Abstract
Spindle and giant cell type undifferentiated carcinoma of the extrahepatic bile duct is an uncommon malignancy. We report a case involving the common bile duct in a 72-year-old male with jaundice who was admitted to our hospital. Diagnostic imaging, including abdominal computed tomography and magnetic resonance imaging, revealed a mass in the distal common bile duct, accompanied by dilatation of both intra- and extrahepatic bile ducts and regional lymph node enlargement. Endoscopic retrograde cholangiography demonstrated stenosis in the distal common bile duct, with a biopsy confirming adenocarcinoma. The patient underwent endoscopic retrograde biliary drainage followed by a subtotal stomach-preserving pancreaticoduodenectomy with regional lymphadenectomy. Microscopic examination revealed that the tumor predominantly comprised spindle and giant atypical cells within the stroma. Immunohistochemical analysis showed the tumor cells expressing cytokeratins and mesenchymal markers, confirming the diagnosis of spindle and giant cell type undifferentiated carcinoma of the common bile duct. Ki-67 labeling index was observed to be above 80%. Postoperatively, intra-abdominal lymph node recurrence was noted at two months, and multiple liver metastases were identified at three months. The patient died seven months post-surgery. The literature pertaining to this rare disease is reviewed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of bile duct cancers are pathologically classified as adenocarcinoma, with a few rare variants also reported. Notably, undifferentiated spindle cell carcinoma is a rare subtype [1, 2]. This paper presents a case of spindle and giant cell-type undifferentiated carcinoma of the common bile duct with rapid postoperative recurrence.
Case report
A 72-year-old male presented with jaundice, brown urine, and grayish-white stool. On admission to our hospital, physical examination revealed icteric sclera and mild epigastric pain. Laboratory investigations revealed elevated hepatobiliary enzyme levels, warranting hospitalization for further assessment. The patient’s medical history included an appendectomy. He had no known allergies, no significant family medical history, and denied habitual alcohol or tobacco use. Liver function tests revealed elevated levels of serum aspartate aminotransferase (185 IU/L), alanine aminotransferase (344 IU/L), alkaline phosphatase (676 IU/L), gamma-glutamyl transpeptidase (489 IU/L), lactate dehydrogenase (278 IU/L), total bilirubin (3.6 mg/dL), and direct bilirubin (2.2 mg/dL). Serum carcinoembryonic antigen (4.0 ng/mL) and serum carbohydrate antigen 19–9 (5.4 U/mL) were within normal limits. Comprehensive routine laboratory assessments including complete blood count, renal function test, coagulation profile, and electrolyte panel were unremarkable.
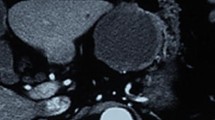
Endoscopic ultrasonography (EUS) (Fig. 1) and contrast-enhanced computed tomography (CECT) (Fig. 2) revealed an enhanced mass in the distal common bile duct (CBD), along with dilatation of the intra- and extrahepatic bile ducts, suggesting biliary tract cancer. Concurrently, EUS and CECT detected enlarged regional lymph nodes at station #13 (Figs. 1 and 2). Magnetic resonance imaging further confirmed a mass in the distal CBD and an adjacent swollen lymph node (#13), exhibiting low signal intensity on T1-weighted imaging and high intensity on diffusion-weighted imaging. Maximum intensity projection showed a stricture in the distal CBD (Fig. 3). Endoscopic retrograde cholangiography also demonstrated a circumferential stricture at the same location, which resembled the appearance of a crab’s claw (Fig. 4). Biopsy of the stricture revealed adenocarcinoma cells, leading to the diagnosis of cholangiocarcinoma. Notably, a biopsy at the junction of the bilateral hepatic ducts did not reveal any malignant cells.
Endoscopic ultrasonography (EUS). a EUS showed a hypoechoic mass and severe stricture of the distal common bile duct (CBD) (arrowhead). b An enlargement of a lymph node (2 cm in diameter, arrow) was detected, located adjacent to the CBD (arrowhead). c Doppler blood flow was detected at the thickened wall of the CBD
Magnetic resonance imaging (MRI). MRI showed a mass in the distal common bile duct (CBD) (arrowhead) and a swollen lymph node (#13) nearby, with low intensity on T1-weighted imaging and high intensity on diffusion-weighted imaging (DWI) (arrow). Maximum intensity projection (MIP) showed a stricture of the distal CBD, accompanied by dilatation of the intra- and extrahepatic bile ducts
An endoscopic retrograde biliary drainage was performed to address the patient's hyperbilirubinemia, followed by a subtotal stomach-preserving pancreaticoduodenectomy. This procedure included regional lymph node dissection and reconstruction of the digestive tract by the modified Child’s method. Intraoperatively, it was observed that the tumor extended to the liver side, surpassing the confluence of the cystic duct. However, intraoperative histological analysis revealed no malignant cells at the surgical margin of the common hepatic duct. There was neither peritoneal dissemination nor distant metastasis detected macroscopically. The surgery lasted 534 min, and the intraoperative blood loss totaled 820 g.
Upon gross examination, it was observed that the distal CBD was significantly obstructed by a nodular infiltrating tumor, measuring 3.2 × 3.0 × 2.8 cm. This tumor extended into the subserosa of the CBD (Fig. 5). Surrounding the constricted CBD, dense tissue was noted (Fig. 6a), which was composed of fibrotic areas and tumorous regions, distributed in a patchy pattern with inflammatory cell infiltration (Fig. 6b). Microscopic examination revealed the tumor as an undifferentiated carcinoma, composed predominantly of spindle and giant cells. A component of adenocarcinoma, situated near the CBD lumen, was also observed (Fig. 6c), along with pleomorphic atypical cells and multinucleated giant cells in the stromal region (Fig. 6d and e). The tumor showed slight infiltration into the pancreatic parenchyma, with malignant cells detected at lymph node stations 12/13, where 6 out of 44 regional lymph nodes were metastatic. The tumor classification, based on the 7th edition of the general rules for clinical and pathological studies on cancer of the biliary tract, was described as BdBp, circ, nodular-infiltrating type, 32 × 30 × 28 mm, pT3a, sci, INFc, Ly0, V1b, Pn1a, pN2(6/44), pDM0, pEM0, pPV0, pA0, pT3(SI)N2M0, pStage IIIA. Notably, there were no rhabdomyoblasts, neoplastic cartilaginous components, or tumor osteoids present. Perineural and venous invasions were noted, but lymphovascular invasion was not evident. The stump of the common hepatic duct and the resected surface were free of malignant cells. Immunohistochemically, tumor cells exhibited positive staining for various cytokeratins (CKs), including AE1/AE3 (Fig. 6f), CK19, and CAM 5.2. Additionally, vimentin staining was present (Fig. 6g), while staining for CD34, desmin, S100 protein, myoglobin, α-smooth muscle actin (αSMA) was negative. The Ki-67 labeling index exceeded 80% (Fig. 6h). The histologic diagnosis confirmed the tumor as a spindle and giant cell-type undifferentiated carcinoma of the CBD. These tumors are often characterized by an adenocarcinoma component with mucus; however, no mucinous components were observed in the resected specimen.
Histopathological examination. a–e Hematoxylin and eosin (HE) stains of the surgical specimen (original magnification; a ×12.5; b ×40, c ×400; d ×400; e ×400). f–h Immunohistochemistry for cytokeratin (AE1/AE3) (f ×400), vimentin (g ×400) and Ki-67 (h ×400). i HE image of biopsy sample (×200). CBD common bile duct
The patient developed a postoperative pancreatic fistula (grade B) and was discharged from our hospital on the 24th day post-surgery. Following a discussion, the patient and his family opted against chemotherapy. Intra-abdominal lymph node recurrences were discovered on the CECT two months post-surgery (Fig. S1a), and multiple liver metastases emerged three months following the resection (Fig. S1b/c/d). The rapid progression of these liver metastases, accompanied by peritoneal dissemination, was noted six months after the operation. The patient died of the primary disease seven months after surgery.
Discussion
Well- to moderately differentiated adenocarcinomas are the most prevalent malignant tumors of the extrahepatic bile duct, whereas undifferentiated carcinoma in this region is exceedingly rare. Albores-Saavedra et al. reported an incidence of undifferentiated carcinoma of the bile duct of 0.38% [1, 2]. At our institute, we encountered two cases of undifferentiated carcinoma among 144 surgical cases of bile tract cancer over the past decade, equating to 1.38%. To date, including our current case, 16 instances of this particular undifferentiated carcinoma of the extrahepatic bile ducts have been reported in the English literature [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] in which one case was identified postmortem and the remaining 15 through surgical specimens. The clinicopathologic characteristics of both this case and previously reported cases are summarized in Table 1.
CKs such as CAM5.2, CK19, and AE1/AE3 are characteristically expressed in epithelial cells, while mesenchymal cells typically express vimentin, desmin, actin, myosin, S100 protein, and αSMA. Immunohistochemical analysis of undifferentiated carcinoma cells reveals the co-expression of various epithelial markers with vimentin in various proportions. Notably, these cells generally do not express mesenchymal markers such as αSMA, desmin, myoglobin, and S100, with two reported exceptions: Kagami et al. documented one case partially positive for desmin [3], and Yoon et al. described another case partially positive for αSMA [9]. Adenocarcinoma was observed in 12 cases, as detailed in prior studies [3,4,5,6, 9, 11,12,13,14, 16, 17], and similarly in our current case. In four of these cases [4, 9, 11], including our own, the adenocarcinoma was predominantly localized to the mucosal side. The tumor in the present case was predominantly composed of spindle and pleomorphic atypical cells, including occasional multinucleated giant cells. Immunohistochemically, these undifferentiated carcinoma cells in our case were positive for both cytokeratin and vimentin in the sarcomatous components, while they did not express other mesenchymal markers and showed no clear differentiation into mesenchymal tissues. Only a few epithelial elements, such as glandular cells, were observed. Based on these findings, we diagnosed this tumor as spindle and giant cell-type undifferentiated carcinoma of the CBD. It is worth noting that deficiencies in SMARCA4/BRG1 and SMARCB1/INI1 can be instrumental in differentiating this tumor subtype [18, 19].
Several reports have demonstrated that undifferentiated spindle and giant cell-type carcinoma possess a high malignancy potential, often leading to frequent metastases [4, 7, 8, 11, 14, 16]. However, the prognosis for undifferentiated carcinoma remains unclear because of the limited number of reported cases. From the 16 documented cases (refer to Table 1), three patients succumbed to postoperative complications: one from liver failure [5], another from a cardiac issue [9], and the third from pulmonary infarction [12]. Additionally, five patients, including the one described in our report, died of local recurrence [6, 10, 15, 17]. Notably, two of these individuals died within four months following surgery, attributed to local recurrence [15, 17]. In the present case, intraoperative findings revealed no peritoneal dissemination and malignant cells were absent at the surgical margins of the common hepatic duct. Nonetheless, histological examination confirmed the tumor’s protrusion into the subserosa of the CBD and its infiltration into the pancreatic parenchyma. The tumor exhibited both vascular/perineural invasion and regional lymph node metastasis. Despite performing radical surgery, intra-abdominal lymph node recurrences appeared two months postoperatively, followed by multiple liver metastases three months later. The patient ultimately died seven months postoperatively from peritoneal dissemination and local recurrence. This outcome, aligning with the tumor’s high metastatic potential, signaled a poor prognosis. The rapid tumor growth in this case, as demonstrated by the high Ki-67 index (> 80%), likely contributed to this unfavorable outcome. Meanwhile, contrasting findings by Yoon et al. regarding undifferentiated carcinoma of the extrahepatic bile ducts, which tested negative for Ki-67 [9], highlight the need for further characterization of the pathophysiology of this cancer.
The differential diagnosis of undifferentiated carcinoma of the extrahepatic bile duct prior to surgery is notably challenging. Despite the detection of adenocarcinomatous tissue in preoperative bile duct biopsies (Fig. 6i), and its consistent identification in surgical specimens (Fig. 6c), the predominant composition of the malignant tissue included spindle and giant cells. Accurate preoperative diagnosis remained elusive in previously reported cases, as well as in the present case. To date, no specific radiographic characteristics have been identified for this type of carcinoma. For a conclusive biopsy-based diagnosis, it is imperative to obtain both sarcomatous and carcinomatous components in the biopsy specimen, followed by confirmation through immunohistochemistry. However, biopsies often reveal only the mucosal aspect of the tumor, potentially missing mesenchymal components that develop beneath the mucosal surface.
The efficacy of chemotherapy in treating undifferentiated carcinoma of the bile duct remains unclear given the scarcity of reported cases. In our case, the patient and his family opted against adjuvant chemotherapy or other treatments following recurrence. However, it is important to note that in two recent cases, postoperative adjuvant chemotherapy (using gemcitabine [14] and a combination of cisplatin with gemcitabine [16]) was administered, leading to comparatively favorable survival outcomes. In addition, a recent multicenter, randomized phase 3 trial revealed that adjuvant S-1 therapy for bile duct cancer was associated with a significant improvement in postoperative survival. Consequently, exploring the potential benefits of S-1 as an adjuvant treatment for undifferentiated carcinoma of the bile duct presents a significant area of interest.
In this report, we presented a challenging case involving rapid postoperative recurrence of spindle and giant cell-type undifferentiated carcinoma of the extrahepatic bile duct. The small volume of the biopsy specimen and limited visibility of only the mucosal side of the carcinoma in the biopsy posed significant diagnostic challenges. Given the rarity of this undifferentiated carcinoma of the extrahepatic bile duct and the need for a clearer understanding of its clinicopathological features, further case studies and discussions are crucial. Advancing our knowledge in this area is essential for developing optimal treatment strategies for such complex and rare cases.
References
Albores-Saavedra J, Scoazec JC, Wittekind C, et al. Carcinoma of the gallbladder and extrahepatic bile ducts. In: Hamilton SR, Aaltonen LA, editors., et al., World health organization classification of tumors. Pathology and genetics of tumors of the digestive system. Lyon: IARC Press; 2000. p. 206–14.
Albores-Saavedra J, Henson DE, Klimstra DS. Tumor of the gallbladder, extrahepatic bile ducts, and ampulla of Vater. Atlas of tumor pathology. Third series fascicle 27. Washington, DC: Armed Forces Institute of Pathology; 2000. p. 191–215.
Kagami A, Nikaidou T, Miyairi M, et al. An autopsy of pseudo sarcoma of the common bile duct. J Gastroenterol. 1994;29:525–30.
Nonomyra A, Mizukami Y, Matsubara F, et al. A case of choledochal cyst associated with adenocarcinoma exhibiting sarcomatous features. J Gastroenterol. 1994;29:669–75.
Yuan CY, Lo HW, Tseng CH, et al. A case of spindle cell sarcomatous change of hepatic ducts manifesting as obstructive jaundice. J Gastroenterol. 1995;30:264–7.
Mokuno Y, Katoh T, Yoshida K, et al. Undifferentiated spindle cell carcinoma of the extrahepatic bile ducts: a case report. Hepatogastroenterology. 2000;47:1234–7.
Nagai E, Shinohara M, Yonemasu H, et al. Undifferentiated carcinoma of the common bile duct: case report and review of the literature. J Hepatobiliary Pancreat Surg. 2002;9:627–31.
Dowaki S, Kijima H, Kashiwagi H, et al. Undifferentiated spindle and giant cell carcinoma of the common bile duct. Tokai J Exp Clin Med. 2003;28:127–30.
Yoon GS, Choi DL. Sarcomatoid carcinoma of common bile duct:a case report. Hepatogastroenterology. 2004;51:106–9.
Kadono J, Hamada N, Higashi M, et al. Carcinosarcoma of the extrahepatic bile duct. J Hepato-biliary Pancreat Surg. 2005;12:328–31.
Oikawa H, Oka K, Nagakura S, et al. Spindle and giant cell type undifferentiated carcinoma arising in the common bile duct: a case report. Pathol Res Pract. 2007;203:179–84.
Nakanishi Y, Ito T, Kubota K, et al. Spindle cell type undifferentiated carcinoma of the common bile duct of the hepatic hilus: report of a case. Surg Today. 2007;37:708–12.
Terada T. Spindle cell carcinoma of the common bile duct: case report with immunohistochemical analysis. Case Rep Gastroenterol. 2010;4:374–80.
Ide T, Miyoshi A, Kitahara K, et al. Spindle and giant cell type undifferentiated carcinoma of the proximal bile duct. Case Rep Gastroenterol. 2012;6:33–9.
Seki T, Saeki S, Hiramatsu K, et al. Undifferentiated carcinoma of the extra hepatic bile duct with rapidly progressive course: Report of a case. Ann Cancer Res Ther. 2014;22:24–7.
Zhang S, Jia J, Bi X, et al. Sarcomatoid carcinoma of the common bile duct: a case report. Medicine (Baltimore). 2017;96: e5751.
Kajioka H, Muraoka A. Rapid recurrence of spindle cell type undifferentiated carcinoma early after radical surgery in a bile duct cancer—a case report. Int J Surg Case Rep. 2021;81: 105800.
Gerber TS, Agaimy A, Hartmann A, et al. SWI/SNF-deficient undifferentiated/rhabdoid carcinoma of the gallbladder carrying a POLE mutation in a 30-year-old woman: a case report. Case Rep Diagn Pathol. 2021;16:52.
Minato H, Yoshikawa A, Katayanagi K, et al. An intrahepatic cholangiocarcinoma with focal rhabdoid features and SMARCA4-deficiency. Case Rep Int J Surg Pathol. 2022;30:581–5.
Acknowledgements
We thank Phoebe Chi, MD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Funding
Funding was provided by Ministry of Education, Culture, Science and Sports (Grant nos. 19K24012 and 22K08749).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008(5).
Informed consent
Informed consent was obtained from all patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagata, K., Nakamura, K., Iida, T. et al. A case of spindle and giant cell-type undifferentiated carcinoma of the extrahepatic bile duct. Clin J Gastroenterol 17, 345–351 (2024). https://doi.org/10.1007/s12328-023-01913-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01913-8