Abstract
A 64-year-old man presented to our hospital with abdominal pain and 4–5 episodes of watery diarrhea per day for 2 months. Abdominal ultrasound examination revealed a mass in the peritoneal cavity, and computed tomography showed a 13.4 cm mass in the mesentery and a 3 cm mass in the mesocolon. The patient underwent laparoscopic partial resection for diagnosis. Microscopically, abundant fibrosis and numerous immunoglobulin (Ig) G4-positive plasma cells were observed. The serum level of IgG4 was 665 mg/dl postoperatively. These findings suggested that the lesion was consistent with IgG4-related sclerosing mesenteritis. Oral steroids resulted in rapid disappearance of symptoms and a decrease in masses. Recently, sclerosing mesenteritis are reported as IgG4-related disease or mimicking IgG4-related disease but multiple lesions rarely occur in the same organ. We report a case of IgG4-related sclerosing mesenteritis with multiple lesions without involvement of other organs, such as the pancreas and salivary glands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a fibroinflammatory condition in affected organs characterized by elevated immunoglobulins and good responsiveness to steroid treatment; immune-mediated entities have often been suggested [1, 2]. However, the pathophysiological mechanisms are not fully elucidated. The disease was described in the pancreas in 2001 [3]. Over the next decade, it became clear that the organs with strong predilection were the pancreas, salivary glands, bile duct, orbits, kidneys, lungs, aorta, retroperitoneum, pachymeninges, and thyroid gland [4, 5]. IgG4-related sclerosing mesenteritis (IgG4-related SM) is rare, and thus limited information is available. Here, we report a case of IgG4-RD that occurred in the intestinal membrane and mesocolon.
Case report
A 64-year-old man presented to our hospital with abdominal pain around the umbilical region and 4–5 episodes of watery diarrhea per day for 2 months. Within this period, he reported reduced appetite and weight loss of 2 kg. He had no history of external wounds, abdominal surgery, allergic disease, or medication before the appearance of symptoms. Body temperature was 36.7℃ on admission. On physical examination the abdomen was soft and slightly tender. Bowel movements were frequent.
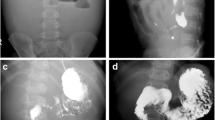
Laboratory findings showed mild anemia with a hemoglobin level of 12.7 g/dL, mild elevation of C-reactive protein (CRP) to 4.20 mg/dL, and an increase in soluble IL-2 receptor (sIL-2R) [1781 U/mL (normal < 496 U/mL)]. Lactate dehydrogenase (LDH) levels were normal. The tumor markers for carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA 19–9) were within normal limits (Table 1). Abdominal ultrasound examination revealed a 10 cm mass in the peritoneal cavity (Fig. 1a). Contrast-enhanced computed tomography (CT) showed soft tissue masses in the upper mesentery (Fig. 2b, c) and transverse mesocolon (Fig. 2d). Multiple small nodules were observed around the masses. On magnetic resonance imaging (MRI), the tumor was visible in the upper mesentery on a low-intensity T1-weighted image (Fig. 2a), a low-intensity T2-weighted image (Fig. 2b), and a mildly high-intensity T2-weighted image with fat saturation (Fig. 2c), as well as a decreased diffusion on diffusion-weighted imaging (DWI) (Fig. 2d). We confirmed absence of other organ involvement using contrast-enhanced CT; we did not perform positron emission tomography (PET) CT in this case. Upper gastrointestinal endoscopy and colonoscopy showed no particular findings that might cause pain or diarrhea. An increased number of IgG4-positive plasma cells was not observed on biopsy of the gastrointestinal mucosa, such as the esophagus, stomach, duodenum, ileum, colon and rectum. Eosinophilic infiltration (less than 10/HPF) was observed in the biopsy specimens of the terminal ileum and ascending colon.
Abdominal magnetic resonance imaging (MRI) (a) MRI T1-weighted image shows low intensity (yellow arrow) in the upper mesentery. b MRI T2-weighted image shows low intensity (yellow arrow) in the upper mesentery. c MRI T2-weighted image with fat saturation shows mild high intensity (yellow arrow) in the upper mesentery. d MRI diffusion-weighted image shows decreased diffusion (yellow arrow) in the upper mesentery
Based on the above findings, we could not exclude malignant lymphoma in the mesentery. Pathological diagnosis was performed via laparoscopy. Intraoperative findings showed that the small intestinal mesentery was involved in the tumor, which became a mass of the peritoneum, with poor mobility. The tumor had no adhesions to the intestinal tract, but was adherent to the retroperitoneum. The tumor itself was hard with redness. Many yellow nodules of approximately 1 cm were found on the peripheral side of the tumor (Fig. 3a). A tumor measuring approximately 3 cm was located on the dorsal side of the transverse mesentery, and the surface was red and covered with many capillaries (Fig. 3b). Murky ascites was observed in the abdominal cavity, and cytology and bacterial culture examination of the ascites were negative for bacterial growth. A part of the main tumor was resected, and intraoperative rapid pathology confirmed that atypical cells were present. On histopathological examination, the resected specimen contained adipose tissue (Fig. 4a), and abundant fibrosis was observed in the adipose tissue septum, accompanied by infiltration of lymphocytes, plasma cells, and histiocytes. Atypical cells and storiform fibrosis were not observed (Fig. 4b). Further immunohistochemical staining (IHC) was performed, and liposarcoma was excluded. Elastica Masson trichrome staining showed that the adipose tissue septum had remarkable fibrosis (Fig. 4c), and Elastica van Gieson staining showed no obliterative phlebitis (image not shown). Abundant IgG4-positive plasma cells were observed in the septa (Fig. 4 d, e). The number of IgG4 + cells/high-power field was > 100. The IgG4 + /IgG1 + plasma cell ratio was 55.4%. These findings suggested that the histological diagnosis was IgG4-related sclerosing mesenteritis. The serum IgG4 and IgG levels were measured after biopsy. IgG4 level was increased to 665 mg/dL (normal range 4–108 mg/dL) and IgG was 2492 mg/dL (normal range 820–1740 mg/dL).
Intra-operative findings contrasted with computed tomography images. a Small intestinal mesentery was involved in the tumor that became a mass of the peritoneum. Yellow nodules were found on the peripheral side of the tumor. b Tumor of about 3 cm on the dorsal side of the transverse mesentery; the surface was covered with many capillaries
Histological examination of resected tissue from mesenteric mass. a Resected tissue is composed of adipose tissue (hematoxylin and eosin staining). b Numerous plasma cells and lymphocytes in fibrosed tissue (hematoxylin and eosin staining, × 400). c Elastica Masson Trichrome staining shows that the adipose tissue septum had remarkable fibrosis (original magnification, × 200). d IgG4 staining shows that abundant IgG4-positive plasma cells are observed (original magnification, × 100). e IgG4 staining shows that abundant IgG4-positive plasma cells are observed (original magnification, × 400)
Oral steroid therapy (prednisolone, 0.5 mg/kg) was administered to treat IgG4-related sclerosing mesenteritis and gradually tapered every 2 weeks. Diarrhea and abdominal symptoms rapidly improved, and a month later, disappeared completely. The serum IgG4 and IgG levels also decreased rapidly. Body weight and nutritional status, such as serum total cholesterol and albumin levels, improved over time (Fig. 5). At 6 months after the start of steroid therapy, no relapse of the symptoms or regrowth of the mass was observed. CT scan performed 1 month later showed that the main tumor size in the upper mesentery had decreased from 13.4 cm to 8.5 cm and the mass in transverse mesocolon was unclear (Fig. 6a–c). MRI performed 6 months later showed that the main tumor and the mass in the transverse mesocolon had become unclear (Fig. 6d–g). Remission continued.
Clinical course IgG4 and IgG decreased and the abdominal symptoms disappeared rapidly after administration of steroid therapy; serum total cholesterol and albumin levels improved over time. EGD esophagogastroduodenoscopy, TCS total colonoscopy, CT computed tomography, MRI magnetic resonance imaging, CRP C-reactive protein, T-cho total cholesterol, Alb albumin
Computed tomography (CT) and magnetic resonance imaging (MRI) after administration of steroid therapy. Contrast-enhanced CT after a month of starting steroid therapy shows decrease in the mass size in the upper mesentery (a, b) and in the transverse mesocolon (c) (yellow arrow). MRI T2-weighted image with fat saturation and diffusion-weighted image after 6 months of starting steroid therapy showed that the mass in the upper mesentery (d, e) and transverse mesocolon (f, g) became unclear
Discussion
IgG4-RD can occur in various organs [1, 2]. It often manifests as multiple organ disease and can be confused with malignancy, infection, or other immune-mediated conditions. Some case reports on the relationship between IgG4-RD and SM have recently been reported. In a previous study, the histopathologic features of IgG4-related sclerosing disease were observed in four of 12 patients with sclerosing mesenteritis [6].
The median age of patients with IgG4-RD was 67 years, and the male to female ratio was 4:1 [7]. In contrast, the mean age of patients with SM was 65 years, and 70% were male [6]. Clinical presentation is non-specific, and patients with IgG4-related SM present with symptoms similar to those of patients with SM, such as abdominal pain, nausea, vomiting, constipation, anorexia, and weight loss [6, 8]. IgG4-RD is typically subacute for months or years [2]. The abdominal pain and diarrhea in our case presented mainly over 2 months, and weight loss was also observed. It is believed that abdominal pain and diarrhea in SM result from compressive effects of the abdominal mass on the bowel, vessels, and lymphatics [8]. Therefore, the widespread lesions of the mesentery and multiple lesions, such as the mesocolon in our case, may have caused abdominal pain and diarrhea due to their compressive effect.
SM on CT is usually described as a heterogeneous, solitary mass localized mainly in the mesentery with delocalization of the small intestine and surrounding structures [9]. IgG4-RD on MRI findings have relatively low signal intensity on T2 imaging owing to their increased cellularity and amount of fibrosis [10]. Mesenteric panniculitis on MRI is characterized by a hyperintense signal on T2W imaging with fat saturation, suggesting the presence of edema [11]. Contrast-enhanced CT and MRI showed that the mesenteric tumor in our case appeared as a finding of intestinal inflammation, such as SM and IgG4-RD. When multiple lesions occur, it is difficult to distinguish them from neoplastic diseases, such as peritoneal dissemination of cancer, malignant lymphoma, desmoid tumor, gastrointestinal stromal tumor, and liposarcoma, and histological diagnosis is needed. In our case as well, multiple lesions were found in the same organ, and malignant tumors and malignant lymphoma could not be ruled out. Therefore, a histological diagnosis was finally performed using a laparoscope. Although PET-CT was not performed in our case, a negative PET has a high diagnostic accuracy in excluding tumoral mesenteric involvement [12].
IgG4-RD is diagnosed based on the combined presence of radiological, serological, and histopathological features. Radiological features tend to form tumefactive lesions at multiple sites. Serological features include elevated serum IgG4 concentrations. The diagnosis of IgG4-RD is based on the combined presence of a characteristic histopathological appearance and an increased number of IgG4-positive plasma cells. The critical histopathological features for diagnosis are storiform fibrosis, obliterative phlebitis, and dense lymphoplasmacytic infiltrate [13]. In our case, storiform fibrosis and iterative phlebitis were not observed. However, histopathological features of an increased number of IgG4-positive plasma cells and fibrosis were observed. Radiological, serological, and histopathological features were observed, and our patient presented with IgG4-related SM.
To the best of our knowledge, 14 cases of SM associated with increased IgG4 plasma cells have been recently described (Table 2) [14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Serum IgG4 levels were measured after resection and biopsy in 9 out of 14 cases, and it is difficult to estimate IgG4-RD based on radiological findings without histological analysis. Even though there were only 7 cases in which the values of IgG4 were > 135 mg/dl, the serum IgG4 value (665 mg/dl) of our case was the highest among these 14 IgG4 related SM cases. Serum IgG4 concentration (280 mg/L) is useful in distinguishing IgG4-RD from other diseases, and the serum IgG4 levels were 2–3 times higher than the normal upper limit, which may serve as a diagnostic marker to improve the diagnostic accuracy of the disease [28].
Steroid therapy is remarkably effective for the treatment of IgG4-RD [1, 2]. Our case also showed rapid improvement in symptoms and lesions with steroid therapy [23,24,25,26]. Among the surgical resection patients [14,15,16,17,18,19,20,21,22], seven maintained remission after resection, one had relapsed symptoms [18], and another with involvement of other organs [19] were improved by steroid therapy. Patients with IgG4-RD are at a risk of recurrent disease or involvement of other organs over time [1]. It is considered that these patients who are diagnosed as IgG4-related SM should also be followed up carefully.
IgG4-related gastrointestinal disease (IgG4-GID), including IgG4-related SM, has not been fully established clinicopathologically. IgG4-GID may have mimickers [29], and there are few cases in which serum IgG4 levels are high that are amenable to histological diagnosis, as in our case. IgG4-RD is a potentially multiple organ disease. According to Koizumi et al. [30], about half of IgG4-RD patients displayed multiple IgG4-RD, and serum IgG4 levels were significantly higher in those with other organ involvement. IgG4-related SM with high serum IgG4 levels and without other organ involvement, despite multiple lesions in the same organ, was considered to be rare.
We present a case of IgG4-related SM with diarrhea as the first symptom of multiple lesions without synchronous organ involvement. The disease is rare, and more cases and studies are needed to clarify its characterization.
References
Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51.
Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet. 2015;385:1460–71.
Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8.
Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty–five patients. Arthritis Rheumatol. 2015;67:2466–75.
Sekiguchi H, Horie R, Kanai M, et al. IgG4-related disease: retrospective analysis of one hundred sixty–six patients. Arthritis Rheumatol. 2016;68:2290–9.
Akram S, Pardi DS, Schaffner JA, et al. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol. 2007;5:589–96 (quiz 523–4).
Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore). 2015;94:e680.
Sharma P, Yadav S, Needham CM, et al. Sclerosing mesenteritis: a systematic review of 192 cases. Clin J Gastroenterol. 2017;10:103–11.
Canyigit M, Koksal A, Akgoz A, et al. Multidetector-row computed tomography findings of sclerosing mesenteritis with associated diseases and its prevalence. Jpn J Radiol. 2011;29:495–502.
Fujita A, Sakai O, Chapman MN, et al. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics. 2012;32:1945–58.
Apostolakis S, Ioannidis A, Tsioga G, et al. A systematic investigation of sclerosing mesenteritis through CT and MRI. Radiol Case Rep. 2016;11:299–302.
Zissin R, Metser U, Hain D, et al. Mesenteric panniculitis in oncologic patients: PET-CT findings. Br J Radiol. 2006;79:37–43.
Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30.
Greenbaum A, Yadak N, Perez S, et al. Surgical management of isolated mesenteric autoimmune disease: addressing the spectrum of IgG 4-related disease and sclerosing mesenteritis. BMJ C Rep. 2017. https://doi.org/10.1136/bcr-2017-220400.
Fukuda M, Miyake T, Matsubara A, et al. Sclerosing mesenteritis mimicking IgG4-related disease. Intern Med. 2020;59:513–8.
Butt Z, Alam SH, Semeniuk O, et al. A case of IgG4-related sclerosing mesenteritis. Cureus. 2018;10:e2147.
Abe A, Manabe T, Takizawa N, et al. IgG4-related sclerosing mesenteritis causing bowel obstruction: a case report. Surg C Rep. 2016;2:120. https://doi.org/10.1186/s40792-016-0248-0 ([EpubOct302016]).
Mejías Manzano MLÁ, Trigo Salado C, Serrano Jiménez M, et al. IgG4-related sclerosing mesenteritis, a rare condition that causes abdominal pain. Rev Esp Enferm Dig. 2018;110:201–3.
Mori E, Kamisawa T, Tabata T, et al. A case of IgG4-related mesenteritis. Clin J Gastroenterol. 2015;8:400–5.
Liu Z, Jiao Y, He L, et al. A rare case report of immunoglobulin G4-related sclerosing mesenteritis and review of the literature. Medicine (Baltimore). 2020;99:e22579.
Nomura Y, Naito Y, Eriguchi N, et al. A case of IgG4-related sclerosing mesenteritis. Pathol Res Pract. 2011;207:518–21.
Minato H, Shimizu J, Arano Y, et al. IgG4-related sclerosing mesenteritis: a rare mesenteric disease of unknown etiology. Pathol Int. 2012;62:281–6.
Takano Y, Niiya F, Kobayashi T, et al. A case of ileocecal IgG4-related sclerosing mesenteritis diagnosed by endoscopic ultrasound-guided fine needle aspiration using forward-viewing linear echoendoscope. Case Rep Gastrointest Med. 2019;2019:2530487.
Hasosah MY, Satti MB, Yousef YA, et al. IgG4-related sclerosing mesenteritis in a 7-year-old Saudi girl. Saudi J Gastroenterol. 2014;20:385–8.
Sakemi R, So S, Morimitsu Y, et al. A case of IgG4-related sclerosing disorders involving the mesentery and the pancreas. Nihon Shokakibyo Gakkai Zasshi. 2011;108:969–77.
Das KK, Clayton EF, Kochman ML. An unusual case of nausea and vomiting. Gastroenterology. 2015;148:515–6.
Lee SJ, Park CK, Yang WI, et al. IgG4-related sclerosing mesenteritis. J Pathol Transl Med. 2016;50:309–11.
Tang J, Cai S, Ye C, et al. Biomarkers in IgG4-related disease: a systematic review. Semin Arthritis Rheum. 2020;50:354–9.
Notohara K, Kamisawa T, Uchida K, et al. Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey. J Gastroenterol. 2018;53:845–53.
Koizumi S, Kamisawa T, Kuruma S, et al. Organ correlation in IgG4-related diseases. J Korean Med Sci. 2015;30:743–8.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MK, ST, KK and KJ. The first draft of the manuscript was written by MK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kozono, M., Tanoue, S., Kiyama, K. et al. A case of immunoglobulin G4-related sclerosing mesenteritis without other organ involvement. Clin J Gastroenterol 14, 1411–1418 (2021). https://doi.org/10.1007/s12328-021-01451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-021-01451-1