Abstract
The majority of cases of Shiga toxin-producing Escherichia coli are self-limited; however, the infection can occasionally be complicated by more severe phenomena, such as thrombotic microangiopathy, with resultant end-organ damage to the kidneys, colon, nervous system, and various other tissues. Shiga toxin-induced hemolytic uremic syndrome (ST-HUS)—the constellation of thrombocytopenia, hemolysis, and renal failure resulting from thrombotic microangiopathy in a subset of infections producing the Shiga toxin—is classically observed in the pediatric population. Nevertheless, the diagnosis should be considered in adults with this presentation, and especially in those with colonic findings suggestive of ischemia. ST-HUS must also be distinguished from other thrombotic microangiopathies and related conditions, such as disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, and complement-mediated HUS, as these diagnoses prompt alternate management strategies. Here, we present a case of ST-HUS in a gentleman following pericardiectomy who was infected with non-O157:H7 E. coli producing Shiga toxin 2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-surgical abdominal pain and diarrhea progressing to hematochezia, particularly following cardiovascular surgery, is often and appropriately attributed to colonic ischemia from intestinal hypoperfusion. Nevertheless, it is critical for clinicians to maintain a broad differential diagnosis for this presentation, which includes infectious, vascular/thrombotic, inflammatory/autoimmune, and medication-induced etiologies [1, 2]. Here, we present a case of a patient who, following a pericardiectomy for lupus-associated constrictive pericarditis, developed post-operative abdominal pain and diarrhea which progressed to hematochezia in conjunction with progressive thrombocytopenia, oliguric renal injury, and hemolytic anemia. He was diagnosed with overlapping post-operative ischemic colitis and Shiga toxin-producing Escherichia coli (STEC) colitis with STEC-associated hemolytic uremic syndrome.
Case report
A 57-year-old man with a 3-year history of systemic lupus erythematosus on hydroxychloroquine and 15 mg prednisone daily underwent elective pericardiectomy for symptomatic, medically refractory constrictive pericarditis. His surgery passed uneventfully, required standard intubation and peri-procedural inopressor support, and was not notable for intra-operative cardiac bypass or hemodynamic instability. He was extubated and weaned from inopressors on post-operative day (POD) 2. On POD 3, he developed abdominal pain, nausea, and non-bloody diarrhea. On POD 4 the patient developed hematochezia, for which a gastroenterology consultation was requested. On evaluation, he had a heart rate of 86 beats per minute, blood pressure of 108/71 mm Hg, and respiratory rate of 12 breaths per minute on room air. Physical examination demonstrated a tired-appearing and diaphoretic gentleman with dry mucous membranes, abdominal distention, active bowel sounds, and diffuse tenderness to palpation without rebound tenderness. Computed tomography of the abdomen and pelvis demonstrated thickening of the rectum and sigmoid colon, with perirectal fat stranding (Fig. 1a). Sigmoidoscopy on POD 5 revealed edematous, ulcerated, and violaceous mucosa from the anal verge to 30 cm proximally that abruptly normalized thereafter (Fig. 1b). Biopsies showed lamina propria hemorrhage with crypt withering and focal pseudomembranes (Fig. 1c). Stool PCR testing for Clostridioides difficile, collected on POD 5, was negative. Given the clinical, endoscopic, and histologic features in a post-operative patient, colonic ischemia from unwitnessed hypoperfusion was the favored diagnosis. Additionally, given the persistence of leukocytosis and fevers with a temperature of 38.7 °C on POD 6, intravenous cefepime and metronidazole were initiated, in accordance with society guidelines [1], in the wake of the clinical, radiologic, and endoscopic findings suggestive of moderate severity colonic ischemia.
Acute pseudomembranous colitis. a Rectosigmoid wall thickening (green arrow) and perirectal fat stranding. b Rectosigmoid colon was edematous, cyanotic, ulcerated, with luminal narrowing by flexible sigmoidoscopy. There was an abrupt transition to normal sigmoid mucosa at 30 cm from anal verge (not shown). c Pathologic specimens from sigmoid colon showed crypt withering (blue arrow), lamina propria hemorrhage (green arrow), and pseudomembrane (yellow arrow)
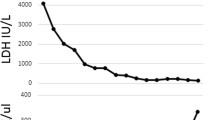
From POD 4 to 8, the patient continued to have nausea, abdominal pain, and bloody diarrhea. Platelets decreased from 142 to 11 × 109/L, and creatinine rose from 1.1 to 2.0 mg/dL in the setting of oliguria (Fig. 2). On POD 6, labs showed lactate dehydrogenase 1,107 U/L, indirect bilirubin 2.7 mg/dL, and undetectable haptoglobin, with hemoglobin decreasing from 14.0 to 12.9 g/dL from POD 7 to 8. Peripheral smear on POD 8 showed schistocytes (Fig. 3). Serum ADAMTS-13, complement levels, and heparin-induced thrombocytopenia panels were negative/normal.
Clinical course of Shiga toxin-induced hemolytic uremic syndrome (ST-HUS). Non-bloody diarrhea started on POD 3, followed by bloody diarrhea on POD 4. The patient’s thrombotic microangiopathy, signified by reduction in platelets and renal injury, began 3–4 days later, illustrating the characteristic delay from diarrhea onset and thrombotic microangiopathy in ST-HUS
On POD 7, broad stool pathogen PCR panel collected from fecal specimen on POD 5 detected Shiga toxin-producing Escherichia coli (STEC), and subsequent PCR showed the toxin was Shiga toxin 2. Stool cultures were negative for the 0157:H7 E. coli strain. The diagnosis of post-operative ischemic colitis with overlapping Shiga toxin-induced hemolytic uremic syndrome (ST-HUS) was made, and antibiotics were stopped on POD 7. The patient clinically improved with intravenous hydration and anti-emetics, without need for hemodialysis. The hemolysis, thrombocytopenia, and acute renal injury, as well as his nausea, abdominal pain, and diarrhea, resolved after 1 week of these supportive measures.
Discussion
E. coli are gram-negative enteric pathogens, of which enterohemorrhagic E. coli (or EHEC) are a subset. The terms EHEC and STEC are often used interchangeably, though STEC is favored because not all EHEC are, in fact, hemorrhagic; while both Shiga toxin 1 and Shiga toxin 2 produced by STEC can induce diarrhea, bloody diarrhea is nearly exclusively associated with the latter toxin [2]. Of the various strains of STEC, O157:H7 is the most common and well known, particularly for the association with ST-HUS. However, it is not the only strain that can produce Shiga toxin 2 and induce hemorrhagic colitis or ST-HUS [3]; indeed, STEC epidemiology has recognized an increase in pathogenic non-O157 STEC over the past decade [4], an instructive phenomenon in our understanding of our presented case.
Fecal shedding from cattle, in which STEC is a commensural organism, is an important source of STEC. STEC outbreaks have been traced to water supply and food products (e.g. meats, sprouts, flour), with sporadic STEC typically acquired from undercooked beef, unpasteurized juice/cider/milk, raw produce vs. contaminated water, and person-to-person [5]. While outbreaks may be easily induced, as the infectious dose of STEC only requires about 50–100 colony-forming units to induce disease in healthy humans, most cases of STEC infection are sporadic rather than outbreak driven [2]. In our case, given the lack of STEC in other hospitalized patients, the infectious source was hypothesized to be from an outside food item. We suspected a hamburger the patient's family member had brought him on POD 2, though notably symptoms developed on POD 3, so this may be too short an incubation period for STEC.
There are two relevant pathogenic mechanisms for STEC. First, STEC bacteria pass through the acidic barrier of human stomachs and form “attaching and effacing” lesions on the mucosal surface of the rectoanal junction [6]. This process creates a molecular syringe to inject protein directly into the host cytoplasm, which co-opts the host’s cytoskeleton to reorganize to actin-based pedestals beneath the offending bacteria, permitting adherence to colonic mucosa [7].
Second, a bacteriophage in the STEC chromosome encodes the Shiga toxin, which is the primary cause of mortality in STEC infections. While Shiga toxins 1 and 2 share structural and mechanistic features, Shiga toxin 2 is associated with bloody diarrhea and the development of HUS [7]. Activation of the lytic cycle is thought to be facilitated by antibiotics, which is why these are recommended against in STEC infections and why potentially offending antibiotics were stopped as soon as the diagnosis was made in this specific case.
Most STEC infections follow a stereotyped course. After exposure, the median incubation time is 3 days, followed classically by abdominal pain with non-bloody diarrhea (as in this case, abdominal pain, nausea, vomiting can precede diarrhea). In 80% of patients, the diarrhea then becomes bloody, often 1–3 days later, as intestinal cells die and slough off [8]. High fever is rare and should point to other dysenteric pathogens, such as Shigella, Campylobacter, or Salmonella) [9].
A small subset of patients with STEC—about 5–7% of cases, primarily in children and the elderly—experience the thrombotic microangiopathy known as Shiga toxin-induced HUS (ST-HUS) [10]. ST-HUS is a direct effect of the destructive properties of the Shiga toxin on vascular epithelium, inducing a vasculitic phenomenon, namely, leukocyte aggregation, apoptosis of the affected cells, platelet aggregation, microthrombi formation, hemolysis, and renal dysfunction [11]. Half of patients with ST-HUS will require dialysis [12, 13].
If it does occur, ST-HUS characteristically develops 5–13 days after the onset of diarrhea in the affected patient. This is a critical distinguishing feature for teasing apart the differential diagnosis; the acute renal injury that may accompany the more commonly observed hypoperfusion-induced colonic ischemia should emerge simultaneously with GI symptoms, rather than in the wake of them. However, given the post-operative setting, relative frequency of colonic ischemia from hypoperfusion compared to STEC, and colonic location (discussed below), we nevertheless favored a contribution to the clinical presentation from hypoperfusion in the post-operative setting in conjunction with STEC infection, rather than STEC infection alone.
In more severe cases of ST-HUS, patients may have jaundice and hepatosplenomegaly, with petechiae or purpura being uncommon findings. Apart from renal injury, other organs may be involved including the gastrointestinal tract, central nervous system, lungs, heart, and pancreas [14]. Neurological manifestations including coma, seizures, and extra-pyramidal symptoms have been reported though are more common with thrombogenic thrombocytopenic purpura, a distinct thrombotic microangiopathy with overlapping clinical features to ST-HUS. Life-threatening gastrointestinal complications, such as colonic necrosis with perforation, intussusception, peritonitis, and sepsis are rare but should remain on the differential for patients with a confirmed microbiologic result and clinically deteriorating course [15].
There are no pathognomonic endoscopic features of colitis secondary to STEC, which may include edema, erosions, ulcerations, and violaceous coloring akin to other causes of colonic ischemia. Histology of this colitis may show pseudomembranes, as was the case with our patient, with lamina propria hemorrhage and crypt withering. The muscularis mucosae, submucosa, and muscularis propria are not usually involved. STEC colitis can be difficult to distinguish based on histology alone as the histological features resemble a pattern similar to ischemic colitis and other toxin-mediated infectious colitis like C. difficile [16]. The specific site of colonic involvement may further help distinguish STEC and ischemic colitis; O157:H7 strains have been observed to more commonly produce right-sided colonic disease, whereas post-operative ischemia characteristically manifests within the left side of the colon [17]. As stated above, in this case, given the involvement of the rectum and sigmoid colon, post-operative ischemic colitis with overlapping STEC infection was favored. Nevertheless, non-O157:H7 strains may deviate from this right-sided predilection, further confusing the picture [18].
Management of ST-HUS includes supportive care with intravenous fluids in hospitalized patients to maintain adequate renal perfusion [19]. Antibiotics ought to be avoided, as stated, given their association with Shiga toxin production by the encoding bacteriophage in the STEC chromosome [20]. Anti-motility agents should also be avoided for the theoretical risk of colonic perforation and toxicity in infectious colitis. Plasmapheresis or plasma exchange has been reported for the treatment of severe, refractory cases [21, 22]. Eculizumab, a monoclonal antibody targeted against complement C5 that is used in another microangiopathy, complement-mediated HUS, may also be used to treat ST-HUS, particularly in those with CNS involvement [23, 24]. Patients with a more severe acute illness, CNS involvement, and need for dialysis are all associated with worse prognosis [3, 24]. Although ST-HUS most commonly affects children, adults may have worse outcomes, and studies have reported up to 15% mortality in certain high-risk, elderly individuals [25, 26]. The most common long-term complications of ST-HUS include hypertension and chronic kidney disease [27, 28]. Fortunately, ST-HUS does not typically recur; future episodes of HUS should lead to investigation of alternative complement pathway disorders.
References
Brandt LJ, Feuerstadt P, Longstreth GF, et al. American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol. 2015;110:18–44.
Valilis E, Ramsey A, Sidiq S, et al. Non-O157 Shiga toxin-producing Escherichia coli-A poorly appreciated enteric pathogen: systematic review. Int J Infect Dis. 2018;76:82–7.
Newell DG, La Ragione RM. Enterohaemorrhagic and other Shiga toxin-producing Escherichia coli (STEC): Where are we now regarding diagnostics and control strategies? Transbound Emerg Dis. 2018;65:49–71.
Bruyand M, Mariani-Kurkdjian P, Gouali M, et al. Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med Mal Infect. 2018;48:167–74.
Frisema I, Van der Zwaluw K, Schuurman T, et al. Emergence of Escherichia coli encoding Shiga toxin 2f in human Shiga toxin-producing E. coli (STEC) infections in the Netherlands, January 2008 to December 2011. Euro Surveill. 2014;19:20787.
Joseph A, Cointe A, Kurkdjian PM, et al. Shiga toxin-associated hemolytic uremic syndrome: a narrative review. Toxins. 2020;12:67.
Melton-Celsa A, Mohawk K, Teel L, et al. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol. 2012;357:67–103.
Manrique-Caballero CL, Peerapornratana S, Formeck C, et al. Typical and atypical hemolytic uremic syndrome in the critically ill. Crit Care Clin. 2020;36:333–56.
Hunt JM. Shiga toxin-producing Escherichia coli. Clin Lab Med. 2010;30:21–45.
Rahman RC, Cobeñas CJ, Drut R, et al. Hemorrhagic colitis in post diarrheal hemolytic uremic syndrome: retrospective analysis of 54 children. Pediatr Nephrol. 2012;27:229–33.
Buelli S, Zoja C, Remuzzi G, et al. Complement activation contributes to the pathophysiology of shiga toxin-associated hemolytic uremic syndrome. Microorganisms. 2019;7:15.
Trachtman H, Austin C, Lewinski M, et al. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol. 2012;8:658–69.
Balestracci A, Martin SM, Toledo I, et al. Laboratory predictors of acute dialysis in hemolytic uremic syndrome. Pediatr Int. 2014;56:234–9.
Nathanson S, Kwon T, Elmaleh M, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5:1218–28.
Khalid M, Andreoli S. Extrarenal manifestations of the hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC HUS). Pediatr Nephrol. 2019;34:2495–507.
Farooq PD, Urrunaga NH, Tang DM, et al. Pseudomembranous colitis. Dis Mon. 2015;61:181–206.
Jessurun J. The differential diagnosis of acute colitis: clues to a specific diagnosis. Surg Pathol Clin. 2017;10:863–85.
Bannas P, Fraedrich K, Treszl A, et al. Shiga toxin-producing E. coli O104:H4 outbreak 2011 in Germany: radiological features of enterohemorrhagic colitis. Rofo. 2013;185:434–9.
Grisaru S, Xie S, Samuel S, et al. Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with Shiga toxin–producing Escherichia coli: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:68.
Freedman SB, Xie J, Neufeld MS, et al. Shiga toxin–producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin Infect Dis. 2016;62:1251–8.
Ninchoji T, Nozu K, Nakanishi K, et al. Clinical characteristics and long-term outcome of diarrhea-associated hemolytic uremic syndrome: a single center experience. Clin Exp Nephrol. 2017;21:889–94.
Harkins VJ, McAllister DA, Reynolds BC. Shiga-toxin E. coli hemolytic uremic syndrome: review of management and long-term outcome. Curr Pediatr Rep. 2020;8:16–25.
Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81.
Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–3.
Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin Infect Dis. 2009;49:1480–5.
Rosales A, Hofer J, Zimmerhackl LB, et al. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis. 2012;54:1413–21.
Gould LH, Mody RK, Ong KL, et al. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis. 2013;10:453–60.
Braune SA, Wichmann D, Von Heinz MC, et al. Clinical features of critically ill patients with Shiga toxin-induced hemolytic uremic syndrome. Crit Care Med. 2013;41:1702–10.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
June Tome, Daniel B. Maselli, Roeun Im, Matthew B. Amdahl, Daniel Pfeifle, Catherine Hagen and Magnus Halland declare that they have no conflict of interest.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tome, J., Maselli, D.B., Im, R. et al. A case of hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli after pericardiectomy. Clin J Gastroenterol 15, 123–127 (2022). https://doi.org/10.1007/s12328-021-01539-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-021-01539-8