Abstract
Neuroendocrine carcinoma (NEC) of the pancreas is very rare, and its origin is not fully elucidated. Here, we present a case of a small-size NEC of the pancreas that is genetically similar to invasive ductal adenocarcinoma (IDA). A 65-year-old man was referred to our hospital due to obstructive jaundice and found to have a 12-mm solid tumor in the pancreas head. The tumor exhibited low vascularity on enhanced computed tomography, and endoscopic retrograde pancreatographic imaging revealed an irregular obstruction in a branch duct of the pancreas. The patient was thereby diagnosed with a pancreatic ductal cancer, and stomach-preserving pancreaticoduodenectomy with regional lymph node resection was performed. Histochemical analysis of the resected tumor showed that the neoplastic cells with scanty cytoplasm and hyperchromatic nuclei strongly expressed chromogranin A and synaptophysin. The Ki-67 index was 40 % in the most proliferative tumor regions, and the tumor was diagnosed as a NEC of the pancreas. However, in the analysis of genetic alterations of the tumor tissue, the neoplastic cells showed altered KRAS, TP53, and SMAD4/DPC4, suggesting that the NEC in our case is genetically related to IDA. Our data suggest that poorly differentiated IDAs may transform into NECs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasms of the pancreas are uncommon, accounting for 1–2 % of all clinically apparent pancreatic neoplasms [1]. In the WHO classification, neuroendocrine neoplasms are classified into well-differentiated (low-to-intermediate grade) neuroendocrine tumors (PanNETs) and poorly differentiated (high grade) neuroendocrine carcinomas (NECs) on the basis of their tumor proliferative rate [1]. Well-differentiated PanNETs are relatively indolent, whereas NECs are highly aggressive; the majority of pancreatic NECs have been already fairly large and metastasized to several distant organs when they are diagnosed [2]. Moreover, due to the rarity of this disease, the literature on poorly differentiated NECs that developed primarily in the pancreas is very limited. Therefore, the origin of poorly differentiated NECs and their genetic characteristics are still uncertain. In this report, we describe a rare case of a small-size NEC of the pancreas harboring molecular alterations similar to invasive ductal adenocarcinoma (IDA).
Case report
A 65-year-old man was initially admitted to a nearby hospital with a history of anorexia and jaundice. An abdominal computed tomography (CT) scan at the hospital disclosed a dilatation of the intrahepatic and common bile duct as well as lower bile duct obstruction. Because of the high serum bilirubin concentration (6.5 mg/dL), he immediately received a percutaneous transhepatic biliary drainage and was referred to our hospital for further investigation. Physical examination showed no specific palpable tumor on his abdomen, but a slight tenderness was present on the right upper abdomen. His laboratory data upon admittance to our hospital were as follows: hemoglobin, 15.2 g/dL; white blood cell count, 8900/mm3; platelets, 446,000/mm3; aspartate aminotransferase, 75 U/L; alanine aminotransferase, 136 U/L; total bilirubin, 3.4 mg/dL; direct bilirubin, 1.8 mg/dL; lactate dehydrogenase, 187 U/L; alkaline phosphatase, 439U/L; and gamma-glutamyl transpeptidase, 183 U/L. Serum DUPAN-2 was elevated to 230 U/ml (normal range < 150 U/ml), but other tumor markers, including CEA, CA19-9, and CA125 were within the normal range. A dynamic CT scan of the abdomen revealed a solid lesion of 12 mm with low vascularity in the head of the pancreas. The tumor appeared to be poorly enhanced compared with the surrounding pancreatic parenchyma in the early phase (Fig. 1a) and was gradually enhanced with delayed image (Fig. 1b, c). Subsequently, endoscopic retrograde pancreatography (ERP) was carried out to evaluate the shape of the pancreatic duct, and to obtain pancreatic juice for a cytological examination. ERP imaging revealed no irregular narrowing of the main pancreatic duct; however, when contrast dye was injected into branches of the pancreatic duct, there was an irregular obstruction in a branch duct of the pancreas head (Fig. 1d). The obtained pancreatic juice did not contain any carcinoma cells. Magnetic resonance imaging revealed a 12-mm irregular mass in the head of pancreas with low signal intensity on a T1-weighted image (Fig. 1e); however, no specific signal was detected on T2-weighed images (Fig. 1f). 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) showed no abnormal accumulation at the pancreas head or any other lesion.
Findings of dynamic computed tomography showing the tumor nodule in the pancreas head (yellow arrow head). The tumor was enhanced poorly compared with the surrounding pancreatic parenchyma in the arterial phase (a) and portal phase (b), and was gradually enhanced with delayed imaging (c). d Endoscopic retrograde pancreatographic imaging showed irregular obstruction in a branch duct of the pancreas head (yellow arrows). e Magnetic resonance imaging revealed a 12-mm irregular mass in the head of pancreas with low signal intensity on a T1-weighted image (yellow arrow head). f No specific signal was detected on T2-weighed images
On the basis of these results, the patient was diagnosed with pancreatic IDA, and stomach-preserving pancreaticoduodenectomy with regional lymph node (LN) resection was performed. Contrary to our preoperative diagnosis, histological examination of the resected pancreas showed an NEC with a micro LN metastasis. Three months after the surgery, multiple recurrences in the liver were detected, and neuron-specific enolase (NSE) was elevated to 68 ng/mL (normal range < 12.0 ng/mL). The patient underwent chemotherapy; however, there was progression of the hepatic tumors and metastatic LNs appeared. The patient’s performance status gradually worsened and he was transferred to another hospital for palliative care 8 months after surgery.
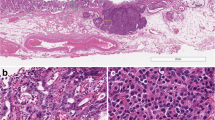
The resected pancreatic specimen showed a solid, whitish, 15-mm tumor (Fig. 2a). Histological examination of samples stained with hematoxylin-eosin (HE) showed that the tumor was composed of extensive desmoplastic stroma and neoplastic cells with high-grade cytological atypia and hyperchromatic nuclei (Fig. 2b, c). Immunohistochemical examination revealed that the neoplastic cells strongly expressed chromogranin A and synaptophysin (Fig. 2 d, e). The Ki-67 index was 40 % in the most proliferative tumor regions (Fig. 2f). The tumor cells did not express serotonin, insulin, glucagon, or trypsin (data not shown).
Pathology of the resected pancreatic specimen. a Resected pancreatic specimen showing a solid, whitish, 15-mm tumor. b Loupe view of the resected specimen stained with hematoxylin eosin (HE) showing that the tumor with marked desmoplastic stroma infiltrates the surrounding parenchyma in a haphazard pattern (yellow arrow heads). c HE staining showing that tumor cells with coarse chromatin and well-visible nucleoli with nested proliferation. Chromogranin A (d) and synaptophysin (e) immunostaining were strongly positive in the neuroendocrine carcinoma cells. f Ki-67 immunostaining was detected in 40 % of the neuroendocrine carcinoma cells
To investigate genetic characteristics of the tumor cells, we analyzed alteration in KRAS, CDKN2A/p16, TP53, SMAD4/DPC4, and Bcl-2 by immunohistochemistry or targeted exomic sequencing. An activating KRAS gene mutation (G12D) was identified by direct sequencing (Fig. 3a). Abnormal diffusely positive nuclear labeling for p53 was detected (Fig. 3b), whereas normal immunolabeling for p16 was observed in the neoplastic cells (Fig. 3c). Immunolabeling for Smad4/Dpc4, which serves as a robust marker of the SMAD4/DPC4 genetic status, revealed that Smad4/Dpc4 expression was lost in the tumor specimen, indicating that SMAD4/DPC4 was inactivated in the neoplastic cells (Fig. 3d). Overexpression of Bcl-2 protein, which is reported to be very frequently found in NEC [3], was not observed in the tumor cells (Fig. 3e).
Genetic features of the tumor cells. a Direct sequencing of PCR product from tumor genomic DNA revealed an activating KRAS mutation (G12D) in the tumor cells. b Abnormal diffusely positive immunolabeling for p53 was detected in tumor nucleus. c Normal immunopositivity was observed in the neoplastic cells. d Lack of Smad4/Dpc4 immunolabeling was observed in the neoplastic cells. e Overexpression of Bcl-2 protein was not observed in the tumor cells
Discussion
Poorly differentiated NECs of the pancreas are uncommon and their clinical course is highly aggressive. Therefore, it is very rare to encounter an NEC of the pancreas in the early stage. A search of the literature from 1984 onward revealed no cases of pancreatic NEC with tumors <2 cm. To our knowledge, this report describes the clinicopathological features of the smallest NEC primarily developed in the pancreas. Typical neuroendocrine neoplasms are often detected as hypervascular tumors. However, in our case the tumor showed low vascularity on an enhanced CT scan and caused an obstruction of the pancreatic branch duct during ERP imaging, mimicking imaging findings of IDA. The neoplastic cells were pathologically accompanied by marked stromal fibrosis in the tumor area, which infiltrated the surrounding pancreatic parenchyma and invaded the common bile duct. These histological features, which are also similar to those of IDA, might have contributed to the radiological findings.
A previous study reported that poorly differentiated NECs were less vascularized than well differentiated NEC [4]. The tumor in our case, which showed high-grade cytological atypia, apparent pleomorphism, and prominent mitotic activity, was diagnosed into poorly differentiated NEC. This poor differentiation of the tumor is also possibly associated with low vascularity of the tumor. The WHO 2010 classification divides NECs into two subtypes, small cell NEC (SCNEC) and large cell NEC (LCNEC). In our case, the tumor showed relatively large nuclei, coarse chromatin, and easily visible nucleoli with nested proliferation, and was categorized as LCNEC. In terms of proliferative activity, Ki-67 index was 40 % at the “hot spot” in our case. Although the morphology of the tumor tissue was compatible to poorly differentiated NEC, 40 % of the Ki-67 index in poorly differentiated NECs was relatively low. Some studies reported that the majority of NECs with lower Ki-67 index (20–55 %) did not respond to platinum-based chemotherapy [5, 6]. Therefore, the patient received everolimus, not a platinum-based regimen as a systemic chemotherapy. However, unfortunately, the tumor did not respond to the therapy.
Shi and colleagues reported that serotonin-producing NETs were frequently accompanied by prominent stromal fibrosis with pancreatic duct obstruction [7, 8], and they called these tumors sclerosing panNETs [9]. Moreover, it is known that some insulinomas contain abundant fibrous tissue [10, 11]. Therefore, we performed immunohistochemical analyses for serotonin and insulin in the tumor tissue, but the tumor showed no immunopositivity for either, suggesting that the present case was not derived from these specific hormone-producing subtypes.
To assess the reason why the tumor in our case showed abundant stromal fibrosis and severe invasive pattern despite being diagnosed as an NEC, we investigated the molecular background of the tumor, analyzing genetic alterations of some oncogenes and tumor suppressor genes that characterize NECs or IDAs. Mutated KRAS, TP53, CDKN2A/p16, and SMAD4/DPC4 are well-known driver mutations frequently identified in IDAs [12, 13], whereas Yachida and his colleagues reported that KRAS mutation and loss of SMAD4/DPC4 were very rare genetic alterations in poorly-differentiated NECs [3]. However, in terms of KRAS gene alteration, there are some controversies because another report suggests that KRAS mutation was frequently found in poorly differentiated NEC of the pancreas [6]. In our case, a mutation in KRAS and altered expressions of TP53, and SMAD4/DPC4 were found in the tumor tissue. On the other hand, Bcl-2 overexpression, which is frequently observed in NEC [3], was not found in this case. These genetic findings suggest that the tumor in our case is genetically similar to an IDA, and might contribute to the clinicopathological findings of the tumor, such as its severe invasive pattern, and extensive desmoplastic stroma.
Although there are some hypotheses concerning the origin of pancreatic NEC, it remains controversial. While dedifferentiation of well-differentiated PanNET has been proposed, the derivation from IDA with neuroendocrine differentiation has also been suggested [14]. In contrast, a recent study suggested that many poorly differentiated NECs are genetically distinct from well-differentiated PanNETs or IDAs [3]. In the pancreatic NEC of our case, genetic alterations in the tumor were very similar to those in IDA. These findings raise the possibility that the tumor in our very rare case of a small-size, early-stage NEC of the pancreas was derived from pancreatic ductal precursors and tumor clones of IDA transformed into a neuroendocrine lineage.
In conclusion, we reported a very rare case of small-size NEC of the pancreas. Our data suggest that poorly differentiated IDA may transform into NEC and provide suggestive information regarding the origin of primary pancreatic NEC cells.
References
Bosman FT, Carneiro F, Hruban RH, et al. World Health Organization (WHO) classification of tumours of the digestive system. Geneva: WHO; 2010.
Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–47.
Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84.
Rodallec M, Vilgrain V, Couvelard A, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85.
Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60.
Hijioka S, Hosoda W, Mizuno N, et al. Does the WHO 2010 classification of pancreatic neuroendocrine neoplasms accurately characterize pancreatic neuroendocrine carcinomas? J Gastroenterol. 2015;50:564–72.
Shi C, Siegelman SS, Kawamoto S, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010;257:107–14.
Kawamoto S, Shi C, Hruban RH, et al. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. AJR Am J Roentgenol. 2011;197:W482–8.
Johnson A, Wright JP, Zhao Z, et al. Cadherin 17 is frequently expressed by ‘sclerosing variant’ pancreatic neuroendocrine tumour. Histopathology. 2015;66:225–33.
Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163–71.
Anaye A, Mathieu A, Closset J, et al. Successful preoperative localization of a small pancreatic insulinoma by diffusion-weighted MRI. JOP. 2009;10:528–31.
Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72.
Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253–60.
Motojima K, Furui J, Terada M, et al. Small cell carcinoma of the pancreas and biliary tract. J Surg Oncol. 1990;45:164–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tetsuo Kimura, Hiroshi Miyamoto, Akira Fukuya, Shinji Kitamura, Koichi Okamoto, Masako Kimura, Naoki Muguruma, Tetsuya Ikemoto, Mitsuo Shimada, Akiko Yoneda, Yoshimi Bando, Makoto Takishita, and Tetsuji Takayama declare that they have no conflict of interest.
Human Rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Kimura, T., Miyamoto, H., Fukuya, A. et al. Neuroendocrine carcinoma of the pancreas with similar genetic alterations to invasive ductal adenocarcinoma. Clin J Gastroenterol 9, 261–265 (2016). https://doi.org/10.1007/s12328-016-0655-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-016-0655-6