Abstract
Background
Leiomyoma of the pancreas is an extremely rare entity. There are currently only three reported cases, all of which were small, asymptomatic, and incidentally found tumor.
Methods
We have reported the first case of leiomyoma of the pancreas in a young woman with a large symptomatic mass.
Results
A 31-year-old woman presented with chronic abdominal pain. Computed tomography scans showed a huge heterogeneously enhancing mass, located between duodenum and pancreatic head. The patient underwent pancreaticoduodenectomy and the histology confirmed leiomyoma of the pancreas.
Conclusions
This case adds the knowledge that this extremely rare entity could be manifested as symptomatic mass in a young patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leiomyoma of the gastrointestinal tract is not an uncommon disease. However, leiomyoma of the pancreas is extremely rare. There have been only three reported cases, all of which were small, asymptomatic, and incidentally found tumors. Herein, we would like to present a case of leiomyoma of the pancreas presenting with a huge symptomatic mass occupying the entire right side of the abdomen, successfully treated by pancreaticoduodenectomy.
Case report
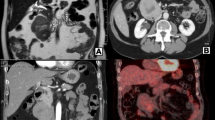
A 31-year-old woman from Lao People's Democratic Republic, presented with chronic abdominal discomfort for 5 years. Her past history was unremarkable. Physical examination revealed a palpable, freely-movable mass on the right side of the abdomen (Fig. 1a). Her blood tests were all within normal limits, including complete blood count, coagulogram, liver tests, hepatitis panels, carcinoembryonic antigen, and carbohydrate antigen 19–9. The patient underwent computed tomography (CT) scan which revealed a huge mass occupying the entire right side of the abdomen. The mass was enhanced heterogeneously in both arterial and venous phases. It had displaced the second part of duodenum laterally without any significant lymphadenopathy or distant metastasis (Fig. 1b–d). Esophagogastroduodenoscopy showed external compression to the junction between the first and second part of duodenum (Fig. 1e). Based on the characteristics of the mass, our differential diagnoses included gastrointestinal stromal tumor (GIST) of the duodenum, pancreatic neuroendocrine tumor, and solid pseudo-papillary epithelial neoplasm (SPEN). Given the patient’s symptom, good performance status, and no sign of metastasis, we decided to perform surgery.
Preoperative findings. a Visible bulging mass on the right side of the abdomen. b–d CT scan of abdomen; b coronal view revealed a huge heterogenous-density mass, occupying nearly the entire right side of abdomen, displacing SMV to the left. c, d Axial view reveal mass situated medially to the C-loop of the duodenum (arrowhead). The mass displaced the ascending colon laterally. e Upper GI endoscopy showed external compression to the junction between the first and second part of duodenum (black circle)
Intraoperatively, the mass was found to locate between the pancreatic head and the medial wall of the duodenum (Fig. 2a, b). The mass was able to be moved freely but unable to be dissected from the pancreatic head. We, therefore, performed pylorus-preserving pancreaticoduodenectomy (PPPD). The surgical specimen revealed a well-circumscribed yellowish-white round mass with a solid whirling pattern, consisting of various sizes of pockets containing clear fluid (Fig. 2c, d) measured 14 × 12 cm. Histopathology reported a spindle cell tumor originating from pancreas (Fig. 3a–c). Hematoxylin–eosin (H–E) staining revealed tumor is comprises of fascicles of spindled cells with fibrillary cytoplasm arranged in interlacing fascicles. Tumor nuclei are bland with elongate blunted end and indistinct nucleolus. Tumor has minimal atypia, few mitotic figures (mitotic rate < 1 per 50 HPF) and no tumor cell necrosis. No tumor infiltration in the intra-tumor ducts and no invasion of the contiguous pancreatic tissue. No lymphovascular involvement is noted. Immunohistochemistry (IHC) results showed strong positive staining for SMA, with negative staining for CK7, CD117, and S-100. While the parenchyma of tumor is negative for CK7, however, the columnar epithelial lining of numerous small slit-like channels dispersed at tumor parenchyma are positive for CK7, which is consistent with pancreatic ducts and branches. The Ki-67 index was 3.4% (17/500 tumor cells). These findings are consistent with leiomyoma of the pancreas. The postoperative period was uneventful and the patient was discharged on postoperative day 11. At 2 months postoperative, the patient no longer experienced any abdominal discomfort and remained recurrence-free. A long-term follow-up program was not applicable, due to the difficulty of travelling abroad.
Intraoperative findings (a, b) and surgical specimen (c, d). a Intraoperative findings of the freely movable mass, originating from the junction between the pancreatic head and the medial wall of the duodenum. b Illustration demonstrating the relations between the mass and its surrounding structures. c Surgical specimen consisted of the head of the pancreas, duodenum, common bile duct, and gallbladder. The mass originated from the pancreatic head. d Illustration of the specimen
Pathology. a The pancreaticoduodenectomy specimen, cut surface at the head of the pancreas showing a circumscribed yellowish-white round mass with a solid whirling pattern, measured 14 × 12 cm. There were various sizes of pockets, containing clear fluid over the surface noted. No evidence of hemorrhagic or necrotic changes. b, c Micrographs in H-E staining of tumor and pancreatic tissue; b At the upper part revealed hypercellular tumor with scattering foci of ductal structures, and the pancreatic tissue with a section of pancreatic ducts (arrow) presented at the lowest part. c) The hypercellular stromal tumor comprised of spindle cells with fibrillar cytoplasm, arranged in fascicles, with the ductal part lining by simple columnar epithelium with focal stratification, no mitosis activity seen. d–f Immunohistochemical staining; d Cytokeratin 7 (CK 7), e Cluster of differentiation 117 (CD 117/c-kit), f Alpha smooth muscle actin (SMA), the epithelium of ductal structures in tumor and at pancreatic duct expressed CK 7(D), inset (magnified area *), and the cytoplasm of spindle tumor cells were positive for SMA (f), both epithelial and stromal tumor tissue were negative for c-kit (e)
Discussion
We have described a case of a young woman with a large symptomatic leiomyoma of the pancreas. Since the pancreas normally only contains a small amount of smooth muscle tissue, leiomyoma formation is extremely rare. We have searched extensively through medical databases, regardless of article language, and found only three reported cases. In all of these cases, the disease was small and asymptomatic, incidentally found in late-middle age to elderly patients [1,2,3] (Table1).
All reported cases of pancreatic leiomyoma, including our case, shared many similar unique features. It is usually incidentally found in female [1, 3] of late-middle age [1,2,3] as an asymptomatic small tumor [1,2,3], with features of early enhancement [1,2,3] and delayed washout [1, 3] on imaging. This enhancement pattern makes it difficult to differentiate between leiomyoma and other more common conditions with a more aggressive nature, such as pancreatic leiomyosarcoma, pancreatic neuroendocrine tumor, gastrointestinal intestinal tumor (GIST), papillary cystic tumor of pancreas.
The etiology of this condition also remains unknown; none of the reported cases were able to identify the exact cause. The tumor might have originated from cells within the wall of blood vessels or pancreatic ducts. In the latter case, performing tumor enucleation might carry a risk of unidentified pancreatic duct injury that eventually leads to postoperative pancreatic fistula. Since leiomyosarcoma is far more common than leiomyoma and none of the larger leiomyoma had been reported, it seemed like leiomyoma could have progressed to leiomyosarcoma as it grows. This is compatible with a previous reported case of malignant transformation of pancreatic leiomyoma to leiomyosarcoma [4].
Regarding our case, preoperative tissue diagnosis using endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) was not performed due to several factors. First, the biopsy result was unlikely to represent the entire histologic makeup of the tumor, due to the heterogeneity of the lesion as seen in the preoperative imaging. Second, the patient was unable to afford the cost of preoperative systemic therapy required if the pathological results turned out to be GIST. She was also unable to travel for multiple visits before definite treatment. Additionally, the mass was freely movable on initial physical examinations, so we decided to perform upfront surgery regardless of the biopsy result.
The decision to perform PPPD was influenced by the intraoperative findings. At first, we attempted dissecting the lesion from the adjacent tissue but were unable to dissect it from the pancreatic head completely. Concerning the risk of unidentified pancreatic duct injury during enucleation or partial pancreatectomy, we decided to perform PPPD which we were much more confident in instead. Taken together, we believe that standard oncologic resection is the suitable treatment for this case because (i) Preoperative investigations are unable to completely exclude other more-aggressive tumor, (ii) Increased difficulty in performing surgery when the tumor grows bigger and the potential malignant transformation of the mass, (iii) There is no effective non-operative treatment for leiomyoma and (iv) Limited resection of the tumors that might have originated from pancreatic duct, could cause a postoperative pancreatic fistula.
To the best of our knowledge, our case was the first reported case of a large symptomatic leiomyoma of the pancreas found in a young patient. Although extremely rare, it should be considered as one of the differential diagnoses for an arterial-enhancing mass found in young patients. Regarding the treatment of this patient, the decision to perform upfront oncologic surgery was quite difficult. Although duodenal GIST, which can be downsized by medication, was on the list of preoperative diagnosis, the patient was unable to afford the additional medical cost. Moreover, there was no evidence of lymph node involvement or distant metastasis despite the big size of the mass, favoring the diagnosis of some kind of tumor with good biology which may gain benefit from surgical resection. Altogether, we decided to performed a standard oncologic resection.
In conclusion, the reported cases of leiomyoma of the pancreas share many unique features. Previous reported cases were present as a small, incidentally found mass in middle age patient. We have reported the first case of leiomyoma of the pancreas in a young woman with a large symptomatic mass.
References
Nakamura Y, Egami K, Maeda S, et al. Primary leiomyoma of the pancreas. IJGC. 2000;28:235–8.
Wisniewski B, Vadrot J, Couvelard A. Léiomyome primitif de la tête du pancréas. Gastroenterol Clin Biol. 2006;30:137–8.
Sato T, Kato S, Watanabe S, et al. Primary leiomyoma of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: LETTERS TECHNIQUES AND IMAGES. Digest Endosc. 2012;24:380–380.
Kant K, Rebala P, Rao GV, et al. Primary Pancreatic leiomyosarcoma: a rare malignant transformation of primary pancreatic head leiomyoma. Cureus [Internet]. 2021 [cited 2023 Feb 15]. https://www.cureus.com/articles/73247-primary-pancreatic-leiomyosarcoma-a-rare-malignant-transformation-of-primary-pancreatic-head-leiomyoma. Accessed 15 Feb 2023.
Acknowledgements
The authors thank the Department of Surgery, Faculty of Medicine, Khon Kaen University for general support of this manuscript.
Funding
This study receives no grant support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analyses were performed by PP, CS and VL. The first draft of the manuscript was written by VL, and CS performed the full writing and editing of the manuscript. All authors read, commented on and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors disclose that there was no conflict of interest for this article.
Human and animal rights
All procedures were approved by the Institutional Review Board, Office of Human Research Ethics, Khon Kaen University.
Informed consent
This study does not contain identifying information about the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petchpiboolthai, P., Suwanprinya, C., Luvira, V. et al. Leiomyoma of the pancreas: an extremely rare entity. Clin J Gastroenterol 16, 495–500 (2023). https://doi.org/10.1007/s12328-023-01788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01788-9