Abstract
A 37-year-old woman was diagnosed by esophagogastroduodenoscopy (EGD) as having a 15-mm subepithelial lesion (SEL) in the gastric body. For 2 years, she experienced epigastric pain and anemia; she then underwent emergency EGD, which revealed a significant morphological change of the lesion. The SEL had a disintegrated tip and its submucosal portion was substantially exposed out of the mucosa, showing an “erect penis like appearance”. Based on the pathological findings of biopsied samples from the exposed portion and the endoscopic features, an inflammatory fibroid polyp (IFP) was suspected. This lesion was considered responsible for the anemia and was removed by endoscopic submucosal dissection (ESD). The pathological findings confirmed the lesion to be IFP. This report presents a case of gastric IFP that showed a marked morphological change and unique endoscopic features and was successfully removed by ESD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory fibroid polyp (IFP) of the stomach is a rare benign lesion which forms in the submucosa. Endoscopically, it appears as a pedunculated or subpedunculated lesion covered by intact mucosa [1], with its tip occasionally being depressed, erosive, ulcerative or covered by a white surface. Lesions with an erosive or ulcerative tip are referred to as having an “penis like appearance”, which is considered to be a characteristic feature of IFP [2], but it is observed in only 20–48.6% of all IFP cases [2, 3].
Pathological features of IFP include proliferation of fibrous tissue, aggregation of small blood vessels and infiltration of inflammatory cells. However, as the main part of the lesion is usually located in the submucosa, it is difficult to diagnose the lesion by forceps biopsy through the mucosal surface [4, 5].
We encountered a case of gastric IFP that showed gradual morphological changes, including disintegration of the tip and exposure of the mass out of the mucosa, and eventually exhibited an “erect penis like appearance”. The lesion was removed by endoscopic submucosal dissection (ESD) and pathologically the definitive diagnosis of IFP was confirmed. This report provides an overview of this case with discussion on the histological features.
Case report
A 37-year-old woman underwent esophagogastroduodenoscopy (EGD) and was diagnosed as having a subepithelial lesion (SEL) in the lower gastric body near the posterior wall of the lesser curvature (Fig. 1a). Endoscopic ultrasonography (EUS) with a 20-MHz thin probe visualized the lesion as a relatively homogeneous, hypoechoic mass measuring 15 mm localized in the submucosa. Although the most likely diagnosis was ectopic pancreas, the findings were not typical and gastrointestinal stromal tumor (GIST) arising from the muscular layer into the submucosa was also considered a differential diagnosis (Fig. 1b). We recommended EUS-guided fine-needle aspiration (EUS-FNA) for a histological diagnosis, but the patient refused to undergo the procedure. We therefore decided to follow-up the lesion with EGD. EGD performed 1 year later revealed no change in the size or shape of the lesion. Then, 1 year later, she experienced abrupt epigastric pain and presented to the emergency department of our hospital. Laboratory findings included a red blood cell count of 287 × 104/µL, hemoglobin 8.0 g/dL, hematocrit 24.3%, white blood cell count 6000/µL, serum albumin 3.2 g/dL, blood urea nitrogen 30.0 mg/dL, serum creatinine 0.64 mg/dL and C-reactive protein 0.03 mg/dL. She tested negative for serum anti-Helicobacter pylori (H. pylori) immunoglobin G antibody (< 3.0 U/mL). EGD was performed on the same day to explore the cause of epigastric pain and anemia.
Initial endoscopic findings. a A 15-mm subepithelial lesion was detected in the lower gastric body near the posterior wall of the lesser curvature. The mucosa appeared intact. b On endoscopic ultrasonography, the lesion was visualized as a relatively homogeneous, hypoechoic mass (measuring 15 mm) localized in the second (mucosal) and third (submucosal) layers
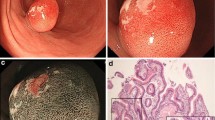
Endoscopically, the background mucosa showed regular arrangement of collecting venules [6] without diffuse redness or enlarged folds or mucus formation, findings consistent with H. pylori-naive mucosa. The basal, raised portion of the lesion in the submucosa had become enlarged to 20 mm in diameter, with marked exposure of the submucosal part of the lesion into the lumen. This exposed part of the lesion had a hard consistency with an “erect penis like appearance” (Fig. 2a). Magnifying endoscopy with narrow-band imaging showed no irregular microvascular pattern or microsurface pattern (Fig. 2b). EUS with an electronic radial scanning scope revealed the basal part to be a hypoechoic mass localized in the submucosa, with no change from the findings obtained 2 years earlier. The part of the lesion exposed into the lumen was as echogenic as the basal part (Fig. 2c). Pathological examination of biopsied samples from the exposed part revealed proliferation of fibroblasts and fibrous tissue (Fig. 2d). Based on these endoscopic and histological findings, IFP was suspected. Based on the judgment that this lesion was for the cause of anemia, we decided to perform ESD for the purpose of diagnosis as well as removal of the lesion.
Endoscopic findings after 2 years. a The basal part of the lesion had enlarged to 20 mm in diameter, the tip of the lesion was disintegrated, and the part previously located in the submucosa was exposed (35 mm in length). b On magnifying endoscopy with narrow-band imaging, the exposed part showed no microsurface pattern or irregular microvascular pattern. c On endoscopic ultrasonography, the lesion was visualized as a relatively homogeneous, hypoechoic mass localized in the second (mucosal) and third (submucosal) layers, as it was 2 years earlier. The exposed part was as echogenic as the basal part. d Pathological examination of biopsied samples from the exposed part (indicated by an arrow in a) revealed proliferation of fibroblasts and fibrous tissue
ESD was performed with a Dual Knife (KD-650L; Olympus Corp., Tokyo, Japan) and an IT Knife 2 (KD-611L; Olympus Corp., Tokyo, Japan) as knives, and a VIO300D (ERBE Elektromedizin GmbH, Tübingen, Germany) was used as the high-frequency generator. A 0.4% sodium hyaluronate (MucoUp; Johnson & Johnson K.K., Tokyo, Japan) was injected into the submucosa using a 26-G injection needle (ImpactFlow; TOP Corp., Tokyo, Japan). Despite the inflamed and thickened mucosa and severe fibrosis of the submucosa, the lesion was successfully removed en bloc. The resected specimen consisted of a 20 × 20-mm basal part and a 35-mm long exposed part (Fig. 3a, b). Histopathological findings included infiltration of eosinophils and other inflammatory cells and proliferation of vessels of varying sizes surrounded by concentric proliferation of fibrous tissue (i.e., onion skin-like appearance) (Fig. 4a, b). Immunohistochemically, the specimen was positive for vimentin and smooth muscle actin and negative for CD34, c-kit, desmin, caldenin and S-100. Based on these findings, the lesion was definitively diagnosed as gastric IFP. No postoperative recurrence of the lesion has been observed in 2-year follow-up period. Both anemia and epigastralgia, which were seen before the treatment, have improved.
Histopathological findings of the specimen obtained by endoscopic submucosal dissection. a Proliferation of fibroblasts and fibrous tissue and infiltration of eosinophils (arrows) were observed. b Proliferation of blood vessels of varying sizes surrounded by concentric proliferation of fibrous tissue (i.e., onion skin-like appearance; indicated by arrowheads) was observed
Discussion
Here, we present a case of IFP whose morphological changes were followed for over 2 years; it eventually showed an “erect penis like appearance”, which was reported for the first time here. In the present case, IFP was not initially considered as a differential diagnosis. It was not until a subsequent endoscopy, performed 2 years after the initial presentation, showed marked morphological changes of the lesion, including exposure of the submucosal part of the lesion, that we considered the possibility of IFP. Since the lesion was considered to be the cause of anemia, ESD was selected as a feasible treatment option, resulting in a definitive diagnosis of IFP.
IFP was first reported in 1949 by Vanek [7] in six cases of the so-called “gastric submucosal granuloma with eosinophilic infiltration”. Subsequently, the lesion was reported under different names, such as “an eosinophilic granuloma” and “an inflammatory pseudo-tumor” [8]. In 1953, Helwig and Ranier [9] proposed the term “inflammatory fibroid polyp (IFP)” and since then, the term has gained wide popularity. Most cases of IFP have occurred in the gastric antrum, with rare cases reported in the gastric body, esophagus, duodenum, small intestine, and colon [10,11,12,13,14]. The lesion in the present case was not located in the antrum and did not show the characteristic endoscopic features, resulting in a delayed diagnosis.
Although the pathogenesis of IFP remains unknown, factors suggested to be associated with IFP development include trauma, allergic reaction, bacterial infection and inflammatory reaction caused by drugs and other stimuli [15]. The involvement of H. pylori has also been suggested [16], although the present case was H. pylori-naive. Recent evidence also suggests the involvement of alterations of the platelet-derived growth factor receptor alpha gene [17], leading to the notion that IFP is a mesenchymal tumor.
IFP is endoscopically visualized as a pedunculated or subpedunculated SEL occasionally with redness or white covering its surface and an erosion or ulcer at its tip [2, 3, 18]. Lesions with an erosive or ulcerative tip are referred to as having an “penis-like appearance”, which is considered a characteristic feature of IFP. “Penis like appearance” is a SEL with slight ulcer on the surface of it. This is a finding similar to the so-called incomplete phimosis which seems to be able to a part of penis from the foreskin. On the other hand, in the present case, it was likely that an ulcer was formed at the tip of the lesion, making it appear like a penis head during the 2-year period. When examined endoscopically after 2 years, the tip of the lesion was disintegrated and the inner part was markedly exposed, resulting in an appearance more impressive than “penis-like appearance”. Therefore, we refer to this unique appearance as an “erect penis like appearance”.
Although there has already been a report of IFP whose gradual increase in size was followed over several years [19], no previous report of IFP has described marked exposure of the mass, as observed in the present case. A possible explanation for the gradual enlargement of IFP was that the inflammatory reaction was induced by certain stimulatory factors, such as food and peristalsis, which triggered an enlargement of the lesion at a certain point in time. This might have caused ischemia and subsequent necrosis of the surface, resulting in abrupt exposure of the submucosal part of the lesion.
On EUS, IFP is visualized as a relatively homogeneous, hypoechoic mass localized in the mucosa to submucosa and can be partially hypoechoic where fibrous tissue is abundant and hyperechoic where microvessels are abundant [20]. However, since these features are also observed in other types of SEL, it is difficult to diagnose IFP based only on EUS findings. In the present case, although in hindsight the initial EUS findings may have suggested IFP, we could not consider IFP as a differential diagnosis at that time.
Pathological features of IFP include proliferation of fibroblasts, fiber cells and connective tissue components, such as collagen fibers, infiltration of inflammatory cells, such as eosinophils, lymphocytes and plasma cells, proliferation of small blood vessels, such as arteriolae and capillaries, and concentric proliferation of fibrous connective tissue around small vessels (onion skin-like appearance). The onion skin-like appearance is reported to be observed in about 50% of all IFP cases [21], and was also observed in the present case. Immunohistochemically, CD34 positivity is considered a characteristic feature of IFP, but it is reported to be negative in 10–15% of cases [21, 22]. The present case was also negative for CD34. Since IFP is located deep in the mucosa, it is difficult to definitively diagnose the lesion by biopsy [4, 5]. Thus, endoscopic resection procedures, such as endoscopic mucosal resection (EMR) [4] and ESD [5, 19, 23], or surgery [24] are often required for a definitive diagnosis. Moreover, en bloc resection is preferred in view of the risks of recurrence [25] and concurrent cancer development [4]. Therefore, endoscopic resection procedures are increasingly used as a minimally invasive procedure replacing open surgery [5, 19, 23]. In this case, the root part seems to be present in a wide range in the submucosa. Furthermore, we thought that there was a possibility that there would be adhesion between the lesion and the muscular layer due to inflammation. In addition, there was concern that a thick artery was present in the lesion. Therefore, we thought EMR would have a possibility of residual tissue in the deep part of IFP, have a risk of perforation, and have a possibility of bleeding after resection. Due to the reasons, we choose ESD because it is more reliable and safer to perform en bloc resection than EMR. Actually, in the present case, the lesion could also be removed en bloc by ESD. H. pylori eradication has also been associated with reduction of gastric IFP [16], although the present case was H. pylori-naive.
In conclusion, in the present case, although the diagnosis of IFP could not be obtained initially, the lesion underwent marked morphological changes over 2 years to present an “erect penis like appearance” and was eventually confirmed to be IFP by diagnostic and therapeutic ESD.
References
Johnstone JM, Morson BC. Inflammatory fibroid polyp of the gastrointestinal tract. Histopathology. 1978;2:349–61.
Chonan A, Mochizuki F, Ikeda T, et al. Nine cases of gastric inflammatory fibroid polyp (IFP) which were endoscopically resected. Gastroenterol Endosc. 1988;30:1504–10.
Klingbeil KD, Balaban A, Fertig RM, et al. Inflammatory fibroid polyp of the gastric antrum presenting as hypovolemic shock: case report and literature review. Intractable Rare Dis Res. 2017;6:304–9.
Hirasaki S, Endo H, Nishina T, et al. Gastric cancer concomitant with inflammatory fibroid polyp treated with endoscopic mucosal resection using an insulation-tip diathermic knife. Intern Med. 2003;42:259–62.
Hirasaki S, Tanimizu M, Tsubouchi E, et al. Gastritis cystica polyposa concomitant with gastric inflammatory fibroid polyp occurring in an unoperated stomach. Intern Med. 2005;44:46–9.
Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39–45.
Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949;25:397–411.
Kuestermann SA, Saleeb SF, Teplick SK. General case of the day. Jejunal intussusception caused by an inflammatory fibroid polyp (IFP). Radiographics. 1999;19:539–41.
Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953;96:335–67.
Bosch O, González Campos C, Jurado A, et al. Esophageal inflammatory fibroid polyp. Endoscopic and radiologic features. Dig Dis Sci. 1994;39:2561–6.
Wysocki AP, Taylor G, Windsor JA. Inflammatory fibroid polyps of the duodenum: a review of the literature. Dig Surg. 2007;24:162–8.
Shimer GR, Helwig EB. Inflammatory fibroid polyp of the intestine. Am J Clin Pathol. 1984;81:708–14.
Hirasaki S, Matsubara M, Ikeda F, et al. Inflammatory fibroid polyp occurring in the transverse colon diagnosed by endoscopic biopsy. World J Gastroenterol. 2007;13:3765–6.
Shimura T, Kataoka H, Sasaki M, et al. Rectal inflammatory fibroid polyp resected with endoscopic submucosal dissection. Intern Med. 2008;47:2029–31.
Abboud B. Vanek’s tumor of the small bowel in adults. World J Gastroenterol. 2015;21:4802–8.
Nishiyama Y, Koyama S, Andoh A, et al. Gastric inflammatory fibroid polyp treated with Helicobacter pylori eradication therapy. Intern Med. 2003;42:263–7.
Schildhaus HU, Cavlar T, Binot E, et al. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008;216:176–82.
Matsushita M, Okazaki K. Characteristic endoscopic features of gastric inflammatory fibroid polyps. J Gastroenterol Hepatol. 2005;20:1310.
Tanaka K, Sakuno T, Yamada R, et al. Gastrointestinal: Gastric inflammatory fibroid polyp that was resected after a 10-year follow-up. J Gastroenterol Hepatol. 2018. https://doi.org/10.1111/jgh.13913.
Matsushita M, Hajiro K, Okazaki K, et al. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997;46:53–7.
Liu TC, Lin MT, Montgomery EA, et al. Inflammatory fibroid polyps of the gastrointestinal tract: Spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol. 2013;37:586–92.
Bjerkehagen B, Aaberg K, Steigen SE. Do not be fooled by fancy mutations: inflammatory fibroid polyps can harbor mutations similar to those found in GIST. Case Rep Med. 2013. https://doi.org/10.1155/2013/845801.
Hattori Y, Kobayashi S, Takahashi H, et al. Gastric inflammatory fibroid polyp treated by endoscopic submucosal dissection. Case Rep Gastroenterol. 2008. https://doi.org/10.1159/000151330.
Zhang C, Cui M, Xing J, et al. Massive gastrointestinal bleeding caused by a giant gastric inflammatory fibroid polyp: a case report. Int J Surg Case Rep. 2014;5:571–3.
Zinkiewicz K, Zgodziński W, Dabrowski A, et al. Recurrent inflammatory fibroid polyp of cardia: a case report. World J Gastroenterol. 2004;10:767–8.
Acknowledgements
We wish to express our deep appreciation to all endoscopy medical staffs for their assistance with endoscopic procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest for this article.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from this patient for being included in the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Watahiki, Y., Hikichi, T., Watanabe, K. et al. A case of inflammatory fibroid polyp of the stomach with an “erect penis like appearance” successfully removed by endoscopic submucosal dissection. Clin J Gastroenterol 12, 279–284 (2019). https://doi.org/10.1007/s12328-019-00935-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-00935-5