Abstract
Neuroendocrine carcinoma in Barrett’s esophagus is rare and its developmental mechanisms remain unclear. Neuroendocrine carcinoma arising in Barrett’s esophagus with adenocarcinoma was detected at an early stage and resected by endoscopic submucosal dissection. Detailed pathological examination revealed that the neuroendocrine carcinoma originated via differentiation of the preexisting adenocarcinoma. A 79-year-old man presented with a flat protruding lesion in the esophagogastric junction. Esophagogastroduodenoscopy revealed a red flat 10-mm protruding lesion in the Barrett’s epithelium and a shallow depression at the distal end. Narrow band imaging with magnification showed that the blood vessels in the protrusion were dilated and meandered irregularly, while those in the depression were small and did not form a network; the blood vessels were missing in some parts of the depression. Well-differentiated adenocarcinoma was diagnosed after analysis of the biopsy specimen of the protrusion, and endoscopic submucosal dissection was performed. The pathological diagnosis was neuroendocrine carcinoma with an adenocarcinoma component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal neuroendocrine carcinoma (NEC) is rarely encountered, accounting for approximately 0.2% of all cases of esophageal cancers [1]. It progresses rapidly and is often detected at an advanced stage and is known to have a poor prognosis [2]. Barrett’s esophagus may potentially be malignant, giving rise, in many cases, to tubular adenocarcinomas [3]. NEC rarely develops in Barrett’s esophagus [4,5,6,7,8,9,10,11,12], and its clinical presentation and developmental mechanism remain unclear. Here, we report on a case of esophageal NEC that developed from an adenocarcinoma arising in Barrett’s esophagus.
Case report
A 79-year-old man visited our facility for detailed examination of a flat protruding lesion in the esophagogastric junction that was detected in an esophagogastroduodenoscopy (EGD) examination during a medical check-up. The patient had a history of hypertension and obsolete cerebral infarction. He had no symptoms associated with gastroesophageal reflux disease. He had never taken proton-pump inhibitors prior to first the EGD examination. The physical examination revealed no abnormalities. Clinically, there were no symptoms of paralysis.
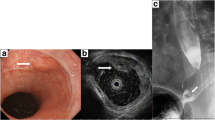
The EGD revealed a flat, red, slightly protruding 10-mm lesion with a small, white protruding center measuring 2 mm, at the 2 o’clock position of the esophagogastric junction. The proximal end of the lesion was covered in white normal squamous epithelium. The lesion was present on a background of Short Segment Barrett’s Esophagus (SSBE), in which columnar-appearing mucosa were accompanied by palisade vessels (Fig. 1a). A biopsy of the white protrusion was performed.
a Initial endoscopic view A flat, red, protruding 10-mm lesion with a small, white, protruding center (indicated by arrow) is visible in the 2 o’clock position of the esophagogastric junction. b Biopsy histology (H&E stain). Stratified squamous epithelium is visible, with deformed cells forming tubules in the deep part (indicated by arrows)
Pathologic evaluation of the biopsy specimen indicated a well-differentiated tubular adenocarcinoma covered with normal stratified squamous epithelium (Fig. 1b).
The blood examination results were normal, including the levels of tumor markers, such as CEA, CA19-9, and SCC, except for serum Helicobacter pylori antibody level was positive at 13 U/mL. No distant metastasis or lymph node involvement was seen on the thoracoabdominal contrast CT examination.
During the preoperative endoscopic examination 4 weeks later, the flat, red, protruding lesion had a depression on the distal end (Fig. 2a). The depression appeared more clearly on acetic acid-indigo carmine chromoendoscopy (Fig. 2b). Magnification using narrow band imaging (NBI) showed dilation and irregular meandering of blood vessels in the protruding area (Fig. 2c) and a clear demarcation line in the depression area separating cancer and non-cancerous mucosae (Fig. 2d). Blood vessels of the depression were small, lacked networking, and missing in parts (Fig. 2e). Prior to endoscopic submucosal dissection (ESD), we determined the depth of cancer invasion endoscopically. In this case, the thickness and deformed shape of the lesion diminished with sufficient air inflation, suggesting the depth of invasion to be confined to T1a-LPM.
Pre-ESD endoscopic view. a A red protruding 10-mm lesion with a small, white, protruding center (indicated by arrow) is visible, and a shallow erosive depression is visible at the distal end. b Chromoendoscopy (acetic acid-indigo carmine). The depression in the lesion (arrow) is more clearly visible. c NBI magnified endoscopy (white protrusion). Irregularly branched, dilated abnormal blood vessels are visible. d NBI magnified endoscopic picture taken by inversion (depression at distal end). A demarcation line between the tumor and normal tissue is visible where indicated by the arrows. e NBI magnified endoscopic picture taken by inversion (depression). Tiny, irregular, poorly networked blood vessels and regions of reduced blood vessel concentration are visible
Clinically, the patient was diagnosed with Barrett’s esophageal adenocarcinoma without invasion of muscularis mucosa, lymph node involvement or distant metastasis, and ESD was performed. The lesion was resected en bloc by ESD without incident. The lesion was located in the center of the resected specimen, and a clear depression was observed on the distal end (Fig. 3a). Staining with Lugol’s iodine revealed that part of the flat protruding lesion was covered by squamous epithelium (Fig. 3b).
An adenocarcinoma covered by normal squamous epithelium was observed in a pathological examination of the resected specimen (Fig. 4). There was submucosal invasion of adenocarcinoma. It was well differentiated in the superficial mucosa, but the tubular configuration gradually became indistinct in deeper parts of the lesion. An area of solid nest configuration contiguous with the adenocarcinoma was observed on the distal end of the lesion. This area composed of small, uniform deformed cells, and its boundary with the adenocarcinoma was indistinct.
Histopathological image of resected specimen (lesion removed along line A in Fig. 3b). An adenocarcinoma (indicated by yellow arrow) and a zone of small, uniform, deformed cells comprising a solid nest configuration (red arrow) are visible
On immunostaining, the area of solid nest configuration was synaptophysin-positive and focally positive for chromogranin A, and 73.8% of all cells stained positive for Ki-67/MIB1; thus, NEC, small cell-type, was diagnosed (Fig. 5).
Synaptophysin-positive and chromogranin A-positive cells were observed in the well-differentiated adenocarcinoma of the superficial mucosa (Fig. 6).
In the area of mixed NEC and adenocarcinoma, the adenocarcinoma had more synaptophysin-positive cells than the adenocarcinoma in the superficial mucosa, and chromogranin A-positive cells were observed in parts (Fig. 7). Cells staining positive for Ki-67/MIB1 in this area accounted for 74.4% of the total tumor, similar to the area of pure NEC at the distal end of the lesion.
Adenocarcinoma near the NEC. (a H&E stain. b synaptophysin stain. c chromogranin A stain.). Tubular configuration of the adenocarcinoma is less distinct, synaptophysin-positive cells are more abundant than in the adenocarcinoma of the superficial mucosa, and chromogranin A-positive cells are visible in parts
At the boundary between the NEC and the adenocarcinoma, p53 immunostaining was positive in scattered cells in both the adenocarcinoma and NEC components (Fig. 8).
In the distal depression of the flat protruding lesion, NEC invasion extensively reached at least 400 μm beyond the muscularis mucosa. Resection margin assessment was vertical margin positive (Fig. 9). Vascular invasion was not seen. As the adenocarcinoma only constituted approximately 10% of the lesion (Fig. 10), the final pathological diagnosis was neuroendocrine carcinoma with well-differentiated type adenocarcinoma.
As additional treatment, laparoscopic distal esophagectomy with D1 + lymphadenectomy was performed. No local tumor residue or lymph node involvement was found during the pathological assessment of the surgical resected specimen.
Postoperative adjuvant chemotherapy was not administered since patient refused consent. The patient was monitored through follow-up. Recurrence has not been observed, 4 years after surgery.
Discussion
NECs were previously diagnosed as endocrine cell carcinoma or small cell carcinoma, but they are currently classified as neuroendocrine neoplasms (NENs) according to the WHO classification [13]. NENs are diagnosed on immunostaining, such as on staining for chromogranin A, synaptophysin, and CD56, and are classified based on nuclear division and Ki-67 index [13]. Gastric NECs with adenocarcinoma in which each component comprises at least 30% of the tumor are diagnosed as mixed adenoneuroendocrine carcinoma (MANEC).
Barrett’s esophagus is characterized by a specific columnar epithelium and has high malignant potential, but the developmental mechanism of NEC from Barrett’s esophagus remains unclear. There are three hypotheses for the developmental mechanism of NEC of the stomach, whose mucosa consists of columnar epithelium-like Barrett’s esophagus. In the first hypothesis, a preexisting low-to-intermediate grade NET progresses into an NEC. Another hypothesis proposes a monoclonal origin of the NEC from a pluripotent cell undergoing biphenotypic differentiation. Finally, clonal differentiation of a pluripotent cell from a preexisting adenocarcinoma into a neuroendocrine phenotype has been suggested as a third hypothesis. Regarding MANEC, in particular, it may be hypothesized that NEC may arise from a preexisting adenocarcinoma in which a clonal differentiation of a pluripotent cell into a neuroendocrine phenotype leads to the development of an NEC [14]. Because neuroendocrine cells are also present in Barrett’s esophagus, the mechanism for the origin of NEC in Barrett’s esophagus should be similar to that in the stomach.
In the present case, although both adenocarcinoma and NEC are present in the lesion, because NEC comprises the greater part of the tumor, the diagnostic criteria for MANEC were not met. However, as an early stage NEC accompanied by adenocarcinoma, the pathological image was striking.
Nishikura et al. [15] analyzed gastric NECs with adenocarcinoma components and reported that both components intermix in a transitional zone between the adenocarcinoma of the superficial mucosa and the NEC of deep mucosa. They report that NECs arise from well-differentiated adenocarcinomas under the influence of the p53 gene. Our case was one of NECs with adenocarcinoma arising in Barrett’s esophagus. Although no specific findings were observed on p53 immunostain of this lesion, pathological findings similar to those in the gastric NECs reported by Nishikura et al. [15] were observed.
In cases where a single lesion is formed from two different malignancies, the possibility of collision carcinoma must be considered. Spagnolo et al. [16] have proposed diagnostic criteria for collision carcinomas, which they have defined as tumors in which carcinomas of two types arise independently of each other and come into contact. These diagnostic criteria are a clear distribution of two distinct histological types and that each histological type is clearly recognizable, though a mixture of the types may be present at the boundary of the two tumors.
In the present case, the flat protruding lesion consisted of a well-differentiated adenocarcinoma in the superficial mucosa and an NEC in the deep mucosa of the lesion’s distal end. The boundary between the two distinct tumors was indistinct, the adenocarcinoma and NEC intermixed in a transition zone, and continuity was observed in both lesions. In addition, a continuous increase in the ratio of synaptophysin-positive cells was observed from the adenocarcinoma to the NEC. Accordingly, the lesion does not appear to be a collision carcinoma.
As in a previous report [17], synaptophysin-positive cells, the greatest proportion of which occurred in the NEC, were also observed at the base of adenocarcinoma tubules in our patient’s tumor. Chromogranin A is highly specific to NEC, but focally positive cases have been reported as common [14], and this tumor’s NEC component was also focally positive. In addition, the chromogranin A-positive cells at the base of adenocarcinoma tubules are an important finding in considering the origin of NEC in this tumor. In the solid nest configuration zone, Chromogranin A was weakly stained, even though synaptophysin was strongly stained. Pathologically, this case showed a continuous transition from well-differentiated adenocarcinoma to NEC. Therefore, the maturity or characteristics of NEC may have been different in the different parts of the lesion, and might have affected the intensity of immunostaining for Chromogranin A.
In conclusion, an NEC arising in Barrett’s esophagus with adenocarcinoma was detected at an early stage and resected en bloc by ESD. Detailed pathological examination revealed that the NEC originated via differentiation of the preexisting adenocarcinoma. Further research is needed to elucidate the molecular biological changes involved in the process by which NEC arises from adenocarcinoma in Barrett’s esophagus.
References
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan 2010. Esohagus. 2017;14:189–21414.
Maru DM, Khurana H, Rashid A, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32:1404–11.
Paraf F, Fléjou JF, Pignon JP, et al. Surgical pathology of adenocarcinoma arising in Barrett’s esophagus: analysis of 67 cases. Am J Surg Pathol. 1995;19:183–91.
Saw EC, Yu GS, Wngner G, et al. Synchronous primary neuroendocrine carcinoma, and adenocarcinoma in Barrett’s esophagus. J Clin Gastroenterol. 1997;24:116–9.
Saint Martin MC, Chejfec G. Barrett eshopohagus-associated small cell carcinoma. Arch Pathol Lab Med. 1999;123:1123.
Trabelsi O, el Mezni F, Kammoun A, et al. Neuroendocrine carcinoma in Barrett’s esophagus. Report of a case. Tunis Med. 1999;77:576–80.
Wilson CI, Summerall J, Willis I, et al. Esophageal collision tumor (Large cell neuroendocrine carcinoma and papillary carcinoma) arising in a Barrett esophagus. Arch Pathol Lab Med. 2000;124:411–5.
Chen KT. Cytology of small-cell carcinoma arising in Barrett’s esophagus. Diagn Cytopathol. 2000;23:180–2.
Bibeau F, Chateau MC, Guiu M, et al. Small cell carcinoma with concomitant adenocarcinoma arising in a Barrett’s esophagus: report of a case with a favorable behavior. Virchows Arch. 2008;452:103–7.
Markogiannakis H, Theodorou D, Toutouzas KG, et al. Small cell carcinoma arising in Barrett’s esophagus: a case report and review of the literature. J Med Case Rep. 2008;2:15.
Kawazoe T, Saeki H, Edahiro K, et al. A case of mixed adenoneuroendocrine carcinoma (MANEC) arising in Barrett’s esophagus: literature and review. Surg Case Rep. 2018;4:45.
Doi S, Matsumoto S, Wakatsuki K, et al. A neuroendocrine carcinoma with a well-differentiated adenocarcinoma component arising in Barrett’s esophagus: a case report and literature review. Surg Case Rep. 2018;4:103.
La Rosa S, Rindi G, Solcia E, et al. Gastric neuroendocine neoplasms: WHO Classification of Tumours’ Editorial Board. WHO Classification of Tumors, 5th Edition: Digestive system. 5th ed. World Health Organization; 2019. pp. 104–9.
La Rosa S, Vanoli A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. 2014;67:938–48.
Nishikura K, Watanabe H, Iwafuchi M, et al. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203–9.
Spagnolo DV, Heenann PJ. Collision carcinoma at the esophago-gastric junction. Cancer. 1980;46:2702–8.
Kloppel G, Couvelard A, Perren A, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancratic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–6.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tsubasa Kinoshita, Shigenao Ishikawa, Tomoki Inaba, Ichiro Sakakihara, Koichi Izumikawa, Sakuma Takahashi, Kumiko Yamamoto, Shigetomi Tanaka, Masaki Wato , Satoko Nakamura and Takashi Yao declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008(5).
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kinoshita, T., Ishikawa, S., Inaba, T. et al. Neuroendocrine carcinoma arising from Barrett’s esophageal adenocarcinoma: a case report. Clin J Gastroenterol 13, 1028–1035 (2020). https://doi.org/10.1007/s12328-020-01210-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-020-01210-8