Abstract
A 69-year-old woman who had no symptoms was found to have an intraperitoneal tumor on abdominal ultrasonography in a medical checkup. Thereafter, she was referred to our hospital for a further detailed examination. Contrast-enhanced computed tomography revealed a thin-walled cystic tumor with a diameter of 8 cm and with a hypervascular solid masses in the cystic wall, along with intraperitoneal multiple nodules. The cystic tumor was contiguous with the stomach wall. For solid mass of cystic lesions, endoscopic ultrasound-fine needle aspiration was performed transgastrically with a 25-gauge Franseen needle. Pathologically, the cells in the tumor were spindle shaped with atypical nucleus and were positive for c-kit, CD34, and smooth muscle actin. The tumor was diagnosed as gastrointestinal stromal tumor (GIST). With the diagnosis of gastric GIST with peritoneal dissemination, imatinib chemotherapy was initiated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and most frequently arise from the stomach [1]. Typically, GISTs appear as solid masses. Although GISTs with some cystic components are occasionally found, GISTs with predominant cystic formation are very rare [2,3,4,5,6,7,8,9,10,11]. Hemorrhage or tumor necrosis is considered to be an important factor involved in the mechanism of cystic space formation in GIST [12]. In previous reports, most cases of GISTs with predominant cystic formation were diagnosed by surgical resection. Here, we report a case of gastric GIST with predominant cystic formation diagnosed by endoscopic ultrasound-fine needle aspiration (EUS-FNA). EUS-FNA is less invasive for patients compared with surgical resection or laparoscopic biopsy and allowed for the early initiation of imatinib chemotherapy.
Case presentation
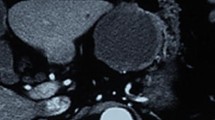
A 69-year-old woman who had no symptoms was found to have an intraperitoneal tumor on abdominal ultrasonography in a medical checkup. Thereafter, she was referred to our hospital for a further detailed examination. Laboratory examinations revealed no abnormal findings other than a slightly increase in the levels of serum potassium (Table 1). Contrast-enhanced computed tomography revealed a thin-walled cystic tumor with hypervascular solid masses in the cystic wall and intraperitoneal multiple nodules in contact with the peritoneum. The diameter of the tumor was found to be 8 cm. The cystic tumor was contiguous with the stomach wall (Fig. 1). On magnetic resonance imaging, the solid masses in the cystic lesion had low signal intensity on T1-weighted imaging, high signal intensity on T2-weighted imaging, high signal intensity on diffusion-weighted imaging, and low signal intensity on apparent diffusion coefficient imaging (Fig. 2). Endoscopic ultrasound (EUS) revealed that the mass was a thin-walled cyst with some solid masses and septum. Considering the malignant tumor with peritoneal dissemination, we decided to conduct EUS-FNA to confirm the pathological diagnosis. For solid mass of cystic lesions, EUS-FNA was performed transgastrically with a 25-gauge Franseen needle (Fig. 3). Since a nodule component was present directly under the scope, puncture could be performed without breaking the cyst. Pathologically, the cells in the tumor were spindle shaped, and hematoxylin and eosin staining revealed cells with atypical nucleus. Immunostaining revealed that the cells were positive for c-kit, CD34, and smooth muscle actin and negative for S-100 protein. Pathologically, the tumor was diagnosed as GIST. The Ki-67 labeling index was 21% (Fig. 4), and the mitotic count was 4/22 per high-power fields (HPFs). With the diagnosis of gastric GIST with peritoneal dissemination, imatinb administration was initiated 15 days after EUS-FNA. Imatinib (400 mg/day) was administered for 6 months, and the size of the tumor remained almost unchanged.
Contrast-enhanced computed tomography revealed a thin-walled cystic tumor with hypervascular solid masses in the cystic wall (arrow) and intraperitoneal multiple nodules (arrowhead), and the cystic tumor was contiguous with the stomach wall. The diameter of the tumor was 8 cm. a Axial image and b Coronal image
On magnetic resonance imaging, the solid mass (arrow) in the cystic lesion had low signal intensity on T1-weighted imaging (a), high signal intensity on T2-weighted imaging (c), high signal intensity on diffusion-weighted imaging (c), and low signal intensity on apparent diffusion coefficient imaging (d)
Histopathological findings of biopsy specimens. Cells in the tumor were spindle shaped with atypical nucleus (hematoxylin and eosin staining). c-kit was diffusely positive and CD34 was also diffusely positive upon immunostaining. Pathologically, the tumor was diagnosed with gastrointestinal stromal tumor. The Ki-67 labeling index was 21%. a Hematoxylin and eosin stain (×200), b c-kit stain (×200), c CD34 stain (×200), and d Ki-67 stain (×200)
Discussion
GISTs are the most common tumors of mesenchymal neoplasms and originate from the interstitial cells of Cajal [13, 14]. The interstitial cells of Cajal are morphologically characterized by a spindle- or stellate-shaped body. Histologically, GISTs vary from spindle-cell tumors to epithelioid and pleomorphic tumors, and 95% of GISTs express kit (CD117) and 70% express CD34 [15]. Most GISTs originate from the stomach (60%) followed by the small intestine (30%) and the colon (5%) [16].
Cystic components are macroscopically identified in less than 50% of GISTs [17]. However, gastric GISTs with predominant cystic formation are very rare. EUS has been reported to be a useful modality for diagnosing cystic components of GISTs [7]. Table 2 presents a summary of cases of gastric GIST with predominant cystic formation including our present case [2,3,4,5,6,7,8,9,10,11]. In previously reported cases that developed predominant cystic changes, many of them were cases involving large-sized GISTs. In some cases, they were misdiagnosed as cystic tumors derived from other organs. In our case, before EUS-FNA, as differential diagnosis of gastric submucosal tumor with cystic formation, neuroendocrine neoplasm, metastatic tumor and GIST were considered. And it was not possible to make a diagnosis only with image findings. Most cases were diagnosed by surgical resection, and there were no other reports of cases diagnosed with EUS-FNA, except our case. Many patients who underwent surgical resection received imatinib after surgery. If tumor necrosis contributes to cystic formation, the Ki-67 index or the mitotic index may be involved in the formation of cysts. In previous reports, there were a few cases where both the Ki-67 index and the mitotic index were listed and the values varied from case to case. The Ki-67 index of our case was the highest compared with previously reported cases. Cystic changes of GIST are considered to be formed by degeneration, necrosis, and bleeding [18]. If the tumor is highly proliferative, it seems to be easy to cause necrosis and form a cyst. However, since factors such as bleeding are also involved, it cannot be explained only by the proliferating ability.
GISTs with dominant cystic components are often difficult to diagnose only by radiological imaging [19], and EUS-FNA is a useful diagnostic tool. The size of the tissue obtained by EUS-FNA is usually small. Moreover, one study reported that EUS-FNA did not reliably reflect the proliferation of GIST, and alternative parameters should be validated for a pre-surgical prognostic classification [20]. The Fletcher’s or Miettinen’s GIST risk classifications require counting the number of mitosis at 50 HPFs [21, 22]. In the present case, 50 HPFs could not be secured because the obtained samples by EUS-FNA of 25-gauge needle were small. The EUS-FNA sample is often inadequate for the risk classification of GIST, and there is also a risk of leakage of cyst contents or needle tract seeding by EUS-FNA. However, the leakage of cyst contents can be technically prevented, and the occurrence of needle tract seeding is very rare. In addition, EUS-FNA is less invasive compared with surgical resection or laparoscopic biopsy. Thus, for cases with peritoneal dissemination, such as our present case, EUS-FNA is very useful for the early initiation of chemotherapy.
In conclusion, we reported a case of gastric GIST with predominant cystic formation diagnosed by EUS-FNA. For intraperitoneal cystic lesions that are continuous with the stomach, the possibility of gastric GIST needs to be considered. EUS-FNA is less invasive for patients compared with surgical resection or laparoscopic biopsy and allows for the early initiation of chemotherapy.
References
Misawa S, Takeda M, Sakamoto H, et al. Spontaneous rupture of a giant gastrointestinal stromal tumor of the jejunum: a case report and literature review. World J Surg Oncol. 2014;12:153.
Park J, Rubinas TC, Fordham LA, et al. Multifocal gastrointestinal stromal tumor (GIST) of the stomach in an 11-year-old girl. Pediatr Radiol. 2006;36:1212–4.
Osada T, Nagahara A, Kodani T, et al. Gastrointestinal stromal tumor of the stomach with a giant abscess penetrating the gastric lumen. World J Gastroenterol. 2007;13:2385–7.
Cruz RJ Jr, Vincenzi R, Ketzer BM, et al. Spontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (%3e 30 cm) in a young patient. World J Surg Oncol. 2008;6:76.
Yu CC, Wu CC, Hwang JI, et al. Thick calcification from a GIST of the stomach penetrating into pericolic soft tissue–report of a case. World J Surg Oncol. 2011;9:45.
Zhu CC, Liu Y, Zhao G. Exophytic gastrointestinal stromal tumor with cystic changes: a case report. Oncol Lett. 2014;7:1427–9.
Okano H, Tochio T, Suga D, et al. A case of a stomach gastrointestinal stromal tumor with extremely predominant cystic formation. Clin J Gastroenterol. 2015;8:197–201.
Hamza AM, Ayyash EH, Alzafiri R, et al. Gastrointestinal stromal tumour masquerading as a cyst in the lesser sac. BMJ Case Rep. 2016; 2016: bcr2016215479.
Sun KK, Xu S, Chen J, et al. Atypical presentation of a gastric stromal tumor masquerading as a giant intraabdominal cyst: a case report. Oncol Lett. 2016;12:3018–20.
Wang L, Liu L, Liu Z, et al. Giant gastrointestinal stromal tumor with predominantly cystic changes: a case report and literature review. World J Surg Oncol. 2017;15:220.
Okagawa Y, Sumiyoshi T, Ihara H, et al. Atypical presentation of a cushion sign-positive stomach gastrointestinal stromal tumor with cystic formation: a case report. Mol Clin Oncol. 2018;9:168–72.
Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304.
Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–83.
Nowain A, Bhakta H, Pais S, et al. Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol. 2005;20:818–24.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78.
Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016;122:3110–8.
Miettinen M, Furlong M, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121–33.
Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68.
Wongta K, Tangsirapat V, Chakrapan Na Ayudhya V, et al. A giant jejunal gastrointestinal stromal tumor misconceived as pancreatic cystic neoplasm: a case report. Int J Surg Case Rep. 2019;60:253–6.
Ricci R, Chiarello G, Attili F, et al. Endoscopic ultrasound-guided fine needle tissue acquisition biopsy samples do not allow a reliable proliferation assessment of gastrointestinal stromal tumours. Dig Liver Dis. 2015;47:291–5.
Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83.
Acknowledgements
The authors would like to thank all the staff involved in the treatments of the patient at Mito Saiseikai General Hospital. We would also like to thank Enago for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takahashi, K., Nihei, T., Aoki, Y. et al. Gastric gastrointestinal stromal tumor with predominant cystic formation diagnosed by endoscopic ultrasound-fine needle aspiration. Clin J Gastroenterol 13, 359–364 (2020). https://doi.org/10.1007/s12328-019-01058-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-01058-7