Abstract
Introduction
Studies reveal that cannabidiol may acutely reduce blood pressure and arterial stiffness in normotensive humans; however, it remains unknown if this holds true in patients with untreated hypertension. We aimed to extend these findings to examine the influence of the administration of cannabidiol on 24-h ambulatory blood pressure and arterial stiffness in hypertensive individuals.
Methods
Sixteen volunteers (eight females) with untreated hypertension (elevated blood pressure, stage 1, stage 2) were given oral cannabidiol (150 mg every 8 h) or placebo for 24 h in a randomised, placebo-controlled, double-blind, cross-over study. Measures of 24-h ambulatory blood pressure and electrocardiogram (ECG) monitoring and estimates of arterial stiffness and heart rate variability were obtained. Physical activity and sleep were also recorded.
Results
Although physical activity, sleep patterns and heart rate variability were comparable between groups, arterial stiffness (~ 0.7 m/s), systolic blood pressure (~ 5 mmHg), and mean arterial pressure (~ 3 mmHg) were all significantly (P < 0.05) lower over 24 h on cannabidiol when compared to the placebo. These reductions were generally larger during sleep. Oral cannabidiol was safe and well tolerated with no development of new sustained arrhythmias.
Conclusions
Our findings indicate that acute dosing of cannabidiol over 24 h can lower blood pressure and arterial stiffness in individuals with untreated hypertension. The clinical implications and safety of longer-term cannabidiol usage in treated and untreated hypertension remains to be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Administration of oral cannabidiol in healthy humans reduces the blood pressure response to acute stressors but not resting blood pressure. |
This study assessed, for the first time, if oral cannabidiol could reduce 24-h ambulatory blood pressure and arterial stiffness in untreated hypertensive participants. |
What was learned from the study? |
Acute administration of oral cannabidiol appeared to reduce systolic and mean arterial blood pressures in untreated hypertensive patients. |
In untreated hypertensive participants, cannabinoids may influence blood pressure via improvements in arterial compliance. |
Introduction
Cannabinoids from Cannabis indica and C. sativa are of growing interest due to their potential therapeutic benefits [1], diverse range of therapeutic properties and their favourable safety and tolerability profile [2]. Compounds of particular interest include, amongst others, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Although poorly established in humans, the purported benefits of non-psychoactive CBD include analgesic, anti-inflammatory, anti-oxidant, anxiolytic, anticonvulsant and antiproliferative cytotoxic effects [reviewed in [3,4,5,6]]. From a cardiovascular perspective, a recent meta-analysis [3] revealed that administration of CBD can attenuate acute stress-induced increases in blood pressure (BP) and heart rate (HR). For example, reductions in BP and HR in rat stress models were observed after administration of CBD intraperitoneally [7], as well as centrally into the cisterna magna [8]. It has been demonstrated that, at least in part, CBD affects stress-related changes in the cardiovascular system via 5-HT1A receptors [9].
In humans undergoing various types of acute stress, conflicting results of CBD administration have been reported. For example, in one early study, although reducing anxiety, CBD (300 or 600 mg, p.o.) did not affect BP and/or HR increased by simulated public speaking [10]. Conversely, in young and healthy volunteers, acute administration of CBD (400–800 mg, p.o.) decreased blood pressure [11] and increased HR during different stressful conditions, such as mental arithmetic tests, isometric hand-grip tests or cold stress [12]. In a recent study—also conducted in otherwise young and heathy volunteers—the same dose of CBD after both acute and chronic treatment (for 7 days) reduced systolic blood pressure (SBP), but did not influence HR (or other cardiovascular parameters) during isometric handgrip stress exercises [13]. Thus, at least in young and normotensive volunteers, the tolerance (observed under resting conditions) for a hypotensive effect of CBD during stress does not develop. However, in the same study, it was demonstrated that the 7-day repeated CBD dosing decreased arterial stiffness and improved endothelial function [13].
It may be that the potential hypotensive effect of CBD may be better observed in individuals with hypertension, a common condition that is associated with reduced arterial compliance and a diminished endothelial function [14]. While previous studies found that chronic (2 weeks) administration of CBD did not influence 24-h ambulatory BP in hypertensive rats [15], to the best of the authors’ knowledge, the influence of acute administration of CBD per se on 24-h ambulatory blood pressure has not been examined in untreated hypertensive individuals. This lack of information is surprising, given the involvement of the endocannabinoid system in several aspects of cardiovascular regulation, including control of blood vessel tone, cardiac contractility, blood pressure, and vascular inflammation [16]. The objective of this current study was to extend the findings from young normotensive volunteers [11, 13] into those individuals with elevated BP, stage 1, or stage 2 hypertension. While utilising a randomised, double-blinded, placebo-controlled, cross-over pilot study, we examined the hypothesis that the hypotensive effects of CBD will be apparent in those with untreated hypertension. We further reasoned that, if CBD administration influenced the magnitude of 24-h blood pressure, this would also be reflected in changes in arterial stiffness and/or cardiac autonomic activity.
Methods
The protocol, and all methods included in the present study, were reviewed and approved by the ethics review board at the University of Split, School of Medicine (certificate number: 2181-198-03-04-21-0001), and conformed to the latest revision of the Declaration of Helsinki, except for registration in a data base. All participants provided written, informed consent prior to enrolling in the study.
Participants
A total of 16 volunteers (8 females) with elevated BP (120–129/< 80 mmHg), stage 1 (130–139/80–89 mmHg), or stage 2 (≥ 140/90 mmHg) untreated hypertension were recruited from the outpatient hypertension clinic (University of Split Hospital) after receiving a hypertension diagnosis from a physician according to the American Heart association guidelines [17]. Hypertension staging was performed based on BP readings obtained in the laboratory only and strictly used for descriptive purposes. All participants completed all experimental sessions described below on both CBD and placebo. The demographics for the volunteers are outlined in Table 1. Exclusion criteria included normal blood pressure (< 120/80), diabetes, history of smoking, medicinal/recreational use of cannabis, or opioid use. In addition, participants were excluded if they had any previous history of cardiopulmonary, liver, gastrointestinal (GI), kidney or cerebrovascular diseases, clinically diagnosed anxiety or depression, or if they were taking prescription drugs.
Experimental Design
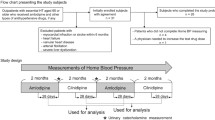
The overall study design is illustrated in Fig. 1. Similar to our previously published protocol [18], the volunteers participated in a randomised, double-blinded, placebo-controlled, cross-over pilot study. All participants completed the full protocol while taking both CBD and placebo. Participants visited the laboratory on three occasions (one for pre-screening and informed consent, two experimental visits). Experimental visits were separated by 4 days. Food and activity logs were kept which participants were asked to adhere to on their subsequent experimental visits. Volunteers were instructed to refrain from caffeine and alcohol-containing drinks for 6 and 24 h, respectively, before each laboratory visit.
Visit 1—Informed Consent and Pre-Screening
On the initial visit, participants provided written, informed consent and completed a Medical Screening Questionnaire to confirm eligibility criteria. Anthropometric and physiologic measurements were collected (height, body weight, blood pressure) for baseline participant characterisation. Following at least 10 min of rest in a quiet room, automated blood pressure was assessed in triplicate [19].
Visit 2 and 3—Experimental Trials
Participants reported to the laboratory after an overnight fast (> 10 h) at around 07:30. A snack high in fat (muffin and/or granola bar) was provided upon initial arrival. This protocol was used as ingestion of CBD with a high fat meal following a fast is shown to increase bioavailability of CBD [20, 21]. Water intake was allowed ad lib.
Following 20 min of supine rest, 150 mg of oral DehydraTECH™2.0 CBD or a placebo control was administered. Volunteers were then instrumented for 24-hour ambulatory BP and electrocardiogram (ECG) monitoring (Schiller BR-102 plus pulse wave velocity and Medilog AR Holter; Schiller, Baar, Switzerland) along with a Fitbit activity and sleep monitor (Fitbit, San Francisco, CA, USA) to monitor steps, distance, calories burned, floors climbed, active minutes, and sleep duration [22]. Participants were then given two more identical doses of either CBD or placebo to be self-administered along with a high-fat snack (i.e. muffin, granola bar) and recorded at 14:00 and 22:00.
Measurements
DehydraTECH TM 2.0 CBD
The production of both placebo and capsules containing CBD was carried out in accordance with well-defined standard operating procedures by skilled personnel using the services of a Good Manufacturing Practices-compliant contract manufacturer located in the United States. The DehydraTECH™ 2.0 CBD formulation was comprised of Lexaria Bioscience’s patented mixture of long chain fatty acid rich triglyceride oil and tetrahydrocannabinol-free, purified cannabidiol distillate oil together with organic substrate powder ingredients, plus other organic constituents included to enhance intestinal absorption and relate delivery performance. The DehydraTECH™ 2.0 CBD formulation was prepared as a powder and filled into Size 00 vegan gel capsules at a target strength per capsule of 25 mg CBD, as independently verified via HPLC upon quality control testing. The placebo capsules were simply filled with an organic substrate powder ingredient devoid of any CBD active drug substance, as also verified upon HPLC testing, and similarly filled into matching Size 00 vegan gel capsules for blinding purposes.
24-h BP
The 24-hour ambulatory BP monitoring was set to take measurements every 30 min through the day (7:00–22:00) and every hour through the night (22:00–7:00). Participants were instructed to keep their arm still at the level of their heart when the device started to take a reading [19].
24-h ECG
From the continuous ECG recordings, we assessed cardiovascular autonomic function by means of heart rate variability (HRV) parameters over 24 h. The detailed methodology behind the acquisition, inspection and processing of the cardiovascular autonomic function data is provided in earlier publications [23, 24]. In brief, for the analysis of HRV in both the time and frequency domain, we processed the R-R interval data by visual inspection (Hearts 1.2, University of Oulu, Oulu, Finland), replaced short artefacts and ectopic beats with the local average, and deleted longer sequences of noise or ectopy (≥ 10 consecutive defective beats). Recordings containing ≥ 80% of adequate data were accepted for further processing. Eligible HRV data (i.e. low-frequency power, high-frequency power, etc.) was not obtained in two subjects because of too short recordings and in one subject because of artefacts; therefore, these subjects were excluded from further analyses. The incidence and type of arrhythmias were screened by a cardiologist who was blinded to the study. Rate pressure product (RPP, HR × SBP), a marker of myocardial oxygen consumption, was calculated.
24-h Arterial Stiffness
The analyses and validation of the 24-h non-invasive measures of arterial stiffness of the device used in the present study (Schiller BR-102 plus PWA; Schiller), in a comparable age group, has been outlined in detail elsewhere [25, 26]. In brief, as a result of aging and pathological changes (e.g. arteriosclerosis or subclinical organ damage), stiffening of vessels and therefore an increase of SBP, as well as an increased peripheral arterial resistance, may occur. As a consequence of these changes, increased and premature pressure reflections emerge, which superimpose the generic pulse wave ejected by the heart earlier and more intensely. Their accumulation leads to an elevation of the SBP and is called augmentation. The percent ratio of augmentation to the aortic pulse pressure is called the augmentation index (AIx).
Subjective Symptoms
The CBD/placebo tolerance for drowsiness and diarrhea was assessed via a follow-up report including any side effects that participants may have experienced.
Statistical Analyses
Statistical analyses were performed in R computing language (R Foundation for Statistical Computing, Vienna, Austria), using the emmeans [27] and lmerTest [28] packages. A linear mixed effects model was used to test relationships among each variable with the drug defined as a fixed factor within the model and the participant ID included as a random effect. For 24-h blood pressure and arterial compliance measurements, time was also included as a fixed effect. Daytime was considered to be 7:00–22:00 while nighttime was defined as 22:00–7:00. Statistical significance was assumed at a level of P < 0.05. Upon achieving a significant F test, pairwise comparisons were made with a Tukey’s post-hoc analysis to determine differences between the estimated marginal means. All data are presented as mean ± SD.
Results
Following screening, the stage of hypertension included those with elevated BP (n = 6), stage 1 (n = 6) and stage 2 (n = 4) hypertension; these stages were combined for the main statistical analyses and all volunteers successfully completed the two experimental conditions. There were no notable differences in reported side effects between the CBD and placebo condition: 2 participants reported mild drowsiness in both conditions, mild diarrhea was noted in 2 participants while taking CBD and in 1 participant while taking the placebo. Both physical activity and sleep patterns were comparable between conditions (Table 2).
Influence of CBD on 24-h Blood Pressure
The mean ± SD of BP, HR, pulse pressure and arterial stiffness over 24 h, as well as during daytime and nighttime, are shown in Table 3. There were no significant interactions between time and drug for any BP parameters when expressed as either absolute values or as a change from baseline. Influence of CBD administration on cardiovascular parameters are illustrated in Fig. 2. There was a main effect of drug on both SBP and MAP, indicating that SBP (133.0 ± 2.6 mmHg) and MAP (103.0 ± 2.0) were lower on CBD than placebo (SBP: 136.0 ± 2.6 mmHg; MAP: 106.0 ± 2.0). In contrast, neither HR nor DBP appeared to be influenced by ingestion of CBD and were comparable between CBD (HR: 74 ± 13 bpm; DBP: 83 ± 16 mmHg) and placebo (HR: 72 ± 13 bpm; DBP: 84 ± 16 mmHg).
Cardiovascular measures following ingestion of either DehydraTECH™ 2.0 (indigo) or placebo (light blue). Values shown are means ± SD. DBP diastolic blood pressure, HR heart rate, MAP mean arterial pressure, SBP systolic blood pressure. Black arrows indicate self-administration of oral CBD (color figure online)
Temporal changes in BP are illustrated in Fig. 3. Following ingestion of DehydraTECH™ 2.0 CBD, there was a smaller increase in ΔSBP from baseline (CBD: 2.5 ± 2.4%, placebo: 9.5 ± 2.4%), and ΔMAP (CBD: 3.3 ± 1.7%, placebo: 8.6 ± 1.7%). In contrast, ΔDBP decreased (0.8 ± 1.6%, placebo: 2.7 ± 1.6%), and ΔHR increased (CBD: 3.2 ± 1.0%, placebo: 0.6 ± 1.0%).
Change in BP over 24-h following ingestion of either DehydraTECH™ 2.0 CBD (indigo) or placebo (light blue). Values shown are mean ± SD. BL, baseline; DBP, diastolic blood pressure; HR heart rate; MAP mean arterial pressure; SBP systolic blood pressure. Black arrows indicate administration of oral CBD (color figure online)
Influence of CBD on 24-h Arterial Stiffness
Continuous measures of arterial stiffness parameters are illustrated in Fig. 4. There was a main effect of drug on all parameters, indicating that pulse wave velocity (PWV) (8.1 ± 0.3 m/s), augmentation index (AIx; 28.4 ± 1.4%), augmentation index corrected to a HR of 75 bpm (AIx.75; 27.8 ± 1.3%), and augmentation pressure (AugP; 12.0 ± 1.0 mmHg) were all lower on DehydraTECH™ 2.0 compared to placebo (PWV: 8.3 ± 0.3 mmHg; AIx: 32.3 ± 1.3%; AIx.75: 30.4 ± 1.3%; AugP: 14.6 ± 1.0 mmHg; P < 0.01 for all comparisons).
Measures of arterial stiffness over 24 h following ingestion of either DehydraTECH™ 2.0 (indigo) or placebo (light blue). Values shown are means ± SD. AIx augmentation index, AIx.75 augmentation index corrected to heart rate of 75 bpm, AugP augmentation pressure, PWV pulse wave velocity. Black arrows indicate self-administration of oral CBD (color figure online)
Heart Rate Variability and Arrhythmias
All indices of autonomic control of HR (measured via HRV analyses) were comparable between DehydraTECH™ 2.0 CBD and the placebo conditions over the 24-h. No significant changes were seen in the different parameters of the 24-h ECG exam and no new sustained arrhythmias developed.
Further statistical analyses were conducted using a linear mixed effects model to examine if there were differences in response to DehydraTECH™ 2.0 CBD based on sex or body mass (i.e. if dosing mg/Kg may have been more effective). Body mass or body mass index (BMI) was not found to be a significant predictor of blood pressure or arterial stiffness response to DehydraTECH™ 2.0 CBD, indicating that a standardised dose of DehydraTECH™ 2.0 CBD should be sufficient to elicit therapeutic effects. The model that was used included an interaction of drug by sex. There was no significant drug by sex interaction on any parameter except for HR, seen in the supplementary figure. On the placebo pill, males had a lower HR than females (females: 77 ± 12 bpm, males: 68 ± 11 bpm; P < 0.05). Males had a significantly higher HR while taking DehydraTECH™ 2.0 CBD than when taking placebo (DehydraTECH™2.0 CBD: 72 ± 13 bpm; P < 0.01).
Discussion
This is the first study to examine the influence of CBD on 24-h BP and arterial stiffness in individuals with untreated hypertension. Our findings provide evidence that CBD attenuates increases in both SBP and MAP more effectively than a placebo over 24 h. It appears as though these changes in BP are mediated, at least in part, by reductions in arterial stiffness. Our findings provide preliminary evidence that CBD doses used over 24 h was safe and well tolerated by the volunteers, and could be useful as a new therapeutic approach to lower BP in the context of untreated hypertension.
Influence of CBD on 24-h BP
CBD is a non-intoxicating major constituent of cannabis which has a potentially diverse range of therapeutic properties and has a favourable safety and tolerability profile [29]. Side effects are generally mild and infrequent, and it is also reported that clinically significant drug interactions pose a low risk [30]. Our finding that CBD lowers blood BP is consistent with reports that: synthetic cannabinoids including synthetic derivatives of Δ9-THC may decrease BP [31]; cannabis users have increased risk of orthostatic hypotension [32]; CBD causes vasorelaxation in human mesenteric arteries [33]; the sympathetic nervous system activity appears to play a role in the cardiovascular depression effects of endocannabinoids [34, 35]; and, although there were no changes in resting BP, acute [11] and 7 days [13] of CBD dosing reduced SBP during isometric handgrip stress exercises that were used to induce transients' hypertension.
Our data show that SBP and MAP are significantly reduced following acute administration of CBD. The modest change that we observed (~ 3 mmHg for both) is slightly lower, but comparable, to changes in BP previously reported following acute dosing in young, healthy males. [12, 13] Similar reductions in BP to those seen in the present study were observed in older hypertensive patients after chronic (5–12 weeks) administration of CBD [36, 37]. Furthermore, our data support findings from previous studies that the reduction in BP is likely mediated by reductions in arterial stiffness [13]. CBD has been shown to exert vasorelaxant effects in rats [38, 39], humans and arteries [33]. Antagonism of the CB1 receptor attenuated CBD-related vasorelaxation, highlighting its involvement in CBD-induced vasorelaxation in the human mesenteric artery [33]. Indeed, activation of the CB1 receptor is associated with increased production of nitric oxide [40], a potent vasodilator [41]. However, as CBD is a weak, partial agonist for the CB1 receptor, it is likely that additional pathways, including transient receptor protein channels, are involved in the hypotensive response to CBD [33]. While peroxisome proliferator-activated receptor gamma activation has been identified as a mediator of CBD-related vasorelaxation in rat conduit arteries [42], this has yet to be demonstrated in humans, and further work is required in order to elucidate the role of this receptor in CBD-dependent vasorelaxation.
Our data also indicate that reductions in BP were greatest at night. Chronic administrated of CBD in hypertensive patients also yielded similar findings [37]. It may be that we observed the greater reduction in BP at night because it was after the final dose of CBD, allowing for peak concentration of CBD concentration in the blood. Another plausible explanation is that, through its sympatho-inhibitory action, CBD may enhance the already diminished sympathetic activity at night [34, 43]. A recent study by Kumric et al. [36] demonstrated that 5 weeks of CBD administration significantly reduced serum catestatin levels. Furthermore, reductions in MAP were correlated with reductions in catetstatin serum levels. Indeed, previous studies show that catestatin may be involved in BP regulation and the development of primary hypertension [44, 45]. Catestatin modulates activity of the sympathetic nervous system via reduced catecholamine secretion [46, 47]. As sympathetic activity is elevated in hypertensive patients, therapies targeting catestatin levels may prove to be useful in treating primary hypertension [48]. However, future studies are needed in order to fully understand this relationship and its potential implications for hypertensive therapies.
Despite promising findings suggesting that CBD may reduce BP at rest and under acute stress [12], these findings are certainly not unanimous. For example, studies looking at the influence of chronic CBD administration have previously reported no change in BP between pre- and post-treatment measures in healthy humans [13], and in both normotensive as well as hypertensive rats [15, 39, 49]. It may be that basal pressure in normotensive individuals may already be too low for CBD to influence [50], as chronic administration of CBD in hypertensive humans reduces ambulatory BP [36, 37]. Interestingly, Remiszewski et al. [15] reporting no impact of CBD in hypertensive rats despite observing similar changes in BP as seen in the present study. It may be that they were underpowered for these modest changes (~ 3–4 mmHg) to be statistically significant, as they used a similar sample size but included more comparisons. Additionally, it is possible that a lack of effect of CBD may result from a developed tolerance to CBD in chronic administration (> 7 days), leading to diminished effects of CBD on BP [13]. Ultimately, more work is needed in order to understand whether a tolerance develops with chronic administration of CBD and how this may influence its potential as a therapeutic agent in hypertension.
We acknowledge that our study did not provide insight into the putative mechanism(s) of action by which CBD may exert potential anti-hypertensive effects. Based largely on pre-clinical studies, other benefits of CBD mentioned in the Introduction (i.e. analgesic, anti-inflammatory, anti-oxidant, anxiolytic, anticonvulsant and antiproliferative cytotoxic), are mediated through signalling mechanisms, including the cannabinoid receptor 1 (weak agonist), the cannabinoid receptor 2 (inverse agonist), the serotonin 1a receptor, G protein-coupled receptor 55, G protein-coupled receptor 18 and the transient receptor potential cation channel subfamily V member 1 receptors (reviewed in [51]). In addition to cannabinoid receptors, there is also preclinical evidence that CBD or its derivatives may act as a potent agonist with nanomolar affinity at α2-adrenoreceptors in peripheral tissues and the central nervous system [52, 53]. These α2-adrenoreceptors are presynaptic autoreceptors found both peripherally and centrally, which inhibit norepinephrine release to reduce sympathetic nervous system activity. Although we did not observe any changes in cardiac autonomic activity (as indexed via HRV), it remains that reduced norepinephrine release onto cardiovascular end organs, such as the vasculature, can reduce vasoconstriction to lower BP [54]. These mechanisms should be explored in future human studies.
Influence of CBD on Arterial Stiffness
In young normotensive volunteers, it was demonstrated that 7 days of CBD administration (600 mg per day) resulted in unchanged resting and 24-h BP; however, in this same study, the repeated CBD dosing decreased arterial stiffness and improved endothelial function in the CBD group [13]. Reduced nitric oxide (NO) bioactivity is associated with increased arterial stiffness in hypertension (reviewed in [55]), and it seems likely that increased NO production in response to CBD administration mediates the observed reductions in arterial stiffness [13, 33, 56]. Activation of the CB1 receptor, as well as Gi and phosphatidylinositol 3-kinase signalling pathways, all lead to increased NO production and, ultimately, to vasorelaxation [33, 56]. Additionally, it is known that arterial stiffness is influenced by the tone of arterial smooth muscle irrespective of the signalling pathway in which it is modulated [57]. Vascular smooth muscle tone is affected by both endothelial cell signalling and the sympathetic nervous system [58, 59]. As noted above, although our findings of a lack of change in cardiac autonomic function—and hence potentially the sympathetic nervous system—the mechanism(s) by which CBD may reduce arterial stiffness remain to be determined in humans. Regardless of the precise mechanism(s), any reductions in arterial stiffness would likely mediate an anti-hypertensive influence.
Influence of Sex on CBD Related Changes in HR
Initial analyses of our data have revealed that there was no significant difference in HR between CBD and placebo conditions, which is consistent with a large amount of data in both humans and mice during resting conditions (summarised in [3, 13]). THC has been shown to increase HR, an effect likely mediated through cannabinoid receptor 1 (CB1; reviewed in [60]). As CBD is a weak, partial agonist of the CB1 receptor, it may be that, when looking at all participants together, its activation of the CB1 receptor may not have been sufficient to elicit an increase in HR. However, further analyses of our data suggest that, while HR is comparable in women in both drug conditions, men had significantly higher HR while taking CBD. Acute administration of CBD has previously been shown to increase resting HR by ~ 10 bpm in young, healthy males [12]. The CB1 receptor appears to be more highly available in the brains of men compared to women [61]. Therefore, it may be that the mild increase in HR seen in males while on CBD may be mediated by the CB1 receptor. However, the HR response to CBD is inconsistent, even in studies using similar dosing protocols, experimental protocols and participants [12, 13], and conversely significantly reduced HR with chronic CBD administration in older hypertensive patients [37]. Ultimately, further studies need to be carried out in order to elucidate the mechanisms by which both acute and chronic dosing of CBD may influence HR.
Perspectives
It has been previously determined that the threshold for BP reduction resulting in decreased composite cardiovascular endpoints was 4.6 mmHg for SBP and 2.2 mmHg for DBP [62]. Moreover, a 1-m/s reduction in aortic stiffness (central PWV) is associated with a reduction in the risk of cardiovascular events and all-cause mortality by 10% [63]. Thus, the averaged effect observed in our study of ~ 5 mmHg (24 h) to ~ 7 mmHg (nighttime) reduction in SBP was statistically and clinically significant during both daytime and nighttime, but the reduction in SBP was more pronounced during the latter. Likewise, arterial stiffness was reduced by ~ 0.7 m/s. Although the clinical implications and safety of longer-term CBD use in hypertension remains to be established, the observed reductions in BP are particularly remarkable, given the fact that many existing drugs used to treat hypertension require several weeks of treatment and/or combination dosing before they produce clinically meaningful reductions in BP [64]. Moreover, the more pronounced reductions in BP during sleep of 10–20% between 03:00 and 04:00 may also have therapeutic value, as these periods are most often associated with cardiac stress and infarct events in hypertensive patients, when people rise suddenly from and/or become increasingly active relative to the supine/sleeping state [65].
Methodological Considerations
The strengths of our study include the double-blinded placebo-controlled crossover trial in volunteers with hypertension, with concurrent measures of 24-h BP, ECG, arterial stiffness, as well as physical activity and sleep patterns. Excluding changes in physical activity and sleep patterns helps support the findings of a direct effect of CBD on BP and arterial stiffness. Measures of 24-h ambulatory BP and ECG monitoring is superior to single office measurements especially for the influence on cardiovascular endpoints and detection of cardiac arrythmias [66]. This was a “real-world” study that included patients with untreated hypertension in the absence of other major comorbidities; however, it should be noted that our volunteers were, on average, classed as overweight or obese. Although neither body mass nor BMI were found to be a significant predictor of BP or arterial stiffness, the influence of CBD on the cardiovascular system in humans might depend not only on dose and duration of administration [review in [13]] but also on the delivery method of CBD. For example, oral CBD at doses of 90 mg did not influence BP, HR and cerebral perfusion; however, the same dose of CBD encapsulated as TurboCBD™ (patented DehydraTECH™ capsule formulation increasing CBD bioavailability) decreased BP and increased cerebral perfusion [67]. TurboCBD™ formulation included 45 mg or 90 mg of CBD in 120 mg or 240 mg of hemp oil, together with 600 mg or 1200 mg American ginseng and 240 mg or 480 mg ginkgo biloba, respectively. The current study used a similar DehydraTECH™ 2.0 patented capsule formulation increasing CBD bioavailability; therefore, a limitation of our study is that we did not collect blood samples to measure CBD and related metabolites of interest that could be acting to influence BP or arterial stiffness.
We also acknowledge that CBD is a known inhibitor of the cytochrome P450 (CYP) system and can therefore increase plasma concentrations of medicines already in use [68], in particular anti-epileptic drugs [69]. Whether there are any drug–drug interactions between CBD and anti hypertensive therapy remains to be determined in further studies before CBD can be recommended as an adjunct therapy for treated hypertension in humans.
Conclusions
Our study suggests that CBD may reduce systolic and mean arterial BP through reducing arterial stiffness. Additional research on CBD is needed to define the precise molecular mechanisms and sites of action, pharmacokinetics, effects of more chronic administration, drug–drug interactions and potential for therapeutic use in human hypertension. Future studies should compare reductions in BP between normotensive and hypertensive patients to assess the whether the magnitude of change in BP is greater in hypertensive patients, as our current data support.
References
Kovalchuk O, Kovalchuk I. Cannabinoids as anticancer therapeutic agents. Cell Cycle. 2020. https://doi.org/10.1080/15384101.2020.1742952.
Klimkiewicz A, Jasinska A. The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Acad Sci Eng Med 2017.
Sultan SR, Millar SA, England TJ, O’Sullivan SE. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol. 2017;8:1–13.
Alfulaij N, Meiners F, Michalek J, Small-Howard AL, Turner HC, Stokes AJ. Cannabinoids, the heart of the matter. J Am Heart Assoc. 2018;7:1–10.
Atalay S, Jarocka-karpowicz I, Skrzydlewskas E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2019;9:1–20.
Richter JS, Quenardelle V, Rouyer O, Raul JS, Beaujeux R, Gény B, et al. A systematic review of the complex effects of cannabinoids on cerebral and peripheral circulation in animal models. Front Physiol. 2018;9:00662.
Resstel LBM, Joca SRL, Moreira FA, Corrêa FMA, Guimarães FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006;172:294–8.
Granjeiro ÉM, Gomes FV, Guimarães FS, Corrêa FMA, Resstel LBM. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol Biochem Behav. 2011;99:743–8. https://doi.org/10.1016/j.pbb.2011.06.027.
Gomes FV, Reis DG, Alves FHF, Corrêa FMA, Guimarães FS, Resstel LBM. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. 2012;26:104–13.
Zuardi AW, Cosme RA, Graeff FG, Guimaraes FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–8.
Arout CA, Haney M, Herrmann ES, Bedi G, Cooper ZD. A placebo-controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br J Clin Pharmacol. 2022;88:347–55.
Jadoon KA, Tan GD, O’sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight. 2017;2:1–11.
Sultan SR, O’Sullivan SE, England TJ. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: a randomised controlled trial. Br J Clin Pharmacol. 2019;86:1125–38.
Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness an expert reappraisal. Hypertension. 2018;72:796–805.
Remiszewski P, Jarocka-Karpowicz I, Biernacki M, Jastrząb A, Schlicker E, Toczek M, et al. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int J Mol Sci. 2020;21:1–24.
Pacher P, Bátkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–8.
Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline with Cardiovascular Events Later in Life. JAMA J Am Med Assoc. 2018;320:1774–82.
Kumric M, Bozic J, Dujic G, Vrdoljak J, Dujic Z. Chronic effects of effective oral cannabidiol delivery on 24-h ambulatory blood pressure and vascular outcomes in treated and untreated hypertension (HYPER-H21–4): study protocol for a randomized placebo-controlled, and crossover study. J Pers Med. 2022;12:1037.
Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019.
Perucca E, Bialer M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs. 2020;34:795–800. https://doi.org/10.1007/s40263-020-00741-5.
Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60:1586–92.
Tedesco S, Sica M, Ancillao A, Timmons S, Barton J, O’Flynn B. Validity evaluation of the fitbit charge2 and the garmin vivosmart HR+ in free-living environments in an older adult cohort. JMIR mHealth uHealth. 2019;7:1–15.
Kiviniemi AM, Perkiömäki N, Auvinen J, Niemelä M, Tammelin T, Puukka K, et al. Fitness, fatness, physical activity, and autonomic function in midlife. Med Sci Sports Exerc. 2017;49:2459–68.
Tulppo M, Huikuri HV. Origin and significance of heart rate variability. J Am Coll Cardiol. 2004;43:2278–80. https://doi.org/10.1016/j.jacc.2004.03.034.
Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9.
Wassertheurer S, Kropf J, Weber T, Van Der Giet M, Baulmann J, Ammer M, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498–504.
Lenth R V. emmeans: estimated marginal means, aka least-squares means. 2022. p. R package version 1.7.4-1.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26.
WHO. Cannabidiol (CBD): World Health Organization Expert Committee on drug dependence thirty‐ninth meeting [Internet]. World Heal. Organ. Tech. Rep. Ser. 2017. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32310508.
Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46:86–95.
Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58–63.
Mathew RJ, Wilson WH, Humphreys D, Lowe JV, Wiethe KE. Middle cerebral artery velocity during upright posture after marijuana smoking. Acta Psychiatr Scand. 1992;86:173–8.
Stanley CP, Hind WH, Tufarelli C, O’Sullivan SE. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res. 2015;107:568–78.
Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:434–43.
Dean C. Cannabinoid and GABA modulation of sympathetic nerve activity and blood pressure in the dorsal periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol. 2011;301:1765–72.
Kumric M, Dujic G, Vrdoljak J, Svagusa K, Kurir TT, Supe-Domic D, et al. CBD supplementation reduces arterial blood pressure via modulation of the sympatho-chromaffin system: a substudy from the HYPER-H21–4 trial. Biomed Pharmacother. 2023;160:114387. https://doi.org/10.1016/j.biopha.2023.114387.
Abuhasira R, Haviv YS, Leiba M, Leiba A, Ryvo L, Novack V. Cannabis is associated with blood pressure reduction in older adults—a 24-hours ambulatory blood pressure monitoring study. Eur J Intern Med. 2021;86:79–85. https://doi.org/10.1016/j.ejim.2021.01.005.
Wheal AJ, Cipriano M, Fowler CJ, Randall MD, O’Sullivan SE. Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. J Pharmacol Exp Ther. 2014;351:457–66.
Baranowska-Kuczko M, Kozłowska H, Kloza M, Sadowska O, Kozłowski M, Kusaczuk M, et al. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: modification by hypertension and the potential pharmacological opportunities. J Hypertens. 2020;38:896–911.
Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–46.
Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–96.
O’Sullivan SE, Kendall DA, Randall MD. Further characterization of the time-dependent vascular effects of Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;317:428–38.
Grassi G, Bombelli M, Seravalle G, Dell’Oro R, Quarti-Trevano F. Diurnal blood pressure variation and sympathetic activity. Hypertens Res. 2010;33:381–5.
Kumric M, Vrdoljak J, Dujic G, Supe-Domic D, Ticinovic Kurir T, Dujic Z, et al. Serum catestatin levels correlate with ambulatory blood pressure and indices of arterial stiffness in patients with primary hypertension. Biomolecules. 2022;12:1204.
Meng L, Ye XJ, Ding WH, Yang Y, Di BB, Liu L, et al. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J Cardiovasc Med. 2011;12:643–7.
Kiranmayi M, Chirasani VR, Allu PKR, Subramanian L, Martelli EE, Sahu BS, et al. Catestatin Gly364Ser variant alters systemic blood pressure and the risk for hypertension in human populations via endothelial nitric oxide pathway. Hypertension. 2016;68:334–47.
Zalewska E, Kmieć P, Sworczak K. Role of catestatin in the cardiovascular system and metabolic disorders. Front Cardiovasc Med. 2022;9:1–14.
Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol. 2021;20(1):12. https://doi.org/10.1186/s12933-021-01356-w.
Pędzińska-Betiuk A, Weresa J, Schlicker E, Harasim-Symbor E, Toczek M, Kasacka I, et al. Chronic cannabidiol treatment reduces the carbachol-induced coronary constriction and left ventricular cardiomyocyte width of the isolated hypertensive rat heart. Toxicol Appl Pharmacol. 2021;411:115368.
Remiszewski P, Malinowska B. Why multitarget vasodilatory (endo)cannabinoids are not effective as antihypertensive compounds after chronic administration: comparison of their effects on systemic and pulmonary hypertension. Pharmaceuticals. 2022;15:1119.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–53.
Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent α 2-adrenoceptor agonist and moderately potent 5HT 1A receptor antagonist. Br J Pharmacol. 2010;159:129–41.
Cathel AM, Reyes BAS, Wang Q, Palma J, Mackie K, Van Bockstaele EJ, et al. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci. 2014;40:3202–14.
Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth Prog. 2015;62:31–8.
Hermann M, Flammer A, Lüscher TF. Nitric oxide in hypertension. J Clin Hypertens. 2006;8:17–29.
McCollum LT, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–7.
Fok H, Jiang B, Clapp B, Chowienczyk P. Regulation of vascular tone and pulse wave velocity in human muscular conduit arteries: selective effects of nitric oxide donors to dilate muscular arteries relative to resistance vessels. Hypertension. 2012;60:1220–5.
Wilkinson IB, Mceniery CM. Arterial stiffness, endothelial function and novel pharmacological approaches. Clin Exp Pharmacol Physiol. 2004;31:795–9.
Bruno RM, Ghiadoni L, Seravalle G, Dell’Oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3(JUL):1–15.
Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol. 2002;42:64–70.
Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio RL, Sormunen E, et al. Sex difference in brain CB1 receptor availability in man. Neuroimage. 2019;184:834–42.
Verdecchia P, Gentile G, Angeli F, Mazzotta G, Mancia G, Reboldi G. Influence of blood pressure reduction on composite cardiovascular endpoints in clinical trials. J Hypertens. 2010;28:1356–65.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. https://doi.org/10.1016/j.jacc.2009.10.061.
SPRINT Research Group. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–16.
Kario K. Morning surge in blood pressure and cardiovascular risk: Evidence and perspectives. Hypertension. 2010;56:765–73.
Diederichsen SZ, Haugan KJ, Kronborg C, Graff C, Højberg S, Køber L, et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation. 2020;141:1510–22.
Patrician A, Versic-Bratincevic M, Mijacika T, Banic I, Marendic M, Sutlović D, et al. Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double-blinded placebo-controlled pharmacokinetics study. Adv Ther. 2019;36:3196–210.
Zendulka O, Dovrtělová G, Nosková K, Turjap M, Šulcová A, Hanuš L, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17:206–26.
Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58:1586–92.
Acknowledgements
Funding
Sponsorship for the present study were funded by Lexaria Bioscience Corp. (Kelowna, BC, Canada). The funds provided by the sponsor to the authors were used to fund the Rapid Service Journal associated with Advances in Therapy.
Author Contributions
Study design: Zeljko Dujic; Data collection: Tanja Dragun, Ante Obad, Mikko Tulppo; Data Analyses: Mikko Tulppo, Courtney Brown. Original draft of manuscript was prepared by Tanja Dragun and Courtney Brown and all authors were involved in reviewing and editing the final version of the manuscript.
Disclosures
All authors, including Tanja Dragun, Courtney V. Brown, Mikko P. Tulppo, Ante Obad and Željko Dujić, declare that the study was sponsored by Lexaria Bioscience Corp., the company which manufactures the DehydraTECHTM2.0 used in the present study. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Compliance with Ethics Guidelines
The protocol, and all methods included, in the present study were reviewed and approved by the ethics review board at the University of Split, School of Medicine (certificate number: 2181-198-03-04-21-0001) and conformed to the latest revision of the Declarataion of Helsinki, except for registration in a data base. All participants provided written, informed consent prior to enrolling in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dragun, T., Brown, C.V., Tulppo, M.P. et al. The Influence of Oral Cannabidiol on 24-h Ambulatory Blood Pressure and Arterial Stiffness in Untreated Hypertension: A Double-Blind, Placebo-Controlled, Cross-Over Pilot Study. Adv Ther 40, 3495–3511 (2023). https://doi.org/10.1007/s12325-023-02560-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02560-8