Abstract
Introduction
The substantial economic burden of acute myeloid leukemia (AML) could be reduced with post-remission maintenance therapies that delay relapse. Real-world healthcare resource utilization (HCRU) data and costs among patients with AML receiving oral azacitidine (Oral-AZA) maintenance therapy or no maintenance are not well understood. We characterize HCRU and costs among these patients in clinical practice in the USA.
Methods
Data from IQVIA PharMetrics® Plus (January 1, 2016–June 30, 2022) were used. Patients ≥ 18 years who were newly diagnosed with AML, received first-line systemic induction therapy, and attained disease remission were eligible. Patients receiving Oral-AZA maintenance and those receiving no maintenance (“watch and wait” [W&W]) were matched 1:3 on baseline characteristics using propensity score matching (PSM) and followed until hematopoietic stem cell transplantation or end of continuous insurance enrollment, whichever occurred first. Outcomes included treatment patterns, inpatient and outpatient visits, and costs.
Results
After PSM, the Oral-AZA cohort included 43 patients and the W&W cohort 129. Of the 43 patients receiving Oral-AZA, 88.4% started at the recommended dose of 300 mg and 11.6% at 200 mg. The Oral-AZA cohort had significantly (p = 0.0025) longer median (95% CI) time to relapse from the index maintenance date (median not reached [NR; 9.0 months–NR] vs 3.3 months [0.8 months–NR]), and fewer per person per month (PPPM) hospitalizations (0.23 vs 0.61; p = 0.0005) and overall outpatient visits (5.77 vs 7.58; p = 0.0391) than the W&W cohort. Despite higher AML drug costs PPPM in the Oral-AZA cohort ($16,401 vs $10,651 for W&W), total healthcare costs PPPM were lower ($25,786 vs $38,530 for W&W; p < 0.0001).
Conclusions
Patients with newly diagnosed AML treated with Oral-AZA maintenance in clinical practice had prolonged remission and lower HCRU and costs than patients receiving no maintenance therapy. These findings underscore the clinical and economic value of Oral-AZA in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
High relapse rates greatly contribute to the substantial economic burden of acute myeloid leukemia (AML). Post-remission maintenance therapies may reduce this burden among patients with AML by delaying relapse; however, real-world healthcare resource utilization (HCRU) and cost data among patients with newly diagnosed AML receiving oral azacitidine (Oral-AZA) maintenance therapy compared with patients without active maintenance therapy in the post-remission period prior to AML relapse/recurrence has not been evaluated. |
This study uses data from the IQVIA PharMetrics® Plus database to compare HCRU and costs in matched cohorts of patients with newly diagnosed AML in remission treated with Oral-AZA maintenance and those who did not receive maintenance therapy (“watch and wait” [W&W]), in clinical practice in the USA. |
What was learned from the study? |
Patients with newly diagnosed AML treated with Oral-AZA maintenance had prolonged remission and lower HCRU and costs than patients receiving no maintenance therapy. |
These findings are in line with the results from the pivotal QUAZAR AML-001 trial, underscoring the clinical and economic benefits of Oral-AZA maintenance therapy versus W&W in clinical practice. |
Introduction
Acute myeloid leukemia (AML) is a heterogeneous malignancy characterized by abnormal proliferation of myeloid progenitors in the bone marrow caused by genetic mutations and cytogenetic aberrations, and is associated with infection, anemia, and bleeding [1,2,3]. AML is the most common form of acute leukemia in adults, with an estimated 20,380 new cases in the USA in 2023 [2,3,4]. Treatment options and outcomes for AML depend on disease subtype, patient age, and overall patient health [5, 6]. The cornerstone of frontline treatment for AML has historically been intensive induction chemotherapy (IC) and consolidation chemotherapy for eligible patients [7, 8], though new combination regimens have been approved for newly diagnosed IC-eligible patients in recent years [9,10,11]. For patients who achieve remission, IC is usually followed by consolidation therapy, with the aim of maximizing the depth and duration of response [12, 13]. While allogeneic hematopoietic stem cell transplantation (HSCT) is a potential curative option in AML [12, 13], many patients are not able to receive HSCT because of advanced age, lack of donor, comorbidities, personal preference, and/or limited access [12,13,14]. Despite treatment advances [15] and the relatively high proportion of patients achieving complete remission (CR) with IC (up to 70% of patients < 60 years of age and 50% of those ≥ 60 years of age), clinical outcomes overall remain poor [16]. Relapse rates can be as high as 70–80%, particularly in older patients with adverse risk factors [16], underscoring the persistent need for new treatment approaches. Research efforts have focused on safe and effective lower-intensity maintenance therapies that can reduce the risk of relapse and prolong survival for patients in remission [17].
Oral azacitidine (Oral-AZA) is the first therapy approved by the US Food and Drug Administration (FDA) for continued treatment of adults with AML who achieve first CR or CR with incomplete recovery (CRi) following IC and are unable to complete intensive curative therapy (e.g., HSCT) [18, 19]. Oral-AZA is a cytosine analog with DNA hypomethylating activity leading to antitumor effects encompassing cell cycle arrest, DNA repair deficiency, and re-expression of silenced genes, including tumor suppressor genes and cellular differentiation genes [17, 19, 20]. FDA approval of Oral-AZA was based on safety and efficacy data from the randomized, double-blind, placebo-controlled phase 3 QUAZAR AML-001 study (NCT01757535) of Oral-AZA maintenance therapy in patients ≥ 55 years of age with AML in first CR or CRi after IC who were ineligible for HSCT [21, 22]. At a median follow-up of 41.2 months, median overall survival and relapse-free survival (RFS) were significantly longer with Oral-AZA compared with placebo (24.7 months vs 14.8 months, and 10.2 months vs 4.8 months, respectively; p < 0.001 for each comparison) [22].
The economic burden of AML is substantial [23,24,25,26,27,28], with inpatient hospitalization and disease relapse contributing to increased healthcare resource utilization (HCRU) and costs [25]. A real-world US study reported patients with newly diagnosed AML who received intensive IC (and no HSCT) to have a median total hospitalization cost of $86,584 per patient in the USA [24]. Prolonged remission has been shown to reduce the economic burden of AML, with mean healthcare costs decreasing from $71,823 to $15,615 per person per month (PPPM) for remission durations of < 3 months and ≥ 12 months, respectively [29]. Since the approval of Oral-AZA, the HCRU and costs among patients with newly diagnosed AML who receive Oral-AZA maintenance compared with patients who receive no maintenance therapy (“watch and wait” [W&W]) in US clinical practice have not been well characterized. This study evaluated HCRU and costs between patients with newly diagnosed AML in remission who received Oral-AZA maintenance therapy compared with matched patients who were eligible for Oral-AZA but did not receive maintenance therapy (W&W) in US clinical practice.
Methods
Data Source
This retrospective, observational cohort study utilized data from the IQVIA PharMetrics® Plus database (January 1, 2016–June 30, 2022) of adjudicated medical and pharmacy claims. The PharMetrics® Plus database is a longitudinal health plan database that includes primarily commercial health plans and contains de-identified Health Insurance Portability and Accountability Act (HIPAA)-compliant data related to inpatient and outpatient services, prescription and office/outpatient administered drugs, and costs, as well as enrollment information for more than 210 million enrollees.
Study Design
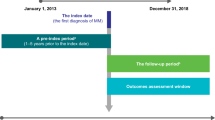
A schematic representation of the study design is shown in Fig. 1. The index diagnosis date was defined as the date of the first claim with an AML diagnosis. The remission date was defined as the date of the first claim with a code for remission in patients who received induction therapy. Index maintenance date was date of remission (for those that did not receive consolidation treatment) or end of consolidation following remission (for those that received consolidation treatment). Patients were followed until HSCT date, or the end of continuous insurance enrollment (due to termination of insurance coverage or death), whichever occurred first. Continuous enrollment in the health insurance plan ensured continuity of an individual patient’s available information in the database, as defined in the Study Sample subsection. The study period was from June 1, 2020 to June 30, 2022.
Study design. Eligible patients were diagnosed with AML, received systemic induction therapy, and attained disease remission. Patients may or may not have received consolidation treatment, defined as taking a regimen involving cytarabine, after remission. The index maintenance date was the date of attaining remission, unless the patient received a consolidation treatment. In such cases, the date following the end of the consolidation treatment was considered the index maintenance date. Treatments received after consolidation and prior to relapse were considered maintenance therapy. Patients were included in the Oral-AZA cohort or in the W&W cohort based on whether they received maintenance therapy with Oral-AZA or received no maintenance therapy, respectively. Patients were followed until HSCT date or the end of continuous insurance enrollment, whichever occurred first. AML acute myeloid leukemia, HSCT hematopoietic stem cell transplantation, Oral-AZA oral azacitidine, W&W watch and wait
Study Sample
Eligible patients were ≥ 18 years of age at index diagnosis date with ≥ 1 inpatient claim or ≥ 2 outpatient claims on different dates within a period of 90 days, with a diagnosis of AML (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] C92.0X, C92.6X, C92.AX), a first-line systemic induction therapy claim on or after the index diagnosis date, and evidence of disease remission (ICD-10-CM C92.01, C92.61, C92.A1) on or after September 1, 2020, and after the start of and within 180 days of induction therapy. Additionally, patients were required to have continuous enrollment in the health insurance plan for ≥ 90 days prior to the index diagnosis date and continuous enrollment with pharmacy benefits (covering reimbursement of prescribed medications) from the index diagnosis date to at least the remission date. Patients could have received consolidation treatment, defined as a cytarabine-containing regimen, although this was not a requirement.
Patients were excluded if they had a diagnosis of acute promyelocytic leukemia (ICD-10-CM C92.4X) or any primary proliferative disorders or malignancies (excluding myelofibrosis, myelodysplastic syndromes, myeloproliferative disorders/neoplasms, chronic myelomonocytic leukemia, and non-melanoma skin cancers); received treatment with arsenic trioxide or all-trans-retinoic acid at any time in the available patient follow-up; received systemic induction therapy for AML or had AML remission (ICD-10-CM C92.01, C92.61, C92.A1) or AML relapse claims (ICD-10-CM C92.02, C92.62, C92.A2) in the 90 days prior to index diagnosis date; or had evidence of HSCT prior to the index maintenance date.
Treatments received after consolidation were considered maintenance, provided they were administered prior to disease relapse (ICD-10-CM C92.02, C92.62, C92.A2) or end of follow-up, if no relapse occurred. Patients who received maintenance therapy with Oral-AZA on or after September 1, 2020 (FDA approval date), were included in the Oral-AZA cohort; patients who did not receive any identifiable maintenance therapy in the same period were included in the W&W cohort.
Baseline Characteristics and Outcomes
The baseline characteristics assessed included patient demographic and clinical characteristics at index maintenance date (e.g., age, sex, region, insurance type, and comorbidities).
Outcomes measured in this study included treatment patterns in the maintenance setting and during the study period (e.g., mono- or combination therapy, prior HSCT, and Oral-AZA dosage and duration), time-to-event characterization of the cohorts (i.e., time from index AML diagnosis to induction therapy initiation, time from induction therapy initiation to remission, time from induction therapy initiation to index maintenance date, time from index AML diagnosis to end of follow-up, and time from index maintenance date to end of follow-up), HCRU (PPPM hospitalizations, emergency room [ER], and overall outpatient visits, which include laboratory visits, office visits, outpatient visits, and other visits) and costs based on health plan paid amounts PPPM (inpatient, outpatient, AML drug costs, and non-AML drug costs) calculated from index maintenance date until the end of follow-up.
Eligible AML drugs for which costs were calculated included azacitidine, cladribine, clofarabine, cytarabine, daunorubicin, decitabine, doxorubicin, enasidenib, etoposide, fludarabine, gemtuzumab, gilteritinib, glasdegib, idarubicin, ivosidenib, midostaurin, mitoxantrone, sorafenib, and venetoclax.
Statistical Analysis
Descriptive analyses were conducted to describe patient characteristics and treatment patterns. Categorical variables were presented as number and percentage of patients; group differences were assessed with chi-squared tests; and continuous variables were summarized as means, standard deviations (SDs), medians, range, and interquartile range (IQR), with differences assessed using t-test.
To minimize between-group differences, the Oral-AZA and W&W cohorts were matched 1:3 on baseline characteristics using propensity score matching (PSM). Characteristics used for matching were age at index maintenance date, age group (≤ 60 years or > 60 years), sex, US region, and insurance type. Propensity scores were estimated using a logistic regression model and the greedy matching algorithm [30]. The latter allows sequential selection of the best match available by generating matches that go from highest to lowest match on the propensity score, thereby enhancing the quality of matching.
Differences between matched groups were modeled using multivariate analyses: a negative binomial distribution (for HCRU) and generalized linear models with a gamma distribution (for costs) with a logarithm link function, clustered on the patient to account for differences in follow-up time. For both HCRU and costs, differences were presented as mean difference PPPM, 95% confidence intervals (CIs), and p values. Significance was defined as p < 0.05. Costs were based on health plan paid amounts and payer adjudicated, adjusted to 2022 US dollars using the Consumer Price Index medical component [31]. All data analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Time to relapse was analyzed from index maintenance date until relapse (event) or end of follow-up (censoring), whichever occurred first, using Kaplan–Meier methodology.
Ethics Statement
This article is based on a study of data derived from the IQVIA PharMetrics® Plus claims database. The database is de-identified and compliant with HIPAA 1996. This article does not relate to any studies with human participants or animals performed by any of the authors.
Results
Demographic and Clinical Characteristics
Overall, 263 patients were included in the analyses; 43 received maintenance therapy with Oral-AZA and 220 had no maintenance therapy (W&W). After PSM, the Oral-AZA cohort included 43 patients and the W&W cohort included 129 patients (Table 1). The demographic and clinical characteristics prior to and post PSM are presented in Table 2. Before matching, the two cohorts differed significantly for median age (IQR) at index maintenance date, which was greater in the Oral-AZA arm, compared with the W&W cohort (60 [49–69] vs 56 [44–63] years; p = 0.0188). After matching, all baseline characteristics were well balanced between cohorts. The median (IQR) age was 60 (49–69) years in the Oral-AZA cohort and 58 (46–64) years in the W&W cohort; 48.8% and 54.3% of patients were female in the Oral-AZA cohort and W&W cohort, respectively. Nearly half of the patients in both cohorts were from the Southern region of the USA.
Treatment Patterns in the Oral-AZA Cohort
Oral-AZA utilization is presented in Table 3. In the Oral-AZA cohort, 88.4% (n/N = 38/43) of patients started treatment at the recommended dose of 300 mg and 11.6% (n/N = 5/43) with a dose of 200 mg. Patients remained on treatment for a mean (SD) of 117.7 (118.3) days. Of the patients who discontinued Oral-AZA use, 51.2% (n/N = 22/43) discontinued Oral-AZA ≤ 30 days prior to end of follow-up, 34.9% (n/N = 15/43) discontinued > 30 days prior to end of follow-up, 11.6% (n/N = 5/43) discontinued as a result of relapse, and 2.3% (n/N = 1/43) discontinued because of HSCT. In this cohort, the majority (76.7%; n/N = 33/43) of patients received Oral-AZA as monotherapy, while the remainder (23.3%; n/N = 10/43) received Oral-AZA in combination with other agents such as venetoclax, enasidenib, or midostaurin.
Analysis of time-to-event characterization of the matched cohorts (Table 4) showed comparable results (median [IQR]) between the matched Oral-AZA and W&W cohorts, including time from index AML diagnosis to induction therapy initiation (1 [1–3] vs 2 [0–3] months), time from induction therapy initiation to remission (1 [0–2] months in each cohort), and time from induction therapy initiation to index maintenance date (1 [0–2] vs 2 [1–3] months), respectively. The median (IQR) follow-up from index AML diagnosis or index maintenance date to end of follow-up was significantly longer with Oral-AZA than with W&W (11 [8–16] vs 5 [4–12] months; p = 0.0041; and (6 [2–12] vs 2 [1–4] months; p < 0.0001). Median (95% CI) time to relapse from index maintenance date (Fig. 2) was significantly shorter in the W&W cohort compared with the Oral-AZA cohort (3.3 months [0.8 to not reached (NR)] vs median NR [9.0–NR]; p = 0.0025).

Adapted from Borate U, et al. Value Health. 2023;26(12):S67. ©2023 Elsevier. All rights reserved [32]. CI confidence interval, IQR interquartile range, NR not reached, Oral-AZA oral azacitidine, SE standard error, W&W watch and wait
Time to relapse A from index maintenance date and B relapse events.
HCRU and Costs
Compared with the W&W cohort, the Oral-AZA cohort had lower HCRU with significantly fewer inpatient visits (0.23 vs 0.61 PPPM for Oral-AZA and W&W, respectively; difference, 0.37 [95% CI 0.16–0.59]; p = 0.0005), overall outpatient visits (5.77 vs 7.58 PPPM for Oral-AZA and W&W, respectively; difference, 1.80 [95% CI 0.16–3.44]; p = 0.0391), and laboratory visits (2.84 vs 4.53 PPPM for Oral-AZA and W&W, respectively; difference, 1.69 [95% CI 0.48–2.91]; p = 0.0100). ER visits were comparable (0.32 vs 0.35 PPPM for Oral-AZA and W&W, respectively; difference, 0.03 [95% CI − 0.16 to 0.23]; p = 0.7584) and low in both groups (Fig. 3).
HCRU. HCRU was analyzed using a generalized linear model with a negative binomial distribution with a logarithm link function; adjusted for age (60 years), sex (male), insurance status (commercial), and region (South). Individual outpatient components do not add up to the total resource use because each component was modeled separately. aLaboratory visits include both visits to a laboratory and lab tests done in an office setting. *Significant difference. ER emergency room, HCRU healthcare resource utilization, Oral-AZA oral azacitidine, PPPM per person per month, W&W watch and wait
Total costs PPPM based on health plan paid amounts from index maintenance date to the earlier of HSCT date or end of follow-up were higher in the W&W cohort than in the Oral-AZA cohort ($38,530 and $25,786 PPPM, respectively; difference, $12,744 [95% CI $8235–17,253]; p < 0.0001) (Fig. 4). This was driven primarily by significantly higher inpatient and outpatient costs in the W&W cohort (difference, $8758 [95% CI $7263–10,253] and $7982 [95% CI $6877–9087] higher, respectively).

Adapted from Borate U, et al. Value Health. 2023;26(12):S67. ©2023 Elsevier. All rights reserved [32]. Costs evaluated over the period from index maintenance date to HSCT date, if applicable, or end of follow-up. Adjusted costs were analyzed using a generalized linear model with a gamma distribution with a logarithm link function; adjusted for age (60 years), sex (male), insurance status (commercial), and region (South). Total costs based on health plan paid amounts were inflated to 2022 US dollars; individual components do not sum up to the total cost because each component was modeled separately. Overall outpatient costs include laboratory costs, office visits, outpatient visits, and other. Laboratory costs could include both visits to a lab and lab tests done in an office setting. aAML drugs included azacitidine, cladribine, clofarabine, cytarabine, daunorubicin, decitabine, doxorubicin, enasidenib, etoposide, fludarabine, gemtuzumab, gilteritinib, glasdegib, idarubicin, ivosidenib, midostaurin, mitoxantrone, sorafenib, and venetoclax. *Significant difference. AML acute myeloid leukemia, ER emergency room, HSCT hematopoietic stem cell transplantation, Oral-AZA oral azacitidine, PPPM per person per month, W&W watch and wait
Healthcare costs.
The Oral-AZA cohort had higher AML drug costs than the W&W cohort ($16,401 and $10,651 PPPM difference, − $5749 [95% CI − $7837 to − $3662], respectively; p < 0.0001) (Fig. 4), with Oral-AZA-specific costs accounting for $9222 (W&W cost for maintenance therapy is $0; difference, − $9222; 95% CI − $10,186 to − $8257) of costs in the Oral-AZA cohort. Costs for other AML drugs (excluding Oral-AZA) were observed in both groups but comprised a greater proportion of the costs in the W&W cohort ($10,646 vs $5825 PPPM in the Oral-AZA cohort; difference, $4821 [95% CI $3606–6036]). Costs for other AML drugs (excluding Oral-AZA) were especially higher in the post-relapse period for the W&W cohort when compared with the Oral-AZA cohort ($12,469 vs $321; difference, $12,148 [95% CI $10,640–13,657]).
When costs for other AML drugs (excluding Oral-AZA) were partitioned by those incurred in the pre-relapse period, costs were $0 in the W&W cohort and $1640 PPPM in the Oral-AZA cohort (some owing to the use of other agents in combination with Oral-AZA; difference, $1640 [95% CI $1470–1810]). In the post-relapse period, total costs were significantly higher in the W&W cohort compared with the Oral-AZA cohort ($28,506 vs $3192 PPPM; difference, $25,314 [95% CI $22,245–28,384]; p < 0.001).
Discussion
This is the first economic study of Oral-AZA use in routine clinical practice since its FDA approval in September 2020. Using data from the IQVIA PharMetrics® Plus longitudinal claims database, we described patient characteristics and treatment patterns, and assessed HCRU and associated costs, in patients with newly diagnosed AML in remission who received Oral-AZA or no maintenance therapy (W&W approach).
Patients receiving Oral-AZA maintenance had longer time to relapse and lower HCRU and total healthcare costs than those in the W&W cohort, indicating that Oral-AZA maintenance may be associated with both clinical and economic benefits for eligible patients with newly diagnosed AML. The observed treatment duration with Oral-AZA in this study was relatively short (mean 117.7 days) considering the time to relapse in the Oral-AZA cohort (median NR, mean 9.8 months), suggesting that some patients may have discontinued Oral-AZA before relapse. Premature treatment discontinuation in clinical practice may have occurred in cases of suboptimal toxicity management; this underscores the importance of implementing consistent antiemetic prophylaxis for all patients, as well as Oral-AZA dose modifications and supportive care as required to mitigate side effects. Moreover, more than half (51.2%) of patients in this study discontinued Oral-AZA ≤ 30 days prior to the end of follow-up, likely indicating that some patients were still on ongoing treatment at the time of the last recorded Oral-AZA use. Therefore, the observed Oral-AZA treatment duration in this analysis should be interpreted with caution.
The clinical benefits observed in this real-world study are in line with those from QUAZAR AML-001 (NCT01757535) [21, 22], which demonstrated significantly longer RFS with Oral-AZA compared with placebo, supporting broad use of Oral-AZA maintenance following IC in clinical practice to reduce relapse risk. The results from this study are also consistent with those from previous real-world research demonstrating that AML is associated with high HCRU and elevated costs in the USA [23,24,25,26,27,28].
In the present study, patients receiving Oral-AZA maintenance had lower overall HCRU and costs compared with W&W. Fewer outpatient and laboratory visits in the Oral-AZA cohort contributed to a lower HCRU in this cohort. Notably, Tabah et al. (2022) found that total HCRU was reduced for patients with a longer remission period, compared with those with a shorter remission period [29]. In an analysis of hospitalization data from the QUAZAR-001 trial [33], Oral-AZA treatment significantly reduced exposure-adjusted hospitalization risk and hospital stay compared with placebo. Given that a previous analysis showed that hospitalization costs can account for approximately 70% of direct AML healthcare costs [34], this could result in substantial cumulative cost savings. As Oral-AZA has been shown to prolong remission [22], this may explain the fewer hospital visits and lower costs with Oral-AZA maintenance observed in the present study.
The total cost of AML for patients in remission treated with Oral-AZA was $25,786 PPPM with total costs for Oral-AZA being over $12,000 PPPM lower than with W&W, underscoring the importance of remission-prolonging treatments in containing AML-related healthcare costs. The Oral-AZA cohort had higher AML drug costs of $16,401 PPPM compared with $10,651 PPPM in the W&W cohort. However, costs for other AML drugs (excluding Oral-AZA) in the post-relapse period were substantial (over $10,000 PPPM) in the W&W cohort, despite the latter incurring no costs for maintenance therapy in the pre-relapse period. While the reasons for treatment claims were not available in the database, higher costs for other AML drugs (excluding Oral-AZA) in the post-relapse period for the W&W cohort were likely related to the significantly higher inpatient and outpatient care observed, which is likely a reflection of earlier relapse. Given the follow-up time of 2 years, earlier relapse in the W&W group would imply a longer time to incur costs for salvage regimens for relapsed disease in the post-relapse period in this cohort when compared with the Oral-AZA cohort.
The primary driver of lower total AML costs with Oral-AZA maintenance versus W&W was decreased inpatient and outpatient costs PPPM in the Oral-AZA cohort, which was likely related to the lower rate of, and longer time to, relapse observed with Oral-AZA maintenance compared with W&W. This is corroborated by the results of a study using medical records data from 27 US institutions, which demonstrated higher HCRU among patients with AML experiencing relapse compared with patients in remission, suggesting that increased duration of remission and decreased relapse rates can minimize the economic burden of AML [35]. Other real-world research has identified relapse as a key contributor to elevated healthcare costs in AML [29]. For example, Potluri et al. reported that in patients with AML ineligible for HSCT, disease relapse was associated with substantial incremental costs [36]. The lower rate of, and longer time to, relapse observed with Oral-AZA maintenance compared with no maintenance therapy is therefore an important aspect to consider when evaluating the cost-effectiveness of Oral-AZA. This is further supported by our finding that post-relapse costs were substantially higher in the W&W cohort compared with the Oral-AZA cohort, which contributed to the higher total expenditures observed in the former group.
Findings from this study should be interpreted in the context of certain strengths and limitations. Retrospective observational research of administrative claims data is valuable for providing a view into real-world clinical treatment patterns and associated outcomes in an insured population that is not possible with controlled clinical trials. In the absence of randomization, this study used PSM to resolve differences between study cohorts as much as possible. Differences may have been present based on variables that were not available in the database and could not be used in the PSM. However, the post-PSM study cohorts were well matched on key demographic and clinical characteristics. This study population was from a real-world claims database and therefore represented a relatively younger AML population than what is generally reported in the literature (post-PSM median ages of 60 and 58 years for the Oral-AZA and W&W cohorts, respectively, compared with 69 years in the literature) [4]. The younger patient population may limit the study’s generalizability to younger patients with AML, who may incur lower overall HCRU and costs than an older population. Future work may include a study population of both patients with commercial health insurance and patients with Medicare (government-funded health insurance for people ≥ 65 years of age) coverage in similar proportions to cover a larger age distribution of patients with AML.
Another limitation is the shorter duration of follow-up for the W&W cohort than the Oral-AZA cohort, which may have limited the observation of available outcomes. Median follow-up time from index maintenance date was < 1 year for both cohorts and < 6 months for the W&W cohort; however, the data in the manuscript represent the totality of available data in the underlying database (i.e., 2 years), and thus we are unable to assess beyond this time frame. While this may limit the view of HCRU and costs to a relatively short time period, this is a relevant time period for a US commercial payer considering differences in costs. Additionally, it should be noted that after the eligibility criteria were applied, the Oral-AZA cohort was relatively small (43 patients). Future work that includes commercial health insurance and Medicare claims may be able to encompass a larger sample size and longer duration of follow-up since, given the age-based enrollment in Medicare (≥ 65 years), patients with AML are likely to move from a commercial health plan to Medicare coverage during the course of their condition and treatment plan, potentially impacting the sample size and available follow-up time. Since the study sample was composed primarily of patients with commercial insurance in the USA, generalizability of these findings to other patient populations (such as patients without insurance) and other health systems and countries should be considered with caution. Despite the limitations, the use of this large, longitudinal, demographically and geographically diverse administrative claims database provides a complementary perspective to controlled clinical trials, as this study population is representative of the adult US patient population with continuous insurance coverage and reflects treatment patterns and outcomes in real-world clinical practice.
Conclusions
Treatment with Oral-AZA maintenance therapy in patients with newly diagnosed AML in remission is associated with significantly longer time to relapse and lower HCRU and costs compared with W&W. Despite no active maintenance therapy, the W&W cohort incurred higher HCRU and total costs than the Oral-AZA cohort, likely related to their shorter duration of remission. Oral-AZA maintenance use may positively impact the healthcare system by reducing the clinical, HCRU, and economic burden of AML in the real world. These real-world findings add to the results of the pivotal QUAZAR AML-001 trial and may assist providers in making informed decisions about the use of active maintenance therapy in clinical practice to prolong remission and survival while reducing HCRU, thereby optimizing patient outcomes and generating cost savings.
Data Availability
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
References
Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2(2):95–107.
Nix NM, Price A. Acute myeloid leukemia: an ever-changing disease. J Adv Pract Oncol. 2019;10(Suppl 4):4–8.
Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(3):502–26.
National Cancer Institute. SEER Cancer Stat Facts: Leukemia — Acute Myeloid Leukemia. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 6 Mar 2024.
Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528–49.
Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767–74.
Cory B, Cohen M, Hernandez K, Saal T, Steinberg A. Emerging trends in frontline treatment for acute myeloid leukemia (AML): a survey of NCI-designated cancer centers. Blood. 2023;142(Suppl 1):5874.
Larson RA. Treatment of relapsed or refractory acute myeloid leukemia. UpToDate. Updated Mar 2023. https://www.uptodate.com/contents/treatment-of-relapsed-or-refractory-acute-myeloid-leukemia. Accessed 11 Mar 2024.
RYDAPT (midostaurin). Package insert. Novartis Pharmaceuticals Corporation. Revised May 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/207997s010lbl.pdf. Accessed 11 Mar 2024.
VANFLYTA (quizartinib). Package insert. Daiichi Sankyo Company, Ltd. Revised Jul 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216993s000lbl.pdf. Accessed 11 Mar 2024.
VYXEOS (daunorubicin and cytarabine). Package insert. Jazz Pharmaceuticals, Inc. Revised Aug 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf. Accessed 11 Mar 2024.
Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–77.
Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(6):697–712.
Eapen M. Since everyone has a donor, why are some eligible patients still not transplanted? Best Pract Res Clin Haematol. 2021;34(4):101321.
Stanchina M, Soong D, Zheng-Lin B, Watts JM, Taylor J. Advances in acute myeloid leukemia: recently approved therapies and drugs in development. Cancers (Basel). 2020;12(11):3225.
Medeiros BC, Chan SM, Daver NG, Jonas BA, Pollyea DA. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol. 2019;94(7):803–11.
de Lima M, Roboz GJ, Platzbecker U, Craddock C, Ossenkoppele G. AML and the art of remission maintenance. Blood Rev. 2021;49: 100829.
ONUREG (azacitidine). Package insert. Bristol-Myers Squibb Company. Revised Oct 2022. https://packageinserts.bms.com/pi/pi_onureg.pdf. Accessed 11 Mar 2024.
Jen EY, Wang X, Li M, et al. FDA approval summary: oral azacitidine for continued treatment of adults with acute myeloid leukemia unable to complete intensive curative therapy. Clin Cancer Res. 2022;28(14):2989–93.
Sorrentino VG, Thota S, Gonzalez EA, Rameshwar P, Chang VT, Etchegaray J-P. Hypomethylating chemotherapeutic agents as therapy for myelodysplastic syndromes and prevention of acute myeloid leukemia. Pharmaceuticals (Basel). 2021;14(7):641.
US Food and Drug Administration. FDA approves Onureg (azacitidine tablets) for acute myeloid leukemia. 1 Sep 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-onureg-azacitidine-tablets-acute-myeloid-leukemia. Accessed 11 Mar 2024.
Wei AH, Döhner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526–37.
Hagiwara M, Sharma A, Chung KC, Delea TE. Healthcare resource utilization and costs in patients with newly diagnosed acute myeloid leukemia. J Med Econ. 2018;21(11):1119–30.
Huggar D, Knoth RL, Copher R, et al. Economic burden in US patients with newly diagnosed acute myeloid leukemia receiving intensive induction chemotherapy. Future Oncol. 2022;18(32):3609–21.
Irish W, Ryan M, Gache L, Gunnarsson C, Bell T, Shapiro M. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33(3):519–27.
Pandya BJ, Medeiro BC, Chen C-C, et al. Real-world occurrence of symptoms and toxicities and associated cost implications in acute myeloid leukemia (AML) treatment episodes: a retrospective database analysis in the US. Blood. 2017;130(Suppl 1):2118.
Preussler JM, Meyer CL, Mau L-W, et al. Healthcare costs and utilization for patients age 50 to 64 years with acute myeloid leukemia treated with chemotherapy or with chemotherapy and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(6):1021–8.
Stein EM, Bonifacio G, Latremouille-Viau D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with acute myeloid leukemia in commercially insured and Medicare populations. J Med Econ. 2018;21(6):556–63.
Tabah A, Brady BL, Huggar D, et al. The impact of remission duration on the long-term economic burden of acute myeloid leukemia among patients without hematopoietic stem cell transplant in the United States. J Med Econ. 2022;25(1):903–11.
Rosenbaum PR. Observational studies. 2nd ed. New York, NY: Springer-Verlag; 2002. https://archive.org/details/observationalstu0000rose_x4u5. Accessed 24 Mar 2024.
US Bureau of Labor Statistics. Updated Nov 2023. https://www.bls.gov/. Accessed 6 Mar 2024.
Borate U, Seiter K, Potluri R, et al. Healthcare resource utilization (HCRU) and associated costs among patients with acute myeloid leukemia (AML) treated with oral azacitidine as maintenance and those eligible but not treated using a US claims database. Value Health. 2023;26(12):S67.
Oliva EN, Kambhampati S, Oriol A, et al. CC-486 reduces hospitalization and associated estimated costs in patients with acute myeloid leukemia (AML) in first remission after intensive chemotherapy: results from the QUAZAR AML-001 maintenance trial. Blood. 2020;136(Suppl 1):14–5.
Pandya BJ, Chen C-C, Medeiros BC, et al. Economic and clinical burden of acute myeloid leukemia episodes of care in the United States: a retrospective analysis of a commercial payer database. J Manag Care Spec Pharm. 2020;26(7):849–59.
Kwon C, Brandt P, Manson S, Fuentes-Alburo A, Forsythe A. Treatment patterns and health care resources use (HCRU) in patients with acute myeloid leukemia (AML): real world evidence (RWE) from 30 US institutions. Blood. 2017;130(Suppl 1):5655.
Potluri R, Papademetriou E, Kiendrebeogo ZN, Liu X, Chen C. EE278 Economic burden of acute myeloid leukemia relapse in US patients. Value Health. 2022;25(7):S388.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Claire Fielden, PhD, of LATITUDE (Powered by AXON), and writing and editorial assistance were provided by Ana Almeida, MSc, of Excerpta Medica funded by Bristol Myers Squibb. Assistance for the conception and design of the study and for the acquisition, analysis, and interpretation of the data was provided by David Rotter, PhD, of Putnam; assistance for the conception and design of the study was provided by Aylin Yucel, PhD, of Bristol Myers Squibb; and assistance for the conception and design of the study and for the acquisition of the data was provided by Willem Heydendael, PhD, of Bristol Myers Squibb funded by Bristol Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this commentary, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was supported by Bristol Myers Squibb. The journal’s Rapid Service and Open Access fees were paid by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
Uma Borate and Karen Seiter were involved in the interpretation of the data. Ravi Potluri and Debasish Mazumder designed the study and were involved in the acquisition, analysis, and interpretation of the data. Manoj Chevli, Thomas Prebet, Maria Strocchia, and Alberto Vasconcelos designed the study and were involved in the interpretation of the data. Lona Gaugler was involved in the interpretation of the data. Jan Sieluk designed the study and was involved in the acquisition and interpretation of the data.
Corresponding author
Ethics declarations
Conflict of Interest
Uma Borate has received grants/research support from and served as an advisory board member for AbbVie; has served as an advisory board member for Agios, Astellas, Blueprint Medicines, Genentech, Kura Oncology, Novartis, Servier, and Takeda; has received grants/research support from Incyte, Jazz Pharmaceuticals, and Pfizer; and has received honoraria from the RUNX1 Foundation. Karen Seiter has received research funding and consulting fees from Bristol Myers Squibb. Ravi Potluri and Debasish Mazumder declare employment with SmartAnalyst/Putnam Associates. Manoj Chevli, Thomas Prebet, Lona Gaugler, Maria Strocchia, and Jan Sieluk declare employment and stock ownership in Bristol Myers Squibb. Alberto Vasconcelos has received research funding support for travel/meeting attendance and declares employment and stock ownership in Bristol Myers Squibb.
Ethical Approval
This article is based on a study of data derived from the IQVIA PharMetrics® Plus claims database. The database is de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). This article does not relate to any studies with human participants or animals performed by any of the authors.
Additional information
Prior Presentation: Related data were presented at the 26th Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research Europe; November 12–15, 2023; Copenhagen, Denmark [32].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Borate, U., Seiter, K., Potluri, R. et al. Healthcare Utilization and Costs Among Patients with Acute Myeloid Leukemia Receiving Oral Azacitidine Maintenance Therapy Versus No Maintenance: A US Claims Database Study. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02947-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02947-1