Abstract
Bilio-enteric fistulization is the aberrant connection between the biliary and luminal digestive tracts. The cholecystocolonic fistula (CCF) is the second most common bilio-enteric fistula (comprising 20% of cases), after the cholocystoduodenal fistula (comprising 70% of all cases). A CCF may result from malignancy or more benign etiologies, such as gallstones, and is thought to arise from a chronic inflammatory cadence of tissue necrosis, tissue perforation, and fistula creation. The combination of chronic watery diarrhea, vitamin K malabsorption, and radiological evidence of pneumobilia in a patient with history of gallstone disease has been suggested as a pathognomonic triad of CCF. Here, we present a case of a 62-year-old woman exhibiting this triad, who was found to have a CCF as a result of chronic gallstone-related disease. Recognition of this rare etiology of chronic diarrhea can enhance clinicians’ diagnostic appraisal and management of this common chief complaint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cholecystocolonic fistula (CCF) is the second most common bilio-enteric fistula (representing 20% of cases), after the cholecystoduodenal fistula (comprising 70% of cases) [1, 2]. The CCF is a more common phenomenon in women (female: male ratio 2.5:1) and typically involves the hepatic flexure of the colon [3]. It may result from malignancy or more benign etiologies, such as gallstones, and is hypothesized to arise from a chronic inflammatory cadence of tissue necrosis, tissue perforation, and fistula creation. This sequence may be further complicated by acute cholangitis, biliary peritonitis, or biliary cirrhosis [3]. Global mortality rate from the CCF and its complications is estimated to be 10–15% [2]. The combination of chronic watery diarrhea, vitamin K malabsorption, and radiological evidence of pneumobilia in a patient with history of gallstone disease has been suggested as a pathognomonic triad of CCF [2]. Here, we present a case of a 62-year-old woman exhibiting this triad, who was found to have a CCF as a result of chronic gallstone-related disease.
Case report
A 62-year-old woman with medical history notable for obesity (body mass index 32 kg/m2), diet-controlled type II diabetes mellitus, and atrial fibrillation presented for evaluation of 6 months of diarrhea. In that time, she had experienced watery, non-bloody, large volume bowel movements at least three times daily, and which were associated with urgency and occasional fecal incontinence. Stools were neither oily nor acholic. She endorsed 3-week history of poor oral intake, decreased appetite, and extreme fatigue. The patient did not have any abdominal pain, fevers or chills, arthritis, uveitis, dermatologic changes, jaundice, or pruritus. She had no recent travel, or exposure to antibiotics or sick contacts. She was a retired bookkeeper with a pet cat. Medications included metoprolol tartrate and warfarin, as well as newly added oral magnesium and potassium supplementation in the month prior to presentation for deficiencies in these electrolytes. Other than her warfarin dose, which she had decreased several times in the preceding months for increasingly supratherapeutic levels, she had no other medication dose changes. She had no recent or remote surgical or endoscopic interventions (except for tonsillectomy in her youth). Family history was not notable for any gastrointestinal or hepatobiliary disorders, including malignancies.
Physical examination showed a hemodynamically stable female. There was no scleral icterus, conjunctival pallor, oral ulcerations, thyroid nodules or bruits. The patient’s cardiac exam showed an irregular rhythm and no murmurs. Her lungs were clear bilaterally. Her abdomen was soft, non-tender, non-distended, with regular bowel sounds. The extremities showed no cyanosis, clubbing, edema, joint irregularity, or tremor. Laboratory evaluation (with normal ranges) showed sodium 141 mmol/L (135–145 mmol/L), potassium 4.3 mmol/L (3.5–5.2 mmol/L), bicarbonate 19 mmol/L (21–32 mmol/L), blood urea nitrogen 10 mg/dL (7–21 mg/dL), creatinine 1.0 mg/dL (0.6–1.1 mg/dL), magnesium 1.4 mg/dL (1.8–2.4 mg/dL), aspartate aminotransferase (AST) 46 U/L (10–31 U/L), alanine aminotransferase (ALT) 35 U/L (7–45 U/L), alkaline phosphatase 110 U/L (55–142 U/L), total bilirubin 0.8 mg/dL (0.2–1.0 mg/dL), hemoglobin A1c 5.7%, thyroid stimulating hormone 1.5 uIU/mL (0.3–4.2 uIU/mL), and C-reactive protein 3.9 mg/dL (0–9 mg/dL).
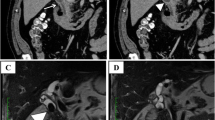
Abdominal Computed Tomography (CT) demonstrated diffuse pneumobilia, with a likely bilio-enteric fistula between the gallbladder fundus and colonic hepatic flexure (Fig. 1). There was slight wall thickening of the gallbladder fundus but no associated stranding, and no definitive mass was identified associated with the fistula. The common bile duct was not dilated but did appear to show sludge near the duodenum.
Two weeks later, as the patient awaited endoscopic evaluation, she was hospitalized for fevers and biliary obstruction, with laboratory values of AST 195, ALT 123 U/L, alkaline phosphatase 238 U/L, and total bilirubin 3.3 mg/dL. Ultrasound showed common bile duct dilatation to 11 mm. Given the concern for cholangitis, the patient underwent an endoscopic retrograde cholangiopancreatography (ERCP), which revealed a small, frond-like mass measuring 12 mm at the major papilla. Endoscopic and histologic evaluation of the mucosa of the first and second portions of the duodenum were unremarkable. Cholangiography revealed multiple stones in the middle third of the main bile ducts, the largest of which was 10 mm in diameter; additionally, a fistula was observed between the gallbladder and colon (Fig. 2). Stones were not extracted due to the papillary mass, and thus a biliary stent was placed to preserve drainage. She tolerated colonoscopy preparation during hospitalization with intake of 4 L of bowel prep while on clear liquid diet for 24 h preceding colonoscopy. Fortunately, there was no concerning signs of symptoms of a retrograde infection or sepsis during periprocedural observation.
Colonoscopy was performed during the same encounter, and it demonstrated an 8 mm diameter fistula at the hepatic flexure, which extended into the gallbladder (Fig. 3). One week later, endoscopic ultrasound (EUS) was performed with evidence of pneumobilia with additional identification of a small ampullary mass measuring 12 mm by 12 mm. There was no evidence of malignant appearing adenopathy, solid hepatic masses, invasive disease or pancreatic extension associated with the ampullary mass. Biliary sludge, stones, and air were also observed alongside the indwelling biliary stent. As such, the mass was resected during the same encounter via snare papillectomy and the area underwent ablation with argon plasma coagulation. Histological review later showed tubular adenoma with low-grade dysplasia. Cholangiogram was performed again by ERCP, revealing choledocholithiasis, and stone retrieval was completed by balloon extraction.
Three weeks later, the patient underwent laparoscopic cholecystectomy with concurrent mobilization of right colon and take-down of the CCF with wedge resection. The surgical resection specimen grossly demonstrated a probe-patent tract that was appreciated from the colonic mucosal surface to the gallbladder fundic mucosa. Pathologic review was notable for abnormal fusion of colonic muscular propria with the gallbladder muscular layer (Fig. 4). In addition, histologic review showed changes consistent with chronic cholecystitis with no evidence of biliary or colonic malignancy. The patient was discharged home in stable condition 2 days post-operatively. Since that time, her diarrhea has improved markedly, and her warfarin dosing and INR values have stabilized.
Microscopic image from surgically resected specimen. Cholecystocolonic fistula. From top to bottom: low power examination demonstrates gallbladder mucosa characterized by a papillary surface and deeper Rokitansky-Aschoff sinuses, with the deeper gallbladder muscular layer. Instead of gallbladder adventitia which would be expected deep to gallbladder muscular layer, there is colonic muscular propria fused with the gallbladder muscular layer. Colonic submucosa and mucosa (Bottom of image) are present overlying the colonic muscularis propria
Discussion
This report described a typical presentation of an uncommon disease state. A 62-year-old woman presented with 6 months of chronic, watery diarrhea associated with vitamin K malabsorption (as suggested by her need to decrease her warfarin dose for supratherapeutic INR values), as well as cross-sectional imaging demonstrating pneumobilia. This triad was offered as pathognomonic for CCF by Savvidou et al., and indeed her constellation of radiologic, endoscopic, and surgical findings confirmed the presence of a CCF [2]. Notably, this triad is not present in all patients and no studies have characterized the specificity/sensitivity of the aforementioned triad for CCF.
Diarrhea is the predominant clinical manifestation of CCF, observed in 71% of the case [3]. The pathophysiology of CCF-associated diarrhea is multifactorial, though bile acids are thought to play a noteworthy role. Bile acids are detergent molecules synthesized in the liver and excreted into the bilio-enteric circulation to facilitate fat and fat-soluble vitamin solubilization to aid in absorption. They are thereafter absorbed in the terminal ileum for recirculation [4, 5]. The framework in understanding causes of bile acid diarrhea can be structured with understanding broad four classification of bile acid diarrhea: (1) ileal dysfunction including Crohn’s disease or ileal radiation and resection (2) idiopathic without clear underlying cause often presenting as chronic diarrhea (3) general malabsorption or secondary to biliopancreatic pathologies (i.e., chronic pancreatitis) and (4) conditions that lead to excessive bile acid synthesis (i.e., hypertriglyceridemia or metformin use) [4, 5].
Excessive bile acids within the colon—from increased production or insufficient ileal reabsorption—affect colonic mucosal permeability with alterations in water and electrolyte secretions at the cellular level. The proposed mechanisms of diarrhea include increased water secretion through activation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) via adenylate cyclase, inhibition of chloride and hydroxide exchange at the apical cell membrane, and increased colonic motility secondary to stimulation of G protein-coupled receptor bile acid receptor 1, and increased mucus secretion through direct effects on the goblet cells [4, 5].
Much like the patient described here, patients with bile acid diarrhea have non-bloody loose bowel movements accompanied by urgency and abdominal cramping [4]. Bile acid diarrhea can be diagnosed with stool collection that quantifies amount of stool bile acids and percentage of specific culprit bile acids [5]. While this was not performed in our patient, it is highly suspected that excessive bile acids directed into the colon and bypassing the typical ileal mucosa for absorption and recirculation was the principal cause of this patient’s diarrhea. While non-anatomic causes of bile acid diarrhea may be ameliorated with bile acid sequestrants, this was unlikely to be helpful in our patient, as these sequestrants would be expected to act in the small bowel, which our patient’s bile acid delivery circumvented by way of CCF.
Our patient additionally was found to have a sporadic duodenal adenoma with low-grade dysplasia at the duodenal papilla. It is not clear if this contributed to the formation of the CCF through backpressure on the gallbladder, but the absence of major hepatobiliary biochemical abnormalities or biliary ductal dilatation at the patient’s baseline (prior to the development of cholangitis from choledocholithiasis) points away from this.
A CCF is reported to be diagnosed incidentally in 1 in every 1000 cases of cholecystectomies [3]. Pre-operative identification of CCF is difficult and rare [6]. The reported pre-operative diagnosis rate of CCF is less than 10%, which leads to a high burden of cases that start as laparoscopic operation which are switched intra-operatively to an exploratory laparotomy which is linked with an increased morbidity due to associated adhesions. A thorough review by Costi et al. identified a pre-operative diagnosis of CCF by ultrasound in 2 cases, barium enema in 4 cases, ERCP in 7 cases, colonoscopy in 4 cases, CT scan in 3 cases, and MRI & EUS in one case each [3].There have been no studies to date comparing the sensitivity of different diagnostic tools in identifying CCF. The diagnostic accuracy of ERCP was found to be 63%, however, this study grouped both cholecystoduodenal and cholecystocolic fistulas together and the study was further limited by a small number of patients in this group (n = 10) [7]. Furthermore, when a CCF is encountered, careful evaluation for malignancy must be undertaken. This involves cross-sectional imaging for gallbladder carcinoma and colonoscopy for colon cancer. Even if gross evidence of malignancy is not apparent during surgery, the resected specimen should be explored for histological evidence of neoplasia.
Given the rarity of CCF, management strategies lack clear guidelines. The majority of the literature supports cholecystectomy in addition to the surgical repair of the fistula to prevent complications, such as sepsis and the link between gallbladder fistula and gallbladder malignancy [8]. The approach further depends on whether the CCF is complicated by destabilizing conditions, such as sepsis or hemodynamic compromise, which may prompt more emergent surgery. For uncomplicated cases, there has been no reported superiority between laparoscopic and laparotomic approaches in terms of rate of complications. In contradistinction, a 17.2–60% rate of conversion has been reported in laparoscopic cases complicated by bleeding, challenges with intestinal suturing, and difficulty with navigating anatomy around an inflamed gallbladder [3, 6, 8].Given that the afflicted demographic is usually elderly with multiple comorbidities, the benefits of a minimally invasive approach ought to be weighed against the possible longer operative times and attendant complications. Of interest, in 2019, Baratta et al. reported first successful robotic repair of CCF and suggested advantages to this approach included greater ability to distinguish and separate chronic inflammatory tissue between the gallbladder and the colon [8]. While an uncommon approach, some physicians have elected to observe asymptomatic patients and pursue symptomatic treatment such as vitamin supplementation and prophylactic antibiotics. The rationale behind the conservative approach is that surgery as prophylaxis against perceived complications is not justified in the context of the limited life expectancy of patients found to have a CCF, particularly those with associated medical comorbidities which commonly accompany the characteristic demographic [9, 10].
Abbreviations
- CCF:
-
Cholecystocolonic fistula
- CT:
-
Computed tomography
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- SIBO:
-
Small intestinal bacterial overgrowth
References
Antonacci N, Taffurelli G, Casadei R, et al. Asymptomatic cholecystocolonic fistula: a diagnostic and therapeutic dilemma. Case Rep Surg. 2013;2013:754354.
Savvidou S, Goulis J, Gantzarou A, et al. Pneumobilia, chronic diarrhea, vitamin K malabsorption: a pathognomonic triad for cholecystocolonic fistulas. World J Gastroenterol. 2009;15:4077–82.
Costi R, Randone B, Violi V, et al. Cholecystocolonic fistula: facts and myths. A review of the 231 published cases. J Hepatobiliary Pancreat Surg. 2009;16:8–18.
Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology. 2019;156:1233.
Camilleri M. Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9:332.
Angrisani L, Corcione F, Fau Tartaglia A, Fau Tricarico A, et al. Cholecystoenteric fistula (CF) is not a contraindication for laparoscopic surgery. Surg Endosc. 2001;15:1023.
Yamashita H, Chijiiwa K, Fau Ogawa Y, Kuroki S, et al. The internal biliary fistula–reappraisal of incidence, type, diagnosis and management of 33 consecutive cases. HPB Surg. 1997;10:143.
Baratta VM, Kurbatov V, Le Blanc JM, et al. Robotic cholecystectomy and cholecystoenteric fistula closure in a female with remote cholangitis. J Surg Case Rep. 2019;2019:rjz231.
Velayos Jiménez B, Gonzalo Molina Ma Fau - Carbonero Díaz P, Carbonero Díaz P Fau - Díaz Gutiérrez F, et al. Cholecystocolic fistula demonstrated by barium enema: an uncommon cause of chronic diarrhoea.
Caroli-Bosc FX, Ferrero Jm Fau - Grimaldi C, Grimaldi C Fau - Dumas R, et al. Cholecystocolic fistula: from symptoms to diagnosis.
Acknowledgements
Special thank you to our patient for giving us the permission to share her story.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Chansong Choi, Christopher Hartley, Daniel Maselli, and Karim Osman declare that they do not have any conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, C., Osman, K., Hartley, C.P. et al. Cholecystocolonic fistula as an uncommon cause of diarrhea: a case-report and review of the literature. Clin J Gastroenterol 14, 1147–1151 (2021). https://doi.org/10.1007/s12328-021-01413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-021-01413-7