Abstract
Esophageal intramural pseudodiverticulosis (EIPD) is an uncommon benign disorder leading to esophageal strictures. The etiology remains unknown; however, anti-fungal treatments or endoscopic balloon dilation can improve early esophageal strictures and these rarely require surgical treatment. We report a case of a 46-year-old male with a 6 cm-long esophageal stricture due to EIPD, which did not improve following treatment with an anti-fungal agent, eventually causing aspiration pneumonia. Therefore, we performed a thoraco-laparoscopic esophagectomy, and his symptoms were improved after surgery. This case suggests that a surgical treatment should be considered in patients with extensive, severe strictures attributable to EIPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal intramural pseudodiverticulosis (EIPD) is a rare benign esophageal disorder which was first described by Mendl et al. in 1960 [1]. EIPD is more common in males and is often associated with candidiasis, diabetes, chronic alcohol abuse, gastroesophageal reflux, esophageal neoplasia, Crohn’s disease, and esophageal motility disorders such as achalasia [2]. In spite of these associations, its etiopathogenesis remains unclear. The primary symptom is chronic progressive or intermittent dysphagia owing to stricture. EIPD is characterized by multiple, small, flask-shaped outpouchings in the wall of the esophagus. Treatment is often restricted to endoscopic balloon dilatation (EBD) and treatment of the underlying esophageal condition, such as acid suppression or fungal infection. Esophagectomy is rarely performed [3]. We herein report a case of EIPD, whose severe stenosis required esophageal resection, and review other cases of EIPD which were treated with surgical resection.
Case report
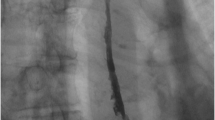
A 46-year-old male was referred to our hospital with a 3-month history of progressive dysphagia. The patient had no remarkable medical or family history and never underwent endoscopic examination. He was a heavy smoker (2 packs per day) and drank regularly for 26 years. Physical examination showed no abnormalities; however, laboratory data were significant for microscopic anemia (hemoglobin 9.4 g/dL). Esophagoscopy (GIF-H260Z, H290, and XP290N; Olympus Medical Systems Corp., Tokyo, Japan) revealed a narrow severe stricture with a submucosal tumor-like elevated lesion at the middle esophagus and multiple tiny orifices with white plaques on the proximal stenosis. We were unable to pass the endoscope into the distal lumen (Fig. 1a–c). Chromoendoscopy with indigo carmine dye improved detection of the orifices (Fig. 1d). No unstained areas were demonstrated by chromoendoscopy using iodine staining. Endoscopic ultrasonography (UM-3R, Olympus Medical Systems Corp., Tokyo, Japan) showed multiple hyperechoic spots with shadows and low echoic wall thickening (Fig. 1e, f). Biopsies from the orifices and stricture produced pathological evidence of inflammatory cell infiltration and Candida infection without malignancy. A gastrografin esophagogram revealed a smooth thoracic esophageal stricture (6 cm in length) with several fine flask-shaped outpouchings from the lumen (Fig. 2a). Computed tomography (CT) showed full circumferential thickening of the esophageal wall with intramural air (Fig. 2b). Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) showed intense FDG uptake of the thickening esophageal wall (Fig. 2c). Oral administration of anti-fungal medicine (amphotericin B 400 mg/day for 12 weeks) did not improve his symptoms or the stricture. He occasionally vomited, resulting in aspiration pneumonia. Nevertheless, he was very careful eating mushy food. Since his symptoms remained very severe and he would require long-term dilation using endoscopic balloons, the possibility of malignant stenosis could not be completely denied. We decided to perform a thoraco-laparoscopic esophagectomy.
Endoscopic images of the esophagus. a Severe stricture with a submucosal tumor-like elevated lesion observed at the middle esophagus. b, c On the oral side of the stenosis, there were multiple tiny orifices with white plaques, which were easily detected using indigo carmine dye (d). a, c, and d were captured using GIF-H260Z, b was captured using GIF-XP290N. Low echoic wall thickening (e) and hyperechoic spots with shadowing (f) at the lesion were observed by endoscopic ultrasonography through a soft balloon via the working channel of GIF-H290
Radiographic images of the esophagus. a Gastrografin esophagography revealed a smooth and long thoracic esophageal stricture (6 cm in length) with several fine flask-shaped lesion outpouchings. b CT showed full circumferential esophageal wall thickening with intramural air. c Intense FDG uptake of the thickening esophageal wall was observed by FDG PET/CT. The average standardized uptake value (SUV) was 8.31 and the maximal SUV was 10.20
Intraoperative findings did not indicate the presence of esophageal malignancy and the frozen sections did not show lymph node metastasis; no further lymph node dissection was performed. Pathological examination of the resected esophagus showed diffuse fibrotic thickening of the submucosa with inflammatory cell infiltration and granulation tissue (Fig. 3). The submucosa contained cystic structures, which were covered with squamous epithelium and were connected to the esophageal lumen as pseudodiverticula. There was no evidence of accompanying candidiasis or malignancy. We finally diagnosed the patient with esophageal stricture due to EIPD. One-year post surgery, the patient is free of symptoms and has not experienced disease recurrence.
Pathological examination of the resected esophagus. a, b Thoraco-laparoscopic esophagectomy was performed. c Diffuse fibrotic hypertrophy was seen in the esophageal wall, and d pseudodiverticula lined with stratified squamous epithelium extended into the submucosa. No evidence of Candida infection was observed by hematoxylin and eosin (H–E) staining
Discussion
The underlying etiology of EIPD remains unclear; however Candida albicans infection, which is frequently present in EIPD, is frequently implicated [4]. In our case, Candida was pathologically detected on the esophageal wall. The primary pathophysiological processes of EIPD were believed to be acquired inflammatory reactions, such as those against Candida. These reactions possibly interfered with the normal submucosal glands, leading to dilatation of the excretory ducts or fibrosis.
The most common symptom is dysphagia, often accompanied by esophageal stricture with acute or chronic mucosal inflammation. Because anti-fungal treatment or endoscopic balloon dilation can help some cases of early esophageal stricture secondary to EIPD, and surgical treatments are rare. Previously, only five cases of EIPD were reported where esophageal surgical resections were performed (Table 1) [5,6,7,8,9]. All patients were male, and the mean age was 52.2 years (range 35–61). The most frequent symptom was dysphagia and Candida infection was frequently present; however, complications relating to aspiration pneumonia were rare.
Thibodeau MP et al. [7] reported on a patient that underwent esophagectomy because treatment with antibiotics, an anti-fungal agent (fluconazole 200 mg iv once daily for 2 weeks), and acid suppressants could not improve his dysphagia symptoms. On the other hand, even in cases with severe esophageal stricture due to EIPD, anti-fungal medications plus EBD can succeed in relieving persistent dysphasia symptoms [4, 10]. In the present case, the diameter of the stricture was longer and more severe, and anti-fungal treatment did not improve his symptoms or stricture, eventually leading to aspiration pneumonia. Notably, we could not do EBD. In addition, candidiasis was not detected within the fibrous thickening of the inflamed resected esophageal wall. These observations suggested that Candida infection could be a trigger of EIPD, followed by irreversible fibrotic stenosis. We agree that anti-fungal medication should be considered as a first-line therapy against a stricture in patients with EIPD; however, esophagectomy should be considered for patients with advanced strictures likely to cause aspiration pneumonia.
There is a known association between EIPD and esophageal carcinoma [2]. Plavsic BM et al. mentioned that the prevalence of EIPD was significantly higher among patients with esophageal carcinoma, compared to patients who underwent esophagography for other indications [11]. In the present case, remarkable FDG uptake was detected in the thickened esophageal wall and we were unable to completely rule out malignant complications preoperatively. If we had been able to select conservative treatment for EIPD, we would have carefully monitored for esophageal carcinoma.
In conclusion, we describe a patient with severe and extensive esophageal stricture owing to EIPD, which ultimately required surgical treatment. Conservative treatments, including anti-fungal medication, did not relieve his symptoms and he soon developed aspiration pneumonia. We evaluated FDG uptake in a patient with EIPD, although esophageal neoplasia was not ultimately identified. FDG PET/CT may not be able to determine whether EIPD is accompanied by malignancy. EIPD is a benign esophageal disorder; however, esophagectomy should be seriously considered in cases with severe, long strictures.
References
Mendl K, McKay JM, Tanner CH. Intramural diverticulosis of the oesophagus and Rokitansky-Aschoff Sinuses in the gall-bladder. Br J Radiol. 1960;33:496–501.
Levine MS, Moolten DN, Herlinger H, et al. Esophageal intramural pseudodiverticulosis: a reevaluation. Am J Roentgenol. 1986;147:1165–70.
Tyberg A. A treatment option for esophageal intramural pseudodiverticulosis. ACG Case Rep J. 2014;1(3):134–6.
Chiba T, Iijima K, Koike T, et al. A case of severe esophageal intramural pseudodiverticulosis whose symptoms were ameliorated by oral administration of anti-fungal medicine. Case Rep Gastroenterol. 2012;6:103–10.
Guccion JG, Ortega LG. Trichomoniasis complicating esophageal intramural pseudodiverticulosis: diagnosis by transmission electron microscopy. Ultrastruct Pathol. 1996;20:101–7.
Murakami M, Tsuchiya K, Ichikawa H, et al. Esophageal intramural pseudodiverticulosis associated with esophageal perforation. J Gastroenterol. 2000;35:702–5.
Thibodeau MP, Brigand C, Ferraro P, et al. Esophagectomy for complications of esophageal intramural pseudodiverticulosis. Dis Esophagus. 2007;20:178–82.
Tsuboi J, Tajika M, Nakamura T, et al. Endoscopic features of short-term progression of esophageal intramural pseudodiverticulosis. Endoscopy. 2010;42:E92–3.
Liu SM, Wu HH, Chang KK, Tseng LJ, Han SC, Mo LR. Esophageal intramural pseudodiverticulosis complicated with stricture. J Formos Med Assoc. 2010;109:241–4.
Koyama S, Watanabe M, Iijima T. Esophageal intramural pseudodiverticulosis (diffuse type). J Gastroenterol. 2002;37:644–8.
Plavsic BM, Chen MY, Gelfand DW, et al. Intramural pseudodiverticulosis of the esophagus detected on barium esophagograms: increased prevalence in patients with esophageal carcinoma. Am J Roentgenol. 1995;165:1381–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest for this article.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Onozato, Y., Sasaki, Y., Abe, Y. et al. Esophageal intramural pseudodiverticulosis complicated with severe stricture requiring surgical resection. Clin J Gastroenterol 12, 292–295 (2019). https://doi.org/10.1007/s12328-019-00940-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-00940-8