Abstract
Although non-small cell lung cancer can metastasize to any part of the body, metastasis to the gallbladder is extremely rare. We present a case of acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer. A 66-year-old man diagnosed with primary stage IV T4N3M1b non-small cell lung cancer was admitted to our hospital to receive chemotherapy, during which he presented with right upper abdominal pain. Abdominal contrast-enhanced computed tomography showed an enhanced mass at the neck of the gallbladder and gallbladder distension with obvious wall thickening. Acute cholecystitis caused by obstruction of the gallbladder neck by malignancy was suspected. Open cholecystectomy, extrahepatic bile duct resection, and Roux-en-Y choledochojejunostomy were performed. Pathological and immunohistochemical examinations revealed gallbladder metastasis originating from non-small cell lung cancer. In conclusion, when a patient with lung cancer presents with acute cholecystitis, the rare possibility of gallbladder metastasis should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) can metastasize to any part of the body. The frequent sites of NSCLC metastasis are the pleura, contralateral lung, bone, liver, adrenal glands, and brain. Gallbladder metastasis from NSCLC is extremely rare; the gallbladder has been recognized as a site of metastasis in only 1.9% of 160 lung cancer cases in a large autopsy review [1], and lung cancer metastasis to the gallbladder was detected in living patients only in a few clinical cases [2,3,4,5]. This report presents a rare case of acute cholecystitis caused by gallbladder metastasis from NSCLC.
Case report
A 66-year-old man with no relevant medical history presented with complaints of back pain, which was treated using nonsteroidal anti-inflammatory drugs. He was referred to our hospital for further examination. Chest contrast-enhanced computed tomography (CT) showed a mass of 7 × 5 × 6 cm in the superior segment of the right lower lung lobe and the enlargement of lymph nodes in subcarinal region and left supraclavicular region (Fig. 1a, b). Abdominal contrast-enhanced CT revealed a mass in the left adrenal gland, indicative of metastasis (Fig. 1c), but there were no specific imaging findings in the gallbladder. Whole-body 18F-2-deoxy-d-glucose (FDG) positron emission tomography (PET) revealed an intense uptake corresponding to the lesions on CT (Fig. 1d, e, f). PET/CT scan showed no FDG uptake in the gallbladder. A transbronchial biopsy did not result in a definitive diagnosis. However, both endobronchial ultrasound-guided transbronchial needle aspiration for the subcarinal lymph node and biopsy of the left supraclavicular lymph node resulted in a definitive diagnosis of non-small cell carcinoma. Although it was difficult to diagnose the histological type of the NSCLC, the tumor cells were negative for thyroid transcription factor-1 (TTF-1), Napsin A, and p40 but partially positive for CK14 immunohistochemically. The final diagnosis was primary stage IV T4N3M1b NSCLC, and the patient was admitted to our hospital to receive first-line chemotherapy with carboplatin (area under the curve: 6), and nab-paclitaxel (170 mg/m2) was subsequently administered. He was discharged from the hospital on the 15th day after starting chemotherapy. After 4 cycles of this regimen for 4 months, follow-up CT revealed increased tumor diameter, leading to suspicion of progressive disease. Repeated biopsy of the left supraclavicular tumors revealed a PD‐L1 tumor proportion score of 95%; therefore, atezolizumab (1200 mg), an anti‐PD‐L1 immune checkpoint inhibitor, was administered every 3 weeks for 2 cycles as second-line treatment.
Radiological findings. a Chest contrast-enhanced computed tomography (CT) showing a mass measuring 7 × 5 × 6 cm in the superior segment of the right lower lung lobe and the lymph node enlargement in the subcarinal region (arrows). b Chest contrast-enhanced CT showing a mass in the left supraclavicular region (arrows). c Abdominal contrast-enhanced CT showing a mass at the left adrenal gland (arrows). d–f A positron emission tomography/CT scan showing an intense 18F-2-deoxy-d-glucose uptake corresponding to the lesions observed on CT
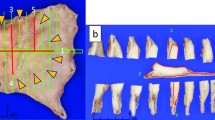
Two months after starting atezolizumab, the patient returned to our hospital with right upper abdominal pain, fever, and nausea. At admission, his vital signs were as follows: temperature, 38.2 °C; blood pressure, 100/60 mmHg; and heart rate, 110 bpm. Laboratory tests revealed an elevated white blood cell count with a left shift (17,400/mm3) and elevated C-reactive protein level (17.09 mg/dL) (Table 1). Abdominal contrast-enhanced CT showed an enhanced mass at the gallbladder neck and gallbladder distension with obvious wall thickening (Fig. 2a). However, CT revealed that the primary tumor and other metastases did not increase in size. Based on these findings, a diagnosis of Grade 1 acute cholecystitis was made according to the TG18 guidelines. Initially, intravenous antibiotics (piperacillin/tazobactam at 13.5 g/day) were administered for 1 week; however, the patient’s condition did not improve sufficiently. Endoscopic retrograde cholangiopancreatography showed severe narrowing of the common bile duct and disruption of the cystic duct (Fig. 2b). These findings indicated compression of the common bile duct by the gallbladder malignancy, which led to a suspicion of Mirizzi syndrome. Repeated exfoliative cytology of the bile and brushing cytology of the bile duct revealed no malignancy, which did not reach a definitive diagnosis. Endoscopic nasal gallbladder drainage (ENGBD) was performed 1 day later. Repeated cytology of the bile from ENGBD also revealed no malignancy. The patient’s systemic condition gradually improved, and laboratory data values returned to normal ranges. Although his Charlson comorbidity index (CCI) score was 8, the American Society of Anesthesiologist physical status classification system (ASA-PS) score was II, which was considered to indicate tolerance to surgery, regardless of the diagnosis of primary stage IV NSCLC. Therefore, cholecystectomy as palliative surgery was selected to treat the obstruction of the gallbladder. Intraoperatively, the mass at the neck of the gallbladder was potentially invaded into the biliary tree, which disabled us to perform only cholecystectomy to confirm the surgical margin; therefore, cholecystectomy, extrahepatic bile duct resection, and Roux-en-Y choledochojejunostomy were performed. The resected gallbladder measured 10 × 5 cm in size. A mass at the gallbladder neck led to the obstruction of the cystic duct macroscopically (Fig. 3). The pathological examination did not prove the existence of tumor invasion to the common bile duct. Immunohistochemically, the tumor cells were negative for thyroid transcription factor-1 (TTF-1), Napsin A, and p40, but were positive for Keratin AE1/3 and partially for CK14 (Fig. 4a-d). The tumor was similar to the past biopsy specimen of the left supraclavicular lymph node; indeed, pathological and immunohistochemical examinations revealed that the histological gallbladder tumor type was similar to that of the lung (Fig. 5a, b).
Preoperative radiological findings. a Abdominal contrast-enhanced computed tomography (CT) showing an enhanced mass at the gallbladder neck (arrow) and gallbladder distension with obvious wall thickening. b Endoscopic retrograde cholangiopancreatography showing severe narrowing of the common bile duct and disruption of the cystic duct (arrow)
Macroscopic findings of the resected gallbladder specimen. a, b Histological examination of the gallbladder tumor diagnosed as poorly differentiated adenocarcinoma (hematoxylin and eosin stain, magnification 10 × (a) and 100 × (b). c, d Immunohistochemical examination showing tumor cells positive for Keratin AE1/3 and partially positive for CK14
Therefore, the final diagnosis was metachronous gallbladder metastasis originating from NSCLC with acute cholecystitis. The postoperative course was uneventful and the patient was discharged on postoperative day 10. The patient was followed up at our hospital’s oncology department. He could restart atezolizumab (1200 mg) 1 month soon after surgery. Although he continued to receive atezolizumab (1200 mg) monthly for 2 cycles, metastases of the lymph nodes and the primary tumor developed rapidly, which was revealed on a follow-up CT 2 months after surgery. The patient died due to disease progression 5 months after surgery.
Discussion
Metastasis to the gallbladder from other malignancies is extremely rare and is associated with an extremely poor prognosis. Metastatic involvement of the gallbladder was found in 5.8% of patients with cancer in a review of one large autopsy series [1]. Among various types of cancer that can metastasize to the gallbladder, malignant melanoma is the most likely to spread to the gallbladder, being present in 20% of cases [1, 5], followed by renal cell carcinoma, breast adenocarcinoma, gastric cancer, squamous cell carcinoma of the cervix, and hepatocellular carcinoma [6,7,8,9,10]. However, lung cancer metastasizing to the gallbladder is exceedingly rare. Abrams et al. reported that autopsy findings showed only 1.9% of patients with lung cancer had gallbladder metastasis [1]. To the best of our knowledge, only five cases of gallbladder metastasis originating from NSCLC with acute cholecystitis have been reported in the English language literature [2,3,4,5, 11]; these previous cases, including our case, are summarized in Table 2.
There are two major routes through which primary tumors can metastasize to the gallbladder, namely direct invasion and a hematogenous route. While hepatocellular carcinomas and pancreatic tumors have been reported to invade the gallbladder by direct invasion [5], melanoma and renal cell, cervical, gastric, breast, and lung cancer metastasize via a hematogenous route [6,7,8,9,10]. Hematogenous gallbladder metastases initially occur as small flat nodules below the mucosal layer, which then grow as pedunculated nodules and rarely surpass several millimeters in size [9]. This is why most gallbladder metastases do not cause any symptoms. Although it may be very difficult to diagnose gallbladder metastasis early, detection of unsuspected metastases is a well-known advantage of F-18 FDG PET/CT; studies using this imaging method have found metastases in 6–17% of patients with lung cancer [12,13,14]. Jeong et al. reported that F-18 FDG PET/CT could be a useful tool for the detection of a usual or unsuspected metastasis during lung cancer staging [4]. Although all patients in previously reported cases had acute cholecystitis, the causal relationship between the development of acute cholecystitis and gallbladder metastasis has remained unclear. In case 5, the volume of tumors was not high enough to cause acute cholecystitis. In our case, acute cholecystitis was caused by the obstruction of the gallbladder neck because of metastatic cancer cells.
It is difficult to differentiate metastatic from primary gallbladder carcinoma when gallbladder malignancy is suspected. Primary gallbladder carcinoma is usually found in aged female patients with long-standing cholelithiasis. It is usually identified at a stage when the entire gallbladder is involved, with diffuse and irregular mural thickening and infiltration of the gallbladder bed and adjacent liver parenchyma. In contrast, acalculous gallbladder is more consistent with a metastatic gallbladder tumor than a primary gallbladder carcinoma [6]. In addition, CT findings of metastatic gallbladder differ among the histologic types of primary tumors; adenocarcinomas metastasized to the gallbladder manifest as infiltrative wall thickenings with persistent enhancement, whereas metastatic gallbladder tumors from melanoma and renal cell carcinoma appear as one or multiple enhancing polypoid lesions with early wash-in or wash-out enhancement [6, 21].
Two methods are most widely available for the pathological diagnosis of a suspected malignant biliary stricture, namely, endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) [22, 23]. A recent systematic review and meta-analysis revealed that both methods have high specificity and positive predictive value in diagnosing suspected biliary strictures, but a combination of both methods is recommended for the tissue diagnosis of a malignant biliary stricture, with a pooled sensitivity of 86% and positive likelihood ratio of 12.50, indicating that a malignant result can be trusted [24].
The most common histologic type of primary gallbladder cancer is adenocarcinoma (75.8%), followed by papillary adenocarcinoma (5.8%) and mucinous (4.8%), adenosquamous (3.6%), and squamous (1.7%) carcinoma [15]. Immunohistochemical staining may be useful to distinguish between primary and metastatic gallbladder tumors and to diagnose these tumors accurately [10]. Although no lung-specific tumor marker has been established, TTF-1 has been shown to play an essential role in discriminating cancers with lung origin with a high degree of reliability. Jagirdar et al. reported that the sensitivity of TTF-1 was 75% for lung adenocarcinoma, followed by 50% for large cell carcinoma and 7% for squamous carcinoma [16]. Moreover, differential expression of TTF-1 in metastatic lung adenocarcinoma was 77%, while that of TTF-1 in primary gallbladder adenocarcinoma was 0% [16]. In three of previously reported cases, immunohistochemical TTF-1 was helpful to diagnose gallbladder metastasis originating from NSCLC.
The TG18 guidelines stipulate that the treatment strategy should be considered and chosen after the cholecystitis severity, patient’s general status, and underlying disease have been assessed [26]. Given the small number of cases reporting gallbladder metastasis from lung cancer with acute cholecystitis, the clinical behavior of such cancers and the optimal treatment strategy remain unclear.
For patients with NSCLC, median overall survival and 5-year survival rates have been poor. In the United States, 5-year survival between 2008 and 2014 was 5.5% for patients with distant metastases [17]. Since the first approval of immune checkpoint inhibitors for NSCLC in 2015, the treatment of metastatic NSCLC has changed completely. The anti-PD-L1 antibody atezolizumab has been shown to provide an overall survival benefit in patients with previously treated metastatic NSCLC [18]. Moreover, patients with high PD-L1 expression (defined as PD-L1 expression on 50% or more of tumor cells or as 10% or more of tumor-infiltrating immune cells) have the greatest benefit from atezolizumab (median overall survival, 20.5 months [95% confidence interval 17.5–not evaluable] vs. 8.9 months [5.6–11.6]; HR 0.41 [95% confidence interval 0.27–0.64]) [18].
In our case, PD-L1 expression was very high (PD‐L1 tumor proportion score of 95%), so a great benefit was expected from atezolizumab administration, and it was extremely essential for the patient to receive atezolizumab continuously and stably. There was a risk of repeated acute cholecystitis due to the tumor, so treatment intervention was required. Percutaneous transhepatic gallbladder drainage or ENGBD as an external drainage procedure is an effective and appropriate method for patients with poor medical condition [19]. However, serious concerns of a drainage tube have the added risk of obstruction after hospital discharge and a limitation in the activities of daily living. Endoscopic transpapillary gallbladder stenting (ETGBS) as an internal drainage procedure is considered to be a superior alternative therapy in terms of the patient’s quality of life, while its inset requires skillful techniques. The complication rate of ENGBD and ETGBS is 8.2%, including stent migration, perforation, biliary leak, bleeding, and post-ERCP pancreatitis [20]. Moreover, a patient with ASA-PS II is considered to be able to tolerate surgery, regardless of the presence of primary stage IV NSCLC (with a CCI score of 8). Therefore, palliative surgery was selected and the patient could receive atezolizumab continuously, which resulted in 13 months of overall survival.
A previous study suggested that the presentation of metastases to the gallbladder with acute cholecystitis is associated with a poor survival, with an overall median survival after the diagnosis of gallbladder metastasis of 8.7 months; however, this study did not include lung cancers [25]. In fact, three previously reported cases of gallbladder metastasis originating from NSCLC with acute cholecystitis died 3 weeks to 5 months after the operation, which indicated that gallbladder metastasis of lung cancer may be associated with a poor prognosis [3, 5, 13]. Considering the clinical behavior and prognosis of lung cancers with acute cholecystitis, the optimal treatment for this case might have been gallbladder drainage, such as with ETGBS.
In conclusion, we report a rare case of acute cholecystitis caused by gallbladder metastasis from NSCLC. When a patient with lung cancer presents with acute cholecystitis, the rare possibility of gallbladder metastasis should be considered.
References
Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85.
Gutknecht DR. Metastatic lung cancer presenting as cholecystitis. Am J Gastroenterol. 1998;93:1986–9.
Nassenstein K, Kissler M. Gallbladder metastasis of non-small cell lung cancer. Onkologie. 2004;27:398–400.
Jeong HT, Yun M, Hong HS, et al. Unusual gallbladder metastasis from non-small cell lung cancer detected by F-18 FDG PET/CT with intravenous contrast enhancement. Clin Nucl Med. 2010;35:635–6.
Jeong YS, Han HS, Lim SN, et al. Gallbladder metastasis of non-small cell lung cancer presenting as acute cholecystitis. Chin J Cancer Res. 2012;24:249–52.
Barretta ML, Catalano O, Setola SV, et al. Gallbladder metastasis: spectrum of imaging findings. Abdom Imaging. 2011;36:729–34.
Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605–12.
Yoon WJ, Yoon YB, Kim YJ, et al. Metastasis to the gallbladder: a single center experience of 20 cases in South Korea. World J Gastroenterol. 2009a;15:4806–9.
Nojima H, Cho A, Yamamoto H, et al. Renal cell carcinoma with unusual metastasis to the gallbladder. J Hepatobiliary Pancreat Surg. 2008;15:209–12.
Aoki T, Inoue K, Tsuchida A, et al. Gallbladder metastasis of renal cell carcinoma: report of two cases. Surg Today. 2002;32:89–92.
Yoshida Y, Shingyoji M, Ashinuma H, et al. Coincidental detection of gallbladder metastasis of lung adenocarcinoma. Jpn J Lung Cancer. 2014;54:73–7.
Weder W, Schmid RA, Bruchhaus H, et al. Detection of extrathoracic metastases by positron emission tomography in lung cancer. Ann Thorac Surg. 1998;66:886–92.
Pieterman RM, van Putten JWG, Meuzelaar JJ, et al. Preoperative staging of non–small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61.
Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493–7.
Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med. 2008;132:384–96.
Noone AM, Howlader N, Krapcho M, et al (2018) SEER Cancer Statistics Review, 1975–2015. https://seer.cancer.gov/archive/csr/1975_2015. (Accessed 18 Jan 2019).
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:3–16.
Itoi T, Takada T, Hwang TL, et al. Percutaneous and endoscopic gallbladder drainage for acute cholecystitis: international multicenter comparative study using propensity score-matched analysis. J Hepatobiliary Pancreat Sci. 2017;24:362–8.
Muller-Horvat C, Radny P, Eigentler TK, et al. Prospective comparison of the impact on treatment decisions of whole body magnetic resonance imaging and computed tomography in patients with metastatic malignant melanoma. Eur J Cancer. 2006;42:342–50.
Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104.
Jo JH, Cho CM, Jun JH, et al. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. J Gastroenterol Hepatol. 2019;34:799–805.
de Moura DTH, Ryou M, de Moura EGH, et al. Endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary strictures: a meta-analysis of same-session procedures. Clin Endosc. 2020;53:417–28.
Yoon WJ, Yoon YB, Kim YJ, et al. Metastasis to the gallbladder: a single-center experience of 20 cases in South Korea. World J Gastroenterol. 2009b;15:4806–9.
Okamoto K, Suzuki K, Takada T, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis [published correction appears in J Hepatobiliary Pancreat Sci. 2019 Nov; 26(11):534]. J Hepatobiliary Pancreat Sci. 2018;25:55–72.
Acknowledgements
We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KI drafted the manuscript and treated the patient. DS treated the patient and helped to draft the manuscript. KI, DS, KO, TY, TK, MI, YC, MY, KN, MH, and HM treated the patient. HI, SS, and MO determined the treatment plan and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, no informed consent is required.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imaoka, K., Satoh, D., Oshita, K. et al. Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report. Clin J Gastroenterol 14, 351–357 (2021). https://doi.org/10.1007/s12328-020-01293-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-020-01293-3