Abstract
Primary gastrointestinal follicular lymphoma is a rare disease. Follicular lymphoma does not cause any typical symptoms, although it usually shows the presence of multiple white granules on endoscopy. Few patients with follicular lymphoma present with the initial symptom of jaundice, which is usually associated with follicular lymphomas located in the papilla of Vater. Herein, we present the first case of a duodenal follicular lymphoma that presented with obstructive jaundice despite not being located in the ampulla, and it did not demonstrate the typical endoscopic findings of multiple white granules. A 72-year-old Japanese man with jaundice was referred to our hospital. Imaging revealed a hypovascular lesion extending into the second part of the duodenum and the pancreatic head, and the common bile duct was dilated upstream of the lesion. Biopsy of the lesion was negative for malignancy. Finally, we suspected the lesion as a pancreatic adenosquamous carcinoma, and not a typical pancreatic ductal carcinoma, because the lesion showed no pancreatic duct dilation and had a partially hyperechoic part within. Therefore, we performed pancreaticoduodenectomy. The final diagnosis was a duodenal follicular lymphoma. The findings of this case may assist in distinguishing between atypical follicular lymphoma and jaundice from pancreatic cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary gastrointestinal follicular lymphoma (FL) is a rare disease, accounting for only 3.6% of cases of gastrointestinal non-Hodgkin lymphoma [1]. No typical symptom is associated with FL, and many patients are incidentally diagnosed while being asymptomatic [2]. The typical endoscopic finding of FL is multiple white granules, observed in 67–100% of the cases [3]. Furthermore, FL rarely causes jaundice; therefore, it is difficult to determine any association between FL and jaundice. Herein, we present the first case of primary non-ampullary duodenal FL without multiple white granules but with obstructive jaundice.
Case report
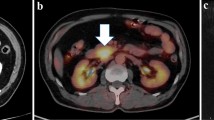
A 72-year-old Japanese man observed yellowing skin for 1 month and was referred to our hospital in September 2017. Obstructive jaundice and hepatic dysfunction were detected based on the following findings on blood tests: aspartate aminotransferase, 112 U/L; alanine aminotransferase, 118 U/L; alkaline phosphatase, 1060 U/L; γ-glutamyl transpeptidase, 284 U/L; total bilirubin, 7.5 mg/dL; and direct bilirubin, 6.1 mg/dL. The carbohydrate antigen 19-9 and carcinoembryonic antigen levels were within normal ranges, pancreatic cancer-associated antigen level exceeded 1600 U/mL, and s-pancreas-1 antigen level was as high as 35 U/mL. Abdominal contrast-enhanced computed tomography (CT) revealed a hypovascular lesion extending into the second part of the duodenum and the pancreatic head, and the common bile duct was dilated upstream of the lesion (Fig. 1). Magnetic resonance imaging revealed that the lesion had a low signal intensity on T1-weighted images, intermediate signal intensity on T2-weighted images, and high signal intensity on diffusion-weighted images (Fig. 2). Duodenoscopy revealed an elevated lesion with a reddish shallow depression at the center in the oral side of the papilla of Vater (Fig. 3). Biopsies of the lesion from the duodenum showed a high number of small lymphoid cells with normal duodenal mucosa; however, no findings indicated that the lesion was malignant. Endoscopic ultrasonography (EUS) showed that the lesion was a hypoechoic mass with clear margins and had a partially hyperechoic part within. Moreover, the lesion was located across the proper muscular layer of the duodenum and spread to the duodenum and the pancreatic head (Fig. 4). There was no pancreatic duct dilation. On endoscopic retrograde cholangiopancreatography, there was severe stenosis at the distal bile duct. Therefore, we performed biliary drainage with a plastic stent. Biopsies of the lesion from the stenosis in the bile duct were negative for malignancy, but the lesion demonstrated increased uptake of 18F-fluorodeoxyglucose on positron-emission tomography (PET)–CT, with a maximum standardized uptake value of 14.26. Abnormally increased uptake of 18F-fluorodeoxyglucose was not noted elsewhere. EUS-guided fine needle aspiration (EUS-FNA) with a standard 22-G needle was performed for the lesion, and we punctured the lesion three times, but the diagnosis revealed an atypical epithelium because there were only a collection of lymphocytes and a few atypical epithelial cells. We considered the lesion to be a pancreatic adenosquamous carcinoma, and not a typical pancreatic ductal carcinoma, because the lesion had no pancreatic duct dilation and a partially hyperechoic part within. Therefore, we performed pancreaticoduodenectomy. Pathological findings showed that a yellowish-white tumor was mainly located in the duodenum, with invasion to the pancreas and the common bile duct. On histological examination, tumor cells were mainly distributed in the submucosal layer and spread beyond the muscular layer. Moreover, many lymphoid follicular formations with centrocyte-like cells and centroblast-like cells were observed (Fig. 5). The elevated lesion that was detected on endoscopy mainly comprised tumor cells, but the mucosal layer of the duodenum remained and there were no tumor cells in the mucosal layer. On immunohistochemical staining, the tumor cells were positive for B cell lymphoma-2 and cluster of differentiation (CD) 10 and negative for CD3, CD5, and cyclin D1. Because the number of centroblast-like cells was more than 15 per high-powered field (40 ×) and centrocyte-like cells were also present, we diagnosed the tumor as a grade 3a FL. The patient was discharged 21 days after surgery. Because the patient had no remaining lesions on contrast-enhanced CT and PET–CT after surgery, adjuvant therapy was not performed. Moreover, the patient did not experience any recurrence for 14 months after surgery, and he has been followed up with contrast-enhanced CT and PET–CT performed by the hematologist.
a Computed tomography (CT) showed that the lesion extended to the second part of the duodenum and the pancreatic head (arrowhead), and dilation of the common bile duct was observed (arrow). b Contrast-enhanced CT showed that the lesion had few enhancements in the early phase. c The lesion showed enhancements in the delayed phase
Discussion
This case report demonstrates two interesting clinical issues. First, the typical endoscopic findings of FL such as multiple white granules are not always observed, even if the tumor protrudes into the duodenum. The relationship between multiple white granules and histological findings is not clear. In our experience, biopsies are usually obtained from the multiple white granules that are typically found in gastrointestinal FL. Therefore, we usually consider multiple white granules to be indicative of lymphoid follicles on the surface of the duodenal mucosa. In the current case, the pathological findings revealed that only a number of small lymphocytes increased on the surface of the duodenal mucosa, and that the follicular structure did not extend to the surface (Fig. 5). This explains the absence of the multiple white granules on endoscopy.
Second, primary FL of the duodenum can present with obstructive jaundice even when the tumor is not located in the papilla of Vater. Among patients with FL, 77% of patients are asymptomatic, but few patients have obstructive jaundice as the initial symptom [4]. A summary of the previous reports on primary FL of the duodenum that presented with obstructive jaundice is shown in Table 1. In these cases, obstructive jaundice appeared owing to the lesion on the papilla of Vater [5,6,7]. To the best of our knowledge, the current case is the first case of primary non-ampullary duodenal FL that invaded the common bile duct and presented with obstructive jaundice. The large size of the lesion could have been responsible for the obstructive jaundice. Most cases of FL grow so slowly that they are usually discovered as small lesions without any symptoms during medical checkups [8]. Therefore, large FL lesions are very rarely detected in developed countries where patients undergo regular medical checkups. In addition, the pathological grade affects the growth and invasion of FL. Only 5% of FL cases are of grade 3, which are more invasive than grade 1 and 2 cases [9]. The FL lesion in this case was large, as it was a pathological grade 3 tumor. In contrast, a population-based cohort study from Sweden showed that the prognosis of grade 3a FL was similar to that of grade 1 and grade 2 FL [10]. Thus, the patient reported herein probably had a good clinical course, as he did not have any other lesions except for the one in the resected duodenum.
As it was difficult to distinguish FL from pancreatic cancer in this case, we were unfortunately compelled to perform surgery. Diagnosis was particularly challenging as none of the biopsies, including EUS-FNA, demonstrated any evidence of FL. The diagnostic accuracy of endoscopic biopsy for gastrointestinal FL is as low as 18% [9]. Although EUS-FNA also has low diagnostic accuracy (47–68%), the combination of EUS-FNA and flow cytometry has improved the diagnostic accuracy to 84–89% [11, 12].
In conclusion, we describe a rare case of a duodenal grade 3a FL, along with obstructive jaundice despite not being located in the ampulla; the FL also did not demonstrate the typical endoscopic findings of multiple white granules. To diagnose such atypical FLs, we should consider lymphomas in the differential diagnosis and evaluate the tissue acquired via EUS-FNA with flow cytometry.
References
Yoshino T, Miyake K, Ichimura K, et al. Increased incidence of follicular lymphoma in the duodenum. Am J Surg Pathol. 2000;24:688–93.
Yamamoto S, Nakase H, Yamashita K, et al. Gastrointestinal follicular lymphoma: review of the literature. J Gastroenterol. 2010;45:370–88.
Iwamuro M, Kondo E, Takata K, et al. Diagnosis of follicular lymphoma of the gastrointestinal tract: a better initial diagnostic workup. World J Gastroenterol. 2016;22:1674–83.
Takata K, Okada H, Ohmiya N, et al. Primary gastrointestinal follicular lymphoma involving the duodenal second portion is a distinct entity: a multicenter, retrospective analysis in Japan. Cancer Sci. 2011;102:1532–6.
Misdraji J, Fernandez del Castillo C, Ferry JA. Follicle center lymphoma of the ampulla of Vater presenting with jaundice: report of a case. Am J Surg Pathol. 1997;21:484–8.
Athanasopoulos PG, Arkadopoulos N, Stafyla V, et al. A rare combination of an endocrine tumour of the common bile duct and a follicular lymphoma of the ampulla of Vater: a case report and review of the literature. World J Surg Oncol. 2011;9:4.
Suzuki S, Tanioka F, Inaba K, et al. A rare collision tumor composed of follicular lymphoma and adenocarcinoma in the ampulla of vater: a case report. Case Rep Pathol. 2014;2014:530727.
Vitolo U, Ferreri AJ, Montoto S. Follicular lymphomas. Crit Rev Oncol Hematol. 2008;66:248–61.
Iwamuro M, Okada H, Takata K, et al. Diagnostic accuracy of endoscopic biopsies for the diagnosis of gastrointestinal follicular lymphoma: a clinicopathologic study of 48 patients. Ann Diagn Pathol. 2014;18:99–103.
Wahlin BE, Yri OE, Kimby E, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156:225–33.
Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91.
Wang J, Chen Q, Wu X, et al. Role of endoscopic ultrasound-guided fine-needle aspiration in evaluating mediastinal and intra-abdominal lymphadenopathies of unknown origin. Oncol Lett. 2018;15:6991–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Junya Sato, Hirotoshi Ishiwatari, Ryo Ashida, Keiko Sasaki, Shinya Fujie, Junichi Kaneko, Tatsunori Satoh, Hiroyuki Matsubayashi, Yoshihiro Kishida, Masao Yoshida, Sayo Ito, Noboru Kawata, Kenichiro Imai, Naomi Kakushima, Kohei Takizawa, Kinichi Hotta, Katsuhiko Uesaka and Hiroyuki Ono declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patients in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, J., Ishiwatari, H., Ashida, R. et al. Primary non-ampullary duodenal follicular lymphoma presenting with obstructive jaundice. Clin J Gastroenterol 13, 214–218 (2020). https://doi.org/10.1007/s12328-019-01033-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-01033-2