocorticoids lessen the clinical symptoms and abnormal liver enzymes but may not prevent the progression of hepatic sarcoidosis [7]. In contrast, corticosteroids reduce the number of hepatic granulomas by suppressing the inflammatory response [17], potentially appearing as suppressed hepatic fibrosis. Liver fibrosis typically improves during corticosteroid treatment in patients with autoimmune hepatitis [18]. Moreover, corticosteroid therapy quickly suppresses the progression of liver fibrosis progression due to immune-related acute hepatitis [19]. These findings may account for attenuated progression of liver fibrosis in this present case.
Other treatments for patients with hepatic sarcoidosis include glucocorticoids and immunomodulators. However, it is unclear whether immunosuppressive drugs prevent the progression of the disease or just reduce its manifestation. Hence, corticosteroids can improve hepatic granulomas formation and clinical parameters [20] but may not ameliorate portal hypertension [21, 22]. Prospective randomized-controlled trials to determine the effectiveness and safety of corticosteroids for treating hepatic sarcoidosis are urgently warranted.
At the recommended daily dose of 13–15 mg/kg, UDCA has positive effects on pruritus reduction and liver biochemistry, supporting the empirical use of UDCA in the treatment of hepatic sarcoidosis [23]. The importance of TNF-α in granuloma formation has led to the proposition that cytokines may be a good therapeutic target in sarcoidosis [24]. UDCA inhibits pro-inflammatory cytokines including IL-6 and TNF-α in both humans [25] and experimental animal models [26]. The pharmacological mechanism of action is thought to be the glucocorticoid-like effects of UDCA. The use of UDCA as steroid-sparing agents has been demonstrated in chronic liver diseases [27], but their effectiveness and usefulness in hepatic sarcoidosis remains unresearched. Corticosteroids and UDCA were considered for the patient discussed in this case report, because combination treatment has been shown to improve biochemical responses in the majority of patients with hepatic sarcoidosis [12, 28]. In conclusion, hepatic sarcoidosis is a rare manifestation of extrapulmonary sarcoidosis. However, multiple mechanisms are involved in hepatic sarcoidosis. This case report describes a patient who underwent repeated liver biopsies, which showed that the extent of both the hepatic granuloma and the portal tract fibrosis was markedly reduced by combination corticosteroid and UDCA treatment. However, minimal lobular inflammation was observed during the treatment course and the patient must be followed up carefully and further observations should be made. Nevertheless, we have shown that the combination of ursodeoxycholic and corticosteroids can be an effective treatment for advanced hepatic sarcoidosis.
References
Hattori T, Konno S, Shijubo N, et al. Nationwide survey on the organ-specific prevalence and its interaction with sarcoidosis in Japan. Sci Rep. 2018;8:9440.
Deutsch-Link S, Fortuna D, Weinberg EM. A comprehensive review of hepatic sarcoid. Semin Liver Dis. 2018;38:284–97.
Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–9.
Karagiannidis A, Karavalaki M, Koulaouzidis A. Hepatic sarcoidosis. Ann Hepatol. 2006;5:251–6.
Studdy P, Bird R, James DG. Serum angiotensin-converting enzyme (SACE) in sarcoidosis and other granulomatous disorders. Lancet. 1978;2:1331–4.
Cremers J, Drent M, Driessen A, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 2012;24:17–24.
Ayyala US, Padilla ML. Diagnosis and treatment of hepatic sarcoidosis. Curr Treat Opt Gastroenterol. 2006;9:475–83.
Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58.
Putra J, Toor A, Suriawinata AA. The utility of repeat liver biopsy in autoimmune hepatitis: a series of 20 consecutive cases. Pathology. 2016;48:449–53.
Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2: 100067.
Atzori SM, Pasha Y, Maurice JB, et al. Prospective evaluation of liver shearwave elastography measurements with 3 different technologies and same day liver biopsy in patients with chronic liver disease. Dig Liver Dis. 2023.
Graf C, Arncken J, Lange CM, et al. Hepatic sarcoidosis: clinical characteristics and outcome. JHEP Rep. 2021;3: 100360.
Ennaifer R, Ayadi S, Romdhane H, et al. Hepatic sarcoidosis: a case series. Pan Afr Med J. 2016;24:209.
Yoshiji H, Kitagawa K, Noguchi R, et al. A histologically proven case of progressive liver sarcoidosis with variceal rupture. World J Hepatol. 2011;3:271–4.
Bhargava P, Vaidya S, Kolokythas O, et al. Pictorial review. Hepatic vascular shunts: embryology and imaging appearances. Br J Radiol. 2011;84:1142–52.
Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–91.
Moller DR. Treatment of sarcoidosis—from a basic science point of view. J Intern Med. 2003;253:31–40.
Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–52.
Imoto K, Kohjima M, Hioki T, et al. Clinical Features of liver injury induced by immune checkpoint inhibitors in Japanese patients. Can J Gastroenterol Hepatol. 2019;2019:6391712.
Tadros M, Forouhar F, Wu GY. Hepatic sarcoidosis. J Clin Transl Hepatol. 2013;1:87–93.
Vannozzi G, Tozzi A, Chibbaro G, et al. Hepatic and mesenteric sarcoidosis without thoracic involvement: a case of severe noncirrhotic portal hypertension and successful pregnancy. Eur J Gastroenterol Hepatol. 2008;20:1032–5.
Cengiz C, Rodriguez-Davalos M, deBoccardo G, et al. Recurrent hepatic sarcoidosis post-liver transplantation manifesting with severe hypercalcemia: a case report and review of the literature. Liver Transpl. 2005;11:1611–4.
Bakker GJ, Haan YC, Maillette de Buy Wenniger LJ, et al. Sarcoidosis of the liver: to treat or not to treat? Neth J Med. 2012;70:349–56.
Amber KT, Bloom R, Mrowietz U, et al. TNF-alpha: a treatment target or cause of sarcoidosis? J Eur Acad Dermatol Venereol. 2015;29:2104–11.
Keely SJ, Steer CJ, Lajczak-McGinley NK. Ursodeoxycholic acid: a promising therapeutic target for inflammatory bowel diseases? Am J Physiol Gastrointest Liver Physiol. 2019;317:G872–81.
Ko WK, Lee SH, Kim SJ, et al. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. Plos One. 2017;12:e0180673.
Angulo P. Use of ursodeoxycholic acid in patients with liver disease. Curr Gastroenterol Rep. 2002;4:37–44.
Ungprasert P, Crowson CS, Simonetto DA, et al. Clinical characteristics and outcome of hepatic sarcoidosis: a population-based study 1976–2013. Am J Gastroenterol. 2017;112:1556–63.
Acknowledgements
The authors would like to thank the Department of Gastroenterology and Hepatology and the Department of Pathology at Nara Medical University. The authors would also like to thank Enago for the English language editing.
Funding
There is no funding to be declared.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by MK and reviewed and edited by TN. All authors reviewed previous drafts and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent amendments.
Informed consent for publication
Written informed consent was obtained from the patient for the publication of all clinical details and the accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12328_2023_1918_MOESM1_ESM.pptx
Fig. S1 (A) Chronological course of contrast-enhanced abdominal computed tomography of a 48-year-old Japanese man with hepatic sarcoidosis. The liver was grossly enlarged with numerous low-contrast nodules in the left and right lobes. The spleen was markedly enlarged and multiple hypointense nodules were apparent after contrast injection. (B) Chronological course of computed tomography of the lung of a 48-year-old Japanese man with hepatic sarcoidosis (PPTX 4198 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kikuchi, M., Koizumi, A., Namisaki, T. et al. Improvement of liver histology in hepatic sarcoidosis due to treatment with corticosteroids and ursodeoxycholic acid: a case report. Clin J Gastroenterol 17, 327–333 (2024). https://doi.org/10.1007/s12328-023-01918-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01918-3